* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Focus on regulatory points
Survey
Document related concepts
Transcript
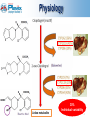
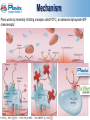
CYP 2C19 Polymorphism How can we ® save Plavix ? Duvernois Julie Dor Julie Fontaine Quentin Le Beherec Ronan Colin Jean-Baptiste 03/02/2011 0 In another world Plavix® would be… Clopidogrel problems only related to CYP 2C19 metabolism Clopidogrel with no patent expiration until the year 2020 …! 1 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 3 Physiology Active metabolite 30% Individual variability 4 Mechanism Plavix works by irreversibly inhibiting a receptor called P2Y12, an adenosine diphosphate ADP chemoreceptor 5 Clinical trials Phase III clinical trials: CAPRIE CURE COMMIT (N=19,185) (N=12,562) (N=45,852) - Trial endpoint: combined MI, ischemic stroke, or vascular death - Treatment regimen: PLAVIX vs aspirin Or PLAVIX+aspirin vs placebo+aspirine - Results: Reducing the risk of ischemic stroke, myocardial infarction (MI), or vascular death 6 www.plavix.com Clinical trials CURE: Primary Endpoint—MI/Stroke/CV Death 7 Indication - patients who have recently had a myocardial infarction - patients who have had a recent ischaemic stroke - patients with peripheral arterial disease - patients who have a condition known as ‘acute coronary syndrome’ including patients who have had a stent inserted 8 http://www.ema.europa.eu Pathology Acute coronary syndrome Set of symptoms linked to erosion or rupture of the atheromatousplaque Consequences : non-ST segment elevation MI - Myocardial infarction ST segment elevation MI - Unstable angina 9 Acute Coronary Syndrome Prevalence 2° leading cause of mortality: 120.000 MI/an, more than 47.000 deaths 10% of total year mortality 10 Market authorization 1997 Centralized procedure 1998 11 Market study Market share Price: 56.90€ Reimbursement: 65% In terms of value Row Product 1 PLAVIX 2 TAHOR 3 SERETIDE 4 INEXIUM 5 ENBREL In terms of quantity: 18° Plavix is the top-selling antiplatelet agent in the market: $9,1bn in 2009 Blockbuster: the 2nd top selling drug in the world 12 www.afssaps.fr - Thomson pharma Plavix presentation Conclusion Because health matters - Efficacy demonstrated with Better Safety profile - Solution for a big public health problem which touch many patients an effective drug which deserves to be fought for ASMR II 13 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 14 Cytochrome P-450 polymorphisms & response to Clopidogrel CARRIERS NON-CARRIERS of at least one CYP 2C19 reduced-function allele → Extended categorical classification: ultrarapid extensive intermediate poor metabolizer CARRIERS of at least one CYP2C19 reduced-function allele = 34% of the study population PHARMACOKINETIC → 32.4% reduction in plasma exposure to the active metabolite Ultrarapid → highest exposure Poor metabolizer → lowest exposure PHARMACODYNAMIC → 25% reduction in platelet inhibition Ultrarapid → greatest platelet inhibition Poor metabolizer → least platelet inhibition 16 CARRIERS of at least one CYP2C19 reduced-function allele = 27,1% of the study population (395 subjects) CARDIOVASCULAR OUTCOMES CARRIERS → Significantly NON-CARRIERS higher risk for cardiovascular outcomes 15 months → 3-times more risk of stent thrombosis 17 Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction CARRIERS *1/*2 & *2/*2 (73 patients) NON-CARRIERS *1/*1 (186 patients) Lancet 2009: 373; 309–17 CARDIOVASCULAR OUTCOMES NON-CARRIERS CARRIERS 19 Lancet 2009: 373; 309–17 CLOVIS-2 Hypothesis Determine whether of clopidogrel can Study design Heterozygous *1/*2 Homozygous *2/*2 Homozygous *1/*1 300 mg 300 mg 900 mg 900 mg 20 Average residual platelet aggregation : the lowest the highest patients *1/*1 patients *2/*2 High clopidogrel LD 21 Conclusions… Heterozygous *1/*2 Homozygous *2/*2 For ± 30% of the population → 25% reduction