* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download X-ray - Pharos University in Alexandria
Survey
Document related concepts
Transcript
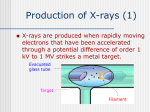
X-Ray Technology By: PROF. Dr. Moustafa Moustafa Mohamed Faculty of Allied Medical Science Pharos University in Alexandria Introduction to Medical Imaging NON Invasive Uses of medical imaging Obtain information about internal body organs or the skeleton to determine a patient’s physical Imaging Modalities: * X-ray (plan , Dental, Panorama, Mammo, Angio, …) * C.T * Ultrasound * MRI * Gamma Camera * PET SPECT Medical Image • Generated by means of radiation – electromagnetic (EM) – ultrasound – electrons • Displayed for interpretation on – Film – photograph or – computer display monitor Types of image 1) Projections 2) Dimensional 3D & 4D 3) Slices • • • • Trans axial - plane normal to a vector from head to toe. Coronal - plane normal to a vector from front to back Sagittal - plane normal to a vector from left to right. Oblique - a slice that is not (at least approximately) one of the above. Inside the atom The NUCLEUS is made up of PROTONS and NEUTRONS PROTONS have a positive charge. NEUTRONS have no electrical charge. Inside the atom ELECTRONS have a negative charge. The number of electrons in an atom usually matches the number of protons, making the atom electrical neutral. Exactly what is an X – Ray? • An x – ray is a form of radiation, that is invisible • Electromagnetic waves of short wavelength with wavelengths between about 0.02 Å and 100 Å (1Å = 10‐10 meters). • Very high energy • Basically gives an “inside view” • The energy of X‐rays, like all electromagnetic radiation, is inversely proportional to their wavelength as given by the Einstein equation: E = hν = hc/λ where E = energy h = Planck's constant, 6.62517 x 10‐27 erg.sec ν = frequency c = velocity of light = 2.99793 x 1010 cm/sec λ = wavelength Since X‐rays have a smaller wavelength than visible light, they have higher energy. • X‐rays can penetrate matter more easily than can visible light. • Their ability to penetrate matter depends on the density of the matter • X‐rays provide a powerful tool in medicine for mapping internal structures of the human body (bones have higher density than tissue, • and thus are harder for X‐rays to penetrate, fractures in bones have a different density than the bone, thus fractures can be seen in X‐ray pictures). History • In 1895 Wilhelm Roentgen, German Physicist, was studying high voltage discharges in vacuum tubes, then he noticed fluorescence of barium platinocyanide screen lying several feet from tube end. • These rays where named – X-rays--invisible penetrating radiation, – X represent unknown in mathematics William Conrad Roentgen • Wilhelm Conrad Rontgen Won the first Nobel Peace Prize for physics in 1901 Early X- Ray Images Right: Mrs. Röntgen's hand, the first X-ray In X-ray of Bertha Roentgen's Hand Types and uses of X-ray Types and uses of X-ray Diagnostic Still picture Continuous picture Therapeutic Still picture scan tomography Various uses of the X - Ray Detect malformations in bones • • • • • Treats disorders such as various cancers Security purposes Alternatives for cancer detection Annual physical exams Pre-surgery evaluations Electromagnetic Spectrum Production of X-rays (1) • X-rays are produced when rapidly moving electrons that have been accelerated through a potential difference of order 1 kV to 1 MV strikes a metal target. Evacuated glass tube Target Filament Production of X-rays (2) • Electrons from a hot element are accelerated onto a target anode. • When the electrons are suddenly decelerated on impact, some of the kinetic energy is converted into EM energy, as X-rays. • Less than 1 % of the energy supplied is converted into X-radiation during this process. The rest is converted into the internal energy of the target. Properties of X-rays • X-rays travel in straight lines. • X-rays cannot be deflected by electric field or magnetic field. • X-rays have a high penetrating power. • Photographic film is blackened by X-rays. • Fluorescent materials glow when X-rays are directed at them. • Photoelectric emission can be produced by X-rays. • Ionization of a gas results when an X-ray beam is passed through it. X-ray Spectra (1) • Using crystal as a wavelength selector, the intensity of different wavelengths of X-rays can be measured. X-ray Spectra (2) • The graph shows the following features. – A continuous background of X-radiation in which the intensity varies smoothly with wavelength. The background intensity reaches a maximum value as the wavelength increases, then the intensity falls at greater wavelengths. – Minimum wavelength which depends on the tube voltage. The higher the voltage the smaller the value of the minimum wavelength. – Sharp peaks of intensity occur at wavelengths unaffected by change of tube voltage. Minimum wavelength in the X-ray Spectra • When an electron hits the target its entire kinetic energy is converted into a photon. • The work done on each electron when it is accelerated onto the anode is eV. • Hence hf = eV and the maximum frequency f max eV h Therefore, min hc eV Continuous (Bremsstrahlung) X-Ray Production Characteristic X-ray Spectra • Different target materials give different wavelengths for the peaks in the X-ray spectra. • The peaks are due to electrons knock out inner-shell electrons from target atoms. • When these inner-shell vacancies are refilled by free electrons, X-ray photons are emitted. • The peaks for any target element define its characteristic X-ray spectrum. Characteristic X-Ray Production Anode Heating • This occurs when projectile electrons excite an atoms outer shell electrons but do not eject them from the atom. • For most X-ray machines, about 99% of the projectile electrons lose energy this way. • Infrared EM radiation (observed as heat energy) is produced when the excited electrons relax and fall back into the original energy level • The amount of anode heating can be reduced by increasing the energy of the projectile electrons so that they cause more ionization rather than excitation. Uses of X-rays • In medicine To diagnose illness and for treatment. • • In industry To locate cracks in