* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download diabetes mellitus
Survey
Document related concepts
Transcript
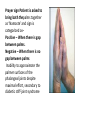
Anesthetic implications of diabetes mellitus Moderator Dr Dara Negi Dr Avinash Goyal Introduction the prevalence of diabetes mellitus (DM) is rapidly increasing throughout the world physicians will be confronted with an increasing population of diabetic patients undergoing anesthesia and surgery A significant number with type 2 DM are unaware that they are diabetic until the time of surgery. Diabetics have higher morbidity and mortality as surgical patients. End-organ effects of diabetes are more important to periop outcome Tighter the control of glucose desired, more frequently glucose levels to be measured PHYSIOLOGICAL IMPACT 6th leading cause of death by disease Decreases life expectancy of middle-aged people by 5-10 years 2-4 x greater risk of death d/t heart disease Leading cause of blindness in 25-74 year olds Leading cause of non-traumatic amputations Responsible for 25-30% of all new dialysis patients Diabetes mellitus results from an inadequate supply of insulin and/or an inadequate tissue response to insulin, yielding increased circulating glucose levels with eventual microvascular and macrovascular complications. DIAGNOSIS As per American Diabetes association (ADA) A normal fasting plasma glucose is 70 to 100 mg/dL. A random plasma glucose concentration of 200 mg/dL or higher, with classical signs and symptoms (polyuria,polydyspia,unexplained weight loss) or A fasting (no calorie intake >8hrs)plasma glucose concentration of 126 mg/dL or higher or An abnormal oral glucose tolerance test (OGTT), in which the glucose concentration is 200 mg/dL or higher 2 hours after a standard carbohydrate load (75 gm of glucose). • Glucose – whole blood values are 10-15% lower than plasma/serum – Venous blood values are 10% lower than capillary/arterial. – Venous blood - loss of 0.33 mmol/L per hour – There is no decrease within 24 h in the presence of sodium fluoride Impaired Fasting Glucose • Any fasting glucose between 101 and 125 mg/dL • Increased risk for DM • Must educate regarding risks and need for lifestyle modifications IMPAIRED GTT • Defined as a plasma blood glucose of >/= to 140 but < 200 after a 2 hour 75 gram glucose tolerance test • 8-10% of population have this problem with a 25 % risk of developing DM 2 • Compounding risk factors effect risk of developing Type 2 DM – – – – Age Activity Comorbidities Weight • Increased risk for macrovascular diseases • Must educate regarding risks and need for lifestyle modifications HEMOGLOBIN A1c provides a valuable assessment of long-term glycemic control Erythrocyte hemoglobin is nonenzymatically glycosylated by glucose HbA1c is stable glycosylated hemoglobin Its percentage concentration indicates average plasma glucose concentration during the preceding 60 to 90 days. Performed 2 times a year. The normal range for Hb A1c is 4% to 6%. Increased risk of microvascular and macrovascular disease begins at a Hb A1c of 6.5%. Lowering HbA1C Reduces Risk of Complications Glucose physiology • Glucose is a crucial fuel source • insulin facilitates glucose movement into cells • Red blood cells, healing wounds, the brain, and the adrenal medulla require glucose approximately @ 2 mg/kg/min. • Daily requirement of glucose for 70 Kg man is 200gm/day(90-100g stored as glycogen) Normal glucose homeostasis is tightly regulated by three interrelated processes (1) glucose production in the liver, (2) glucose uptake and utilization by peripheral tissues, chiefly skeletal muscle, (3) actions of insulin and counter-regulatory hormones (e.g., glucagon). Metabolic problem • Diabetes is generalised catabolic state • Lipids are mobilized with release of fatty acids • Ketogenesis leads to acidosis & loss of cellular potassium • Gulconeogenesis & glycogenolysis ,with decreased peripheral glucose utilization causes hyperglycemia • Glycosuria causes osmotic polyuria & dehydration • Plasma hyperosmolarity ,acidosis,hyperkalemia results leading to coma & death GLUCOTOXICITY: high levels of glucose causes nonenzymatic glycosylation reactions that lead to the formation of abnormal proteins which weaken endothelial junctions and decrease elastance, which is responsible for the stiff joint syndrome (and difficult intubation secondary to fixation of the atlanto-occipital joint), as well as decrease wound-healing tensile strength. increase macroglobulin production by the liver (which would increase blood viscosity) and promote intracellular swelling by favoring the production of nondiffusible, large molecules (such as sorbitol). Glycemia also disrupts autoregulation. Glucose-induced vasodilation prevents target organs from protecting against increases in systemic BP. DM TYPE I Constitutes 5-10% of DM diagnosed Mostly appears in children and young adults caused by a T cell–mediated autoimmune destruction of beta cells of the pancreas. At least 80% to 90% of beta-cell function must be lost before hyperglycemia occurs A long preclinical period (9–13 years) presentation of clinical disease is often sudden and severe , Patients demonstrate hyperglycemia over several days to weeks associated with fatigue, weight loss, polyuria, polydipsia, blurring of vision, and signs of intravascular volume depletion The diagnosis is based on • : a random blood glucose greater than 200 mg/dL • hemoglobin (Hb) A1c level greater than 7.0%. • ketoacidosis indicates severe insulin deficiency and unrestrained lipolysis. Beta-cell destruction is complete within 3 years of diagnosis , with the process being slower in adults. TREATMENT-INSULIN t DM TYPE II Most common form of diabetes Involves about 90-95% of people with DM most type 2 diabetics have had the disease for approximately 4 to 7 years before it is diagnosed Associated with: older age obesity family history of DM prior history of gestational diabetes physical inactivity Ethnicity Patient with type II DM usually makes enough insulin but the body cannot use it effectively => insulin resistance Gradually insulin production decreases over the following years Symptoms are similar to type I but develop more gradually TREATMENT- diet control ( wt loss),exercise & medications (OHGs) Gestational diabetes Hyperglycemia first diagnosed during pregnancy Occurs in 2-5% of pregnancies Occurs due to placental hormone changes that effect insulin function (greater resistance) Screening usually occurs during the 24th-28th week in high risk patients Criteria for diagnosis is different than for Type 1 and Type 2 Dietary changes are initial treatment and insulin is the only BG lowering agent used Better maternal glycemic control lowers the incidence of neonatal hypoglycemia and hyper bilirubinemia Postpartum BG levels usually return to normal Women with a history of gestational diabetes have a 20-50% chance of getting type II DM within 5-10 years SECONDARY DM • DM occurs as a result of another problem (primary) – Medications • • • • Thiazides Diuretics Beta blockers Steroids • Treatment of the primary cause may resolve the DM but lifestyle modifications and medications may be needed as well DRUG THERAPY IN DM • Oral Diabetic Medications – Sulfonylureas-gliclazide,tolbutamide increases insulin release longer duration of action – Biguanides-(Metformin) potentiates insulin causes lactic acidoses in dehydrated pts – Alpha-Glucosidase (acarbose) Inhibitors-decrease absorption of glucose – Nonsulfonylurea Secretagogues • Meglitinide • D-phenylalanine derivatives – Thiazolidinediones (TZDs)-rosiglitazone • Insulins – Short acting - Soluble / Neutral insulin Insulin aspart Insulin lispro – Intermediate acting - Isophane – Long acting - Insulin Zinc suspension new insulin analogue - Glargine Detemir – Biphasic- mixture of short and intermediate Biphasic lispro Biphasic Isophane Types of insulin Insulin Lispro Aspart Neutral/ regular Isophane ultratard Glargine Onset 10-20′ 30′ 1h 4h 2-4h Peak 1h 1-3h 4-6h 6-18h peak less Duration 3-5h 