in platelet inhibition → Significantly higher risk for cardiovascular events Heterozygous *1/*2 For ± 25% of the population → 3-times the dosage = 1 time the dosage in *1/*1 NORMAL metabolizer 22 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 23 Pharmacogenomics CYP 2C19 genotype CYP2C19*1 allele FULLY functional CYP2C19*2 and *3 alleles NO functional 24 Distribution Homozygous NORMAL metabolizer *1/*1 Heterozygous INTERMEDIATE metabolizer *1/*2 Homozygous POOR metabolizer *2/*2 ± 70% ± 25% ± 5% 25 Ethnical distribution CYP2C19*2 and *3 alleles Majority of reduced Function alleles in White (85%) & Asian (99%) poor metabolizers Poor metabolizer patients possess 2 loss-of-funtion alleles Poor CYP 2C19 metabolizer Genotypes are approximately White : 2% Black : 4% Asian : 14% 26 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 27 FDA Black Box Warning In March 2010: Plavix received a boxed warning to its US label WARNING: Diminished effectiveness in poor metabolizers The Boxed Warning in the drug label will include information to: - Warn about reduced effectiveness in patients who are poor metabolizers of Plavix. Poor metabolizers do not effectively convert Plavix to its active form in the body - Inform healthcare professionals that tests are available to identify genetic differences in CYP2C19 function - Advise healthcare professionals to consider use of other anti-platelet medications or alternative dosing strategies for Plavix in patients identified as poor metabolizers An appropriate dose regimen for « poor metabolizers » has not been established 28 http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm European Medicines Agency European Public Assessment Reports No safety alert about CYP2C19 29 http://www.ema.europa.eu/ European Medicines Agency Summary of product characteristics: 30 http://www.ema.europa.eu/ SUMMARY Plavix® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 31 Competitor No CYP2C19 polymorphism Prasugrel Significant increase in serious bleeding Centralised Procedure French market February 2009 January 2010 Price 56,04 € (30 tablets) ASMR V 32 NICE guidance Prasugrel is prefered to Clopidogrel for treating ACS Guidance based on Triton-Timi 38 study Fewer hospitalisations = savings 33 http://guidance.nice.org.uk/TA182 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 34 Focus on Medco (USA) Medco is a PBM (Pharmacy benefit manager) « improving quality and reducing costs in order to extend access » 2C19 test for each clopidogrel prescription (after patient consentment) 35 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 36 Why is it important to save Plavix® ? Plavix® really doesn’t work in only 5% of the population POOR metabolizer For 95% of the population very good and known DRUG ! EXCLUSION of this small proportion Better trust in the drug & in Sanofi Better compliance Only 5% loss of the market ! 37 Our clinical Investigation 38 Our Clinical Investigation 2 cardiologists Biopathology center Lille Evaluation of prescribing cardiologists deal with nonresponders Plavix® Dijon 4 cardiologists 4 cardiologists Marseille 39 Questionnaire to CARDIOLOGIST (city & hospital) 40 What we observed… Cardiologists know there is a problem with Plavix® But the origin of the problem isn’t really known Information at medical meeting, studies but not by Sanofi, Afssaps or EMA They know that genotyping test exist in hospital Phenotyping test : VASP, VerifyNow® In case of emergency Efient®, no genotyping test ! If the test is recommended, so they will They are saying that currently there are no answers to this problem 41 Currently Response to Plavix® No response to Plavix ® ? Efficacy tests CYP 2C19 TEST 42 Biopathology center Most important biopathology center in Europe Interview of Pr BROLY 2C19 test is already available (2009) DNA sequencing : 2C19 + ABCB1 43 In practice in Lille Test prescripted Informed consent