metals. X-ray crystallography To explore the structure of materials. Conditions for x ray production • • • • Separation of electrons Production of high speed electrons Focusing of electrons Sopping of high speed electrons in target COLD GAS CATHODE TUBE • glass tube with partial vacuum with small amount of gas, • two electrodes, one negative (cathode) • & another positive (Anode). Hot Cathode Diode tube • In 1913 W.D. Coolidge invented new type of tube on Edison principal called • Hot Cathode Diode tube. • It made possible the control of mA and kV independently and there by controlling the quantity and quality of x-rays. PARTS OF X RAY TUBE • Glass Tube • Cathode – Filament – Supporting wires – Focusing cup • Anode – Stationary – Rotating Main components of x-ray unit are: •· X-ray tube •· X-ray electrical power generator •· Control unit •· Film or digital system In addition to: •· Table unit •· Bucky film tray and grid system •· Suspension system Introduction X-ray image is a shadow picture produced by x-rays emitted from a point source Image contrast is (((proportion with))) 1- Mass attenuation coefficient of the imaged part 2- Density 3- Thickness Principles of X-ray tube Battery Main components of a modern xray tube • A heated filament releases electrons that are accelerated across a high voltage onto a target. • The stream of accelerated electrons is referred to as the tube current. • X rays are produced as the electrons interact in the target. • The x rays emerge from the target in all directions but are restricted by collimators to form a useful beam of x rays. • A vacuum is maintained inside the glass envelope of the x-ray tube to prevent the electrons from interacting with gas molecules. X-Ray Tubes X‐ray Absorption • When the x‐rays hit a sample, the oscillating electric field of the electromagnetic radiation interacts with the electrons bound in an atom. • Either the radiation will be scattered by these electrons, or absorbed and excite the electrons. • A narrow parallel monochromatic x‐ray beam of intensity I0 passing through a sample of thickness x will get a reduced intensity I according to the expression: • I = I0 e‐μ x or Ln (I0 /I) = μ x • where μ is the linear absorption coefficient, which depends on the types of atoms and the density ρ of the material. How X‐ray Lose Energy within Matter? • • Photoelectric effect • X‐ray interacts with an electron by giving all its energy to the electron near the nucleus. • It is the most probable way of losing energy • X‐ray energy must be greater than or equal to the electron binding energy to the nucleus • Compton effect • X‐ray (of energy at least 511 KeV) colloids with a loosely bound outer electron. • The electron receive part of the energy and the rest goes in different direction as a scattered photon radiations each of 511 KeV Pair production • X‐ray (of energy at least 1.02 MeV) penetrates the intense electric field of nucleus. It is converted to an electron and a positron each of 511 KeV. • The positron will then colloids with one electron and results in the production of two annihilation Bragg's Law • • • • According to Bragg, X‐ray diffraction can be viewed as a process similar to reflection from planes of atoms in the crystal. In Bragg's construct, the planes in the crystal are exposed to a radiation source at a glancing angle θ and X rays are scattered with an angle of reflection also equal to θ. The incident and diffracted rays are in the same plane as the normal to the crystal planes. Bragg reasoned that constructive interference would occur only when the path length difference between rays scattered from parallel crystal planes would be an integral number of wavelengths of the radiation. When the crystal planes are separated by a distance d, the path length difference would be 2d sin θ. Thus, for constructive interference to occur Bragg’s law must be fulfilled. • • • • • For constructive interference: nλ = 2a From trigonometry: a = d sin θ or 2a = 2 d sin θ thus, nλ = 2d sin θ What it says is that if we know the wavelength ,λ , of the X‐rays going in to the crystal, and we can measure the angle θ of the diffracted X‐rays coming out of the crystal, then we know the spacing (referred to as d‐spacing) between the atomic planes. d = nλ /2 sin θ Checkpoint Question 1 What are x-rays? • X-rays are high-energy waves that travel at the speed of light. X-rays can penetrate fairly dense objects, such as the human body. They cannot be seen, heard, felt, tasted, or smelled. Checkpoint Question 2 • Why is it important to schedule a barium enema before an upper GI or barium swallow? Answer • Barium enemas involve filling only the large intestine with barium. Because the large intestine is the last part of the GI tract, this barium can be eliminated more quickly. If an upper GI examination is performed first, it may be days later before barium can be eliminated, thus delaying other examinations. Radiation Safety • X-rays have potential to cause cellular or genetic damage • At highest risk – Pregnant women – Children – Reproductive organs of adults 1. 2. 3. 4. 5. Radiation Safety Procedures for Patients Reduce exposure amounts as much as possible Avoid unnecessary examinations Limit area of body exposed Shield sensitive body parts Evaluate potential pregnancy status Radiation Safety Procedures for Clinical Staff 1. 2. 3. 4. 5. 6. Limit amount of time exposed to x-rays Stay far away from x-rays Use available shielding Avoid holding patients during exposure Wear individual dosimeters Ensure proper working condition of equipment Diagnostic Procedures • Routine radiographic examinations • Named for part of body involved • Performed for viewing bone structure or abnormalities Mammography • • • • Specialized x-ray examination of the breast Screening tool for breast cancer Breast compressed in specialized device Has become vital adjunct to biopsy procedures Contrast Media Examinations • Radiographic contrast media helps differentiate between body structures • Used to evaluate structure and function Contrast Media • Introduced into body in several ways – Swallowing – Intravenously – Through a catheter • Medical assistant must ensure that patients understand preparation instructions Checkpoint Question 3