4-8h 8-14h 24h 20-24h Complications of DM ACUTE – Metabolic • Diabetic ketoacidosis(DKA) • Hyperosmolar Hyperglycemic Nonketotic Syndrome(HHNS) • Hypoglycemia – Infections CHRONIC – Microvascular • Retinopathy • Nephropathy – Macrovascular • CAD • CVD • PVD – Neuropathies – Diabetic Foot Disorders – Psychosocial concerns DIABETIC KETOACIDOSIS (DKA) Signs and symptoms – – – – – – – – – Hyperglycemia (>300mg/dl) Elevated serum ketones Acidosis as evidenced by pH of 6.8-7.3 HCO3 < 18 Osmolarity <320 Dehydration Electrolyte depletion GI distress (abdominal pain, N/V) Respiratory distress (Kussmaul’s respirations and acetone breath odor) TREATMENT OF DKA Managing diabetic ketoacidosis (DKA) in an intensive care unit during the first 24-48 hours always is advisable. • Correction of fluid loss with intravenous fluids • Correction of hyperglycemia with insulin • Correction of electrolyte disturbances, particularly potassium loss • Correction of acid-base balance • Treatment of concurrent infection, if present Correction of Fluid Loss • Rehydration alone reduces plasma glucose by 30-50% • Initial correction of fluid loss is either by isotonic sodium chloride solution or by lactated Ringer solution. The recommended schedule for restoring fluids is as follows: • Administer 1-3 L during the first hour.(15-20ml/kg) • Administer 1 L during the second hour. • Administer 1 L during the following 2 hours • Administer 1 L every 4 hours, depending on the degree of dehydration and central venous pressure readings • When blood sugar decreases to less than 250 mg/dL, isotonic sodium chloride solution is replaced with 5-10% dextrose with half isotonic sodium chloride solution. Insulin therapy • Loading dose of 0.1U/kg i.v. of regular insulin • Followed by infusion of 0.1U/kg per hour • Average decline of plasma glucose should be 75-100ml/hr with target values around 250ml/dl Electrolyte Correction • If the potassium level > 6 mEq/L, do not administer potassium supplement. • If the potassium level is 4.5-6 mEq/L, administer 10 mEq/h of potassium chloride. • If the potassium level is 3-4.5 mEq/L, administer 20 mEq/h of potassium chloride. • Monitor serum potassium levels hourly, Correction of Acid-Base Balance • Bicarbonate typically is not replaced as acidosis will improve with the other treatments alone. • Administration of bicarbonate has been correlated with cerebral edema in children. • Sodium bicarbonate is infused if decompensated acidosis starts to threaten the patient's life(pH <7.1) • If sodium bicarbonate is indicated, 100-150 mL of 1.4% concentration is infused initially. This may be repeated every half hour if necessary. • Treatment of Concurrent Infection Hyperosmolar Hyperglycemic Nonketotic Syndrome(HHNS) • Four Clinical Features – – – – Hyperglycemia (> 600-2000) Mild or no ketosis Hyperosmolality of plasma or serum (>340) Profound dehydration • 10-20% mortality rate with HHNS • Risk factors include – Elderly with Type 2 – Undiagnosed DM – Prolonged hyperglycemia • Signs and Symptoms – – – – – Hypotension Dehydration Tachycardia Decreased mentation Neurologic abnormalities (focal) Treatment • If the plasma osmolarity > 320 mOsm/L, large volumes (1000–1500 mL/hr) of 0.45% normal saline should be administered until the osmolarity is less than 320 mOsm/L,followed by 0.9% normal saline Insulin infusion @ 0.1U/kg hr Electrolyte disturbances are less severe compared to DKA hypoglycemia plasma glucose level less than 50 mg/dL. May occur in all types of DM and is usually related to illness Causes Excessive exogenous insulin or oral diabetes medications that increase insulin production Excessive alcohol consumption especially w/o adequate food intake Impaired hepatic or renal function Too little food Excessive exercise Signs and symptoms adrenergic (sweating, tachycardia,palpitations, restlessness, pallor) and neuroglycopenic (fatigue,confusion, headache, somnolence, convulsions,coma). TREATMENT OF HYPOGLYCEMIA • Mild (40-60 gm/dl) – Ingestion of 15 grams of Carbohydrate – Monitor BG levels and treat until euglycemia – Educate to prevent • Moderate (20-40 gm/dl) – Ingestion of 15-30 grams of CHO and follow with a meal or snack containing protein – Monitor BG levels and treat until euglycemia – Educate to prevent • Severe (<20 gm/dl) – Ingestion not possible-must use IV glucose 0.5 g/kg IV or IM glucagon 0.5 to 1.0 mg preferably. – May use glucose gel, honey, syrup, or jelly inside cheek – Monitor BG levels and treat until euglycemia – Educate to prevent • Each ML of 50% glucose will raise BG by 2 mg/dl WOUND HEALING & INFECTIONS Wound healing is impaired in diabetic pts Infections are the common cause of diabetic complications – Alterations in leukocyte function– ↓ chemostasis – Impaired phagocytic activity – Reduced intracellular killing • Commonly seen infections – – – – – Cutaneous-furunculosis and carbuncles Vulvovaginits Cellulitis UTI Ear • Must be treated aggressively MICROVASCULAR COMPLICATIONS • Microangiopathies affecting the capillaries and arterioles of tissues – Retina (retinopathy) – Renal glomeruli (nephropathy) • RETINOPTHY – Development and progression largely depend on the duration and severity of hyperglycemia – Early detection and treatment can be beneficial in visual restoration – Uncontrolled HTN is an aggravating factor – 3 categories • Nonproliferative • Preproliferative • proliferative NEPHROPATHY • • • • • 20-40% chance of developing with a diagnosis of DM Earlier occurrence in Type 2 than Type 1 DM Glomerulosclerosis with glomerular basement membrane thickening 39-54% decrease in development with tight BG control (DCCT) Signs and symptoms – Microalbuminuria/proteinuria – Elevated creatinine clearance • Aggrevating conditions – HTN – Neurogenic bladder/UTI/Nephrotoxic drugs • Treatment – Improving BG control – HTN management (ACE inhibitors) – Protein restriction – Dialysis/kidney transplant – Educate to prevent MACROVASCULAR COMPLICATIONS • Responsible for 80% of deaths in people with DM • Major vessels affected – Coronary arteries – Cerebral arteries – Peripheral arteries • CORONARY – MI risk is 2x> in men and 3x> in women with DM – Treatment-Aimed at reducing modifiable risk factors • Smoking cessation • BG, HTN, and lipid control – Medications may include ACE or ARBs, statin drugs, and ASA • Exercise (focus on wt reduction) • CEREBRAL – 2x> risk of CVD in patients with DM • PERIPHERAL – 2-3x> risk in patients with DM – Signs and symptoms include IC, diminished peripheral pulses, lower extremity hair loss, and nocturnal rest pain – Major factor in the development of gangrene and amputations in patients with DM – Treatment • • • Aimed at modifiable risk factors as listed with CAD Medication Rest during periods of exertion Peripheral neuropathy • 50% of diabetics develop peripheral neuropathy after 25 years • Distal symmetrical diffuse sensorimotor polyneuropathy is most common(stocking glove neuropathy) • Foot ulcers develop due to loss of cutaneous sensitivity to pain and temp and impaired perfusion • Treatment includes optimal glucose control ,NSAIDS,Antidepressants,anticonvulsants for pain relief Diabetic Autonomic Neuropathy • affect any part of the autonomic nervous system. • Autonomic disturbances can be subclinical or clinical • Subclinical DAN can occur within a year or two after diagnosis • Cardiovascular autonomic neuropathy is a common type of DAN • A resting tachycardia and a loss of heart rate variability (HRV)(<10 beats per min)during deep breathing are early signs. • in the advance stages, severe orthostatic hypotension (>30 mm Hg with standing) is present. • Impaired respiratory reflexes and impaired ventilatory responses to hypoxia and hypercapnia are also present Gastrointestinal effects of DAN • Affects 25% of diabetics • impair gastric secretion and gastric motility, causing gastroparesis diabeticorum • nausea, vomiting, early satiety, bloating, and epigastric pain. • In DAN, the counterregulatory hormone responses to hypoglycemia are impaired and the warning signs eliminated, creating