Information by prescriptor 5 days x2 Results reported by the biologist DNA sequencing 44 Prescription conditions Genetic testing needed Submitted to bioethical law Patient written consent required 45 Biopathology Center CYP2C19 polymorphisms study Prescriptors’ opinions doesn’t convince agree with genomic test studies differ from practice Pr Broly follows FDA recommendation: FDA recommends, but does not require Today: no consensus on interpretation because doctors think that there are others factors involved in clopidogrel response Interpretation-decision is prescriptor-dependant BHN 730 = 197 € (no refund) 46 Conclusion of our study Analysis….to find solutions! No technical or availability barrier Interpretation by cardiologist ? Inform every prescriptor about genomic test - test must be inserted in practice - modify Plavix® prescription habits Who will pay ? the test seems to be too expensive for patient 47 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 48 Safety concerns ? Clopidogrel response variability Disminished activity Increased risk of CV event ? Important potential risk Risk Management Plan Scientific advice 49 PV plan Routine PharmacoVigilance ADR collection systems Reports – ADR PSUR Plavix® variability ? Distinguish normal incidence & response variability related events Additional PV activities 50 PV plan Additional PV activities Mesure variability rates incidence Active surveillance Patients follow-up Comparative observational studies Epidemiologic methods Test assessment PASS Clinical Trial 51 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 52 What about drugs with combined genotyping test ? 53 Extract of ABACAVIR SmPC 54 4.4 switch to 4.1 Clinical study required 55 Abacavir study PREDICT 1 RESULTS 56 N Engl J Med 2008; 358:568-579, Price : 102 € Charged to the patient 57 Extract of MARAVIROC SmPC TROFILE test only available in California (price : 800 $) 58 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance, Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 59 How can we do with ? We must show a significant difference between doing the test or not. CLINICAL STUDY60 Our study simulation The Prospective Randomized Evaluation of CYP2C19 Genotype Screening in a Clinical Trial hypothesis prospective pharmacogenetic screening for CYP 2C19 exclusion of those patients carrying the loss of function allele from Clopidogrel treatment as compared with 61 STUDY 1 DESIGN Recruitment : ≥ N sufficient Control group patients received VS prospective-screening Group CYP 2C19 TEST NORMAL metabolizer patients Allele 1*1 CYP 2C19 TEST POOR metabolizer INTERMEDIATE metabolizer patients Allele 1*2 and 2*2 30% exclusion 62 STUDY 2 DESIGN Recruitment : ≥ N sufficient What about intermediate metabolizer ? INTERMEDIATE metabolizer patients Allele 1*2 Only 5% excluded 63 Consequence on the SmPC If the results confirm the hypothesis Possible switch from 4.4 to 4.1 by the EMA 4.1 Therapeutic indications […] « Before initiating treatment with clopidogrel, screening for carriage of the CYP 2C19 *2 and *3 alleles should be performed in any patient, irrespective of racial origin . Screening is also recommended prior to re-initiation of clopidogrel in patients of unknown CYP 2C19 *2 and *3 status who have previously tolerated clopidogrel. » […] 64 THEN Obligation for the cardiologists to prescribe a test prior the first administration of Clopidogrel Any test possible (no information about that in SmPC!) Written consent of the patient required Importance of the test disponibility ! 65 In practice… RESULTS NORMAL metabolizer INTERMEDIATE metabolizer POOR metabolizer ± 70% ± 25% ± 5% 66 Evaluation of the need for RMA New indication condition Genetic testing Suficient for this purpose ? Solution found but implementation needed Additionnal risk minimisation actions needed COMMUNICATION 67 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 68 Communication What? Genetic variation Test Still a significant benefit Support AE notification 69 Cardiologists Information letter to the cardiologists sales representatives Information to the cardiologists 70 Healthcare professionals Direct Indirect 71 Public Healthcare professionals Patients association 72 Risk min assessment Test prescribed ? Reliable ? Test performed ? False - ? False + ? Patients agreement ? Still variability related CV events ? WHY ? 73 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation What already exists ? Risk Management Plan Study simulation Communication Focus on regulatory points Pharmacoeconomy 74 Focus on regulatory points Regarding diagnostics tests Tests are evaluated by FDA CDRH (Center for Devices and Radiological Health) is part of FDA Tests must be approved 75 Focus on regulatory points interactions Center for Drugs Evaluation and Research (CDER) Center for Biologics and Evaluation and Research (CBER) Center for Devices and Radiological Health (CDRH) Center for veteran medicine (CVM) Center for Food Safety and Applied Nutrition (CFSAN) AmpliChip CYP 450 array (Roche) 2D6 : 31 mutations and deletions/duplications 2C19 : 2 mutations 24/12/04 : AmpliChip CYP450 test is the first FDA approved pharmacogenetic test 76 Focus on regulatory points Regarding diagnostics tests Tests are evaluated by FDA CDRH (Center for Devices and Radiological Health) is part of FDA Tests must be approved 24/12/04 : AmpliChip CYP450 test is the first FDA approved pharmacogenetic test Tests are not evaluated by EMA AMM for tests doesn't exist CE label is enough Directive on in vitro Diagnostic Medical Devices (IVDD 98/79/EC) « Member States shall presume compliance with the essential requirements » 77 Focus on regulatory points Regarding diagnostics tests in SPC To use a diagnostic test can be labelled in SPC Directive on in vitro Diagnostic Medical Devices (IVDD 98/79/EC) Pharmacogenomic not mentionned Test supposed/said reliable by RMS Test performances not evaluated Non-proprietary tests (if not co-developed with the drug, Celsentri-Trofile) « with a reliable and robust method » as demonstrated by... 78 SUMMARY Plavix ® presentation Studies Pharmacogenomics FDA & EMA actions Threat : Prasugrel, NICE guidance Medco Clinical investigation Risk Management Plan What already exists ? Study simulation Communication Focus on regulatory points Pharmacoeconomy 79 Pharmacoeconomy Who will pay the CYP2C19 genomic test ? Medco $120 Biopathology center 197€ Patient Insurance Health In US Medco refund the80test Cost-Effectiveness Prasugrel vs Clopidogrel TRITON-TIMI 38 Study: VS Evaluation of the cost-effectiveness of Prasugrel vs Clopidogrel from the perspective of the US healthcare system by using data from TRITON-TIMI 38 Medication costs: $4.62/d $5.45/d 81 Journal of American Heart Association Cost-Effectiveness Prasugrel vs Clopidogrel Rate of hospitalization for MI, stroke, death higher for Clopidogrel (12,1 VS 9,9 P<0,001) Rate of hospitalization for major bleeding higher for Prasugrel (2,4 VS 1,8 P=0,03) 82 Journal of American Heart Association Cost-Effectiveness Prasugrel vs Clopidogrel Gastrointestinal hemorrhage 83 Journal of American Heart Association Cost-Effectiveness Prasugrel vs Clopidogrel Hospitalization cost Clopidogrel > Prasugrel 84 Journal of American Heart Association Cost-Effectiveness Prasugrel vs Clopidogrel Conclusion Drug costs Rehospitalizations costs Life-years lost $4.62/d $24 734 0.530 $5.45/d $24 205 0.428 Treatment with Prasugrel vs Clopidogrel appears to be an economically dominant strategy, resulting in both lower costs and greater life expectancy BUT no stratified patients with 2C19 genetic testing 85 Journal of American Heart Association Cost-Effectiveness Prasugrel vs Clopidogrel STUDY 1 DESIGN STUDY 2 DESIGN We can calculate the hospitalization costs between the prospectivescreening group and the