a dangerous situation of hypoglycemia unawareness. Diabetes and accelerated physiologic ageing(“RealAge”) (‘X’ yrs for each choronologic yr of disease) Diabetes cause accelerated aging Thus, the risks involved with diabetes are similar to those for someone much older, someone who has a much higher physiologic age (or “RealAge”). Poor control of glucose levels Tight control glucose levels Type 1 1.75 1.25 Type 2 1.5 1.06 Acute hyperglycemia causes • dehydration, • impaired wound healing, • an increased rate of infection, • worsening central nervous system/spinal cord injury with ischemia, • hyperviscosity with thrombogenesis Preop evaluation • History & general physical exam – MEASURE BLOOD PRESSURE AND PULSE RATE- BOTH WHEN THE PATIENT IS LYING DOWN AND STANDING – ASSESS THE PATIENT'S MUSCLE STRENGTH, DEEP TENDON REFLEXES, AND SENSE OF TOUCH. • HBA1c levels • Pulmonary,Cardiovascular & renal assessment • CXR-cardiac enlargement,pleural effusion, pulmonary vascular congestion • ECG • TM & C-spine mobility • No insulin ,no glucose for short procedures Pre-op evaluation Autonomic Neuropathy • typical symptoms and signs of postural hypotension, gastroparesis, gustatory sweating, and nocturnal diarrhoea. • It is worth assessing all diabetic patients for autonomic neuropathy. • The easiest way is to assess heart rate variability. The normal heart rate should increase by over 15 beats/minute in response to deep breathing. Neuropathy is likely is there is less than 10 beats/minute increase Peripheral Neuropathy • The commonest type of peripheral neuropathy is the “glove and stocking” type. • However diabetics are also prone to mononeuritis multiplex and some particularly painful sensory neuropathies. • Poor patient positioning is likely to result in pressure sores that are often slow to heal given poor peripheral blood flow. • Documentation of existing neuropathy is prudent, especially if considering a regional technique. • Cardiovascular • Diabetics are more prone to ischaemic heart disease (IHD), hypertension, peripheral vascular disease, cerebrovascular disease, cardiomyopathy and perioperative myocardial infarction. • Ischaemia may be “silent” as a result of neuropathy. Routine ECG should be performed and appropriate stress testing if in doubt. • Autonomic neuropathy can result in sudden tachycardia, bradycardia, postural hypotension and profound hypotension after central neuraxial blockade Respiratory • Diabetics, especially the obese and smokers are more prone to respiratory infections and also have abnormal spirometry. • Chest physiotherapy, humidified oxygen and bronchodilators should be considered Gastrointestinal • Gastroparesis is characterised by a delay in gastric emptying without any gastric outlet obstruction. Increased gastric contents increase the risk of aspiration. • Always ask about symptoms of reflux and consider a rapid sequence induction with cricoid pressure even in elective procedures. • If available prescribe an H2 antagonist such as ranitidine150mg plus metoclopramide 10mg, at least 2 hours preoperatively. Airway • Glycosylation of collagen in the cervical and temporo-mandibular joints can cause difficulty in intubation. Prayer sign Patient is asked to bring both thepalms together as ‘Namaste’ and sign is categorized as– Positive – When there is gap between palms. Negative – When there is no gap between palms Inability to approximate the palmer surfaces of the phalangeal joints despite maximal effort, secondary to diabetic stiff-joint syndrome Palm print The patient is made to sit; Palm and fingers of right hand are painted with blue ink, patient then presses the hand firmly against a white paper placed on a hard surface. It is categorized as: Grade 0 – All the phalangeal areas are visible. Grade 1 – Deficiency in the interphalangeal areas of the 4th and 5th digits. Grade 2 – Deficiency in interphalangeal areas of 2nd to 5th digits. Grade 3 – Only the tips of digits are seen Renal • Diabetes is one of the commonest causes of endstage renal failure. • Check urea, creatinine and electrolytes. Specifically check the potassium especially in view of the possible need for suxamethonium as a result of gastroparesis. • If unavailable, proteinuria is likely to indicate kidney damage. • Ensure adequate hydration to reduce postoperative renal dysfunction. Immune system • Diabetics are prone to all types of infection. Indeed an infection might actually worsen diabetic control. • Tight glycaemic control will reduce the incidence and severity of infections and is routine practice in the management of sepsis and diabetic foot infections. • Perform all invasive procedures with full asepsis. • Autonomic neuropathy predisposes to hypothermia under anaesthesia • Diabetics are prone to cataracts and retinopathy. Prevent surges in blood pressure, for example at induction, as this might cause rupture of the new retinal vessels. • Diabetes affects oxygen transport by causing glucose to covalently bind to the hemoglobin molecule, decreasing oxygen saturation and red blood cell oxygen transport in diabetic patients Anesthetic agents and diabetes mellitus • etomidate inhibits adrenal steroid genesis and may induce a decrease in the glycemic response to surgery. • alpha 2 agonist, clonidine, reduces sympathetic tone and the release of norepinephrine from nerve terminals. clonidine decreased circulating catecholamines & improved blood glucose control and decreased insulin requirements • High doses of opiates induce hemodynamic ,hormonal and metabolic stability • Avoid lactate containing solutions-cause hyperglycemia • Diabetics less able to metabolise lipid emulsionsprolonged infusions of propofol should be avoided-can lead to hyperglycemia • volatile anesthetics, such as halothane, enflurane,and isoflurane, inhibit insulin response to glucose • Halothane or sevoflurane, produced greater negative inotropic effects in myocardium of diabetic compared with nondiabetic patients • BZDs reduce the secretion of adrenocorticotrophic hormone (ACTH) and consequently cortisol, and stimulate basal secretion of growth hormone • Minimal in usual sedative doses but significant in continuous infusion to patients in intensive care units Anaesthetic Management General principals • Avoid hypoglycaemia (under 4mmol/l) as this can cause irreversible cerebral damage • Avoid severe hyperglycaemia (over 14mmol/l) to minimise dehydration and metabolic upset • Type 1 diabetics need insulin to prevent ketogenesis • • Aim for a blood glucose between 6 and 10mmol/l • Accurate, easy-to-use glucose monitors, make the practice of “permissive hyperglycaemia” unacceptable given the known outcome benefits of tight control • Diabetic patients should be placed first on the operating list to shorten the preoperative fast and potentially allow normal oral intake later that same day • Tight metabolic control is important for both type 1 and type 2 patients. • The best marker for recent control is the percentage of glycosylated haemoglobin (HbA1C). If available, levels less than 7% indicate good control whilst levels over 9% and particularly 12%, indicate poor control and likely associated electrolyte and water loss. • These patients should be admitted preoperatively for correction of these abnormalities and stabilisation of blood sugar levels before the addition of surgical stressors. • Continue all diabetic medication until the day of surgery except: a.) Chlorpropamide (stop 3 days prior as long acting, substitute with a shorter acting sulphonylurea) b.) Metformin only if major surgery as risk of lactic acidosis c.) Glitazones c.) Long acting insulin – substitute with short/intermediate acting Measure blood sugar preoperatively – 4 hourly if on insulin, 8 hourly if not If the patient is expected to eat within 4 hours of the operation then treat this group as having “Minor” surgery. Otherwise, surgery is “Major Minor surgery, type 2 diabetes NOT on insulin (diet/tablet controlled) • 1st on list • Preoperative blood sugar <180mg/dl Take normal medication inc. evening dose • Preoperative blood sugar >180mg/dl Treat as if “Major” surgery • Omit oral hypoglycaemic on morning of surgery • Monitor blood glucose 1 hour preop; intraoperatively if over 1 hour; and 4hourly postop until eating. • Recommence oral hypoglycaemics with first meal Minor surgery, type 2 diabetes ON insulin/type 1 diabetes, • • • • • 1st on list Take normal medication on day prior* Omit morning SC insulin if glucose < 126mg/dl Give half normal insulin if glucose >126mg/dl Monitor blood glucose 1 hour preop; intraoperatively at least once; 2 hourly until eating and then 4 hourly. • Recommence normal SC insulin with first meal • *If taking a long acting insulin, either convert to short/intermediate acting several days prior or ½ the dose the day prior to surgery Major surgery, all types of diabetes, • 1st on list • Normal medication on day prior (unless very poorly controlled, in which case, establish sliding scale below 3 days prior) • Day of surgery, omit oral hypoglycaemics/ normal SC insulin • Check blood glucose 1 hour preop; • hourly intraop until 4 hours • postop; 2 hrly thereafter and • 4 hourly once stabilised. • Insulin is the key infusion. With close monitoring, and adjustment according to a sliding scale, there is no absolute requirement for concurrent dextrose containing solutions, • However, commonly 5% or 10% dextrose solutions containing 20mmol/l of potassium are infused at a steady rate of 100ml/hr to provide carbohydrate substrate. • • Titrate the insulin infusion (through a dedicated line with one-way valve) as below. Blood glucose (mmol/l) Insulin infusion rate (unit/hr) If poor control* (unit/hr) <4 0 0 4.1 – 9 1 2 9.1-13 2 3 13.1-17 3 4 17.1-28 4 6 >28 6 (check infusion running) 8 • *If glucose not maintained <10mmol/l then increase the rates, Classic “Non-tight Control” Regimen • Aim-to prevent hypoglycemia,ketoacidosis and hyperosmolar states • protocol – NPO after midnight – 6AM –start i.v. dextrose 5% @ 125ml/hr/70 kgBW – ⅟2 usual morning insulin dose s/c – Cont 5D intra op – Post op -monitor glucose and give insulin on sliding scale “Tight Control” Regimen 1 • Aim-to keep glucose levels b/w 79-120 mg/dl. it Improves – wound healing & prevent wound infection – Neurologic outcomeafter global or focal ischemic insults – Weaning from CPB • Protocol (evening before surgery) – – – – Determine preprandial glucose levels Begin i.v. infusion of 5%Dextrose @ 50ml/hr/70kgBW Piggyback an infusion of regular insulin with infusion pump (50U in 250 ml NS) Infusion rate (U/hr)= plasma glucose(mg/dl)/150 (100 if pt taking steroids or BMI >35) – blood glucose monitoring 4 hrly – At the start of surgery -determine blood glucose levels and then, every 1-2 hrly for 24 hrs • Hypoglycemia(<50mg/dl) is treated with 15 ml of 50% dextrose • Tight control regimen 2 requires “feedback mechanical pancreas” Alberti GIK Regimen • Initial solution: 500 ml 10% glucose+15U insulin+10 mmol KCl • Infusion rate @ 100 ml/ h( 3U/h) • Check BG every 2 hr BG (mmol/L) action <6.5 10U insulin in 500 ml (2U/h) >6.4-<11.1 15U insulin in 500ml(3U/h) >11 20U insulin in 500ml(4U/h) • Adjust in 5-U steps Regional techniques • are not contraindicated in the diabetic patient • offer some potential advantages such as the avoidance of intubation, having an awake patient to warn of impending hypoglycaemia, and an earlier return to normal eating patterns. • Document any existing motor/sensory neuropathies prior to performing any blocks • look for evidence of autonomic neuropathy. If present, expect increased hypotension after neuraxial blocks. • The chances of epidural abscess are also increased. • high spinal anesthesia (dermatome level T2– T6) induced a reduction in the acute insulin response to glucose, whereas low spinal anesthesia (dermatome level T9–T12) induced no such reduction. future • Non-invasive BS testing • Continuous BS monitor + insulin pump • “Artificial Pancreas” • Islet cell transplants • Stem-cell research • Energy homeostasis breakthroughs thanks