control group to prove the test could reduce the hospitalization cost 86 Medco Analysis Genetic test is NOT cost-effective > $ 380 $ 380.0 Genetic test is cost-effective < $ 380 $ 4,62 87 www.medcoresearch.com Medco Analysis Genetic test is NOT cost-effective > $ 200 $ 200.0 Genetic test is cost-effective < $ 200 As the cost of clopidogrel decreases, the cost of genetic testing also has to decrease to keep the cost-effectiveness 88 Medco Analysis Cost-effectiveness calculated with following criterias : Cost of the hospitalizations Cost of genetic test Cost of the switch : clopidogrel → prasugrel (for 30% of patients max) Cost-effectiveness ≠ prasugrel-clopidogrel Cost of the genetic test ↓ cost of clopidogrel → ↑ ≠ ↓ cost of the genetic test 89 Who will pay ? TODAY : patients have to pay the test but savings because fewer hospitalisation with clopidogrel company image, still have strong sales Patient part of his genetic ID, French mentality ? 90 Back in reality 91 CONCLUSION GENETIC Factors • Polymorphisms of P2Y12 • Polymorphisms of CYP 3A4 • Polymorphisms of CYP 2C19 CLOPIDOGREL RESPONSE VARIABILITY CLINICAL Factors CELLULAR Factors • Poor absorption • Drug-drug interactions (IPP) • Diabetes mellitus/insulin resistance • Elevated body mass index • Accelerated platelet turnover • Increased ADP exposure • Up-regulation of the P2Y12 pathway 92 SWOT Strengths Good tolerance if it is active Prescriptors habits with Plavix More indications than Prasugrel Already tested in USA by Medco Only 5% of market lost with test Opportunities Risk of major bleeding with Prasugrel Tests are available Already leader Weaknesses Cost of new study to change SPC Test is expensive and no reimboursment Cardiologists need to be informed Threats Concurrence of Prasugrel and others NICE recommendation in favour of prasugrel Many others variability problems Study results ??? 93 Thank you for your attention ! 94 Back-up slides 95 The Thienopyridine Family Ticlopidine (1st generation) P2Y12 ADP receptor antagonism: antithrombotic treatment of choice for coronary stenting Side effects: neutropenia, thrombocytopenia, rash, diarrhea, not use if patient has severe liver disease Delayed time frame to achieve full antiplatelet effects Solution to these problems: Better Safety profile - Fewer side effects (CLASSICS trial. Bertrand NE et al. Circulation. 2000;102:624-629.) Clopidogrel (2nd generation) Rapid onset of action with a loading dose (Cadroy Y et al. Circulation. 2000;101:2823-2828.) Better clinical outcomes (Bhatt DL et al. J Am Coll Cardiol. 2002;39:9-14.) 96 Where perform the Abacavir HLA test ? Laboratory with special approval from the Ministry of Health for research of genetic markers (11 in France) Laboratory known as "HLA" (33 in France) - HLA laboratories of EFS - HLA laboratories specializing in marrow or organs, - HLA laboratories of hospitals 97 Test VASP •Échantillon sanguin, dure 24h •Mesure de l’état de phosphorylation du VASP = vasodilator stimulated phosphoprotein (protéine exprimé sur les plaquettes) •Plus le CLOPIDOGREL se fixe moins le VASP est phosphorylé < 50% ok > 50% résistance au clopidogrel 98 Test VerifyNow® •Échantillon sanguin •Detection optique mesure de l’agrégation plaquettaire (niveau de blocage du R P2Y12) 99 Prasugrel Indication • syndrome coronarien aigu (angor instable ou IM avec ou sans sus-décalage du segment ST) • chez les patients traités par angioplastie primaire ou retardée • CI chez > 75ans et < 60kg 100 GECCO by Medco Genotype Guided Comparison of Clopidogrel and Prasugrel Outcomes Study Objective Compare the effectiveness of clopidogrel in CYP2C19 extensive metabolizers with prasugrel in adults recently hospitalized for acute coronary syndrome with primary, delayed, or planned percutaneous coronary intervention. Study design Group with 5 or 10 mg of prasugrel VS Group with 75 mg of clopidogrel, extensive metabolizers CYP 2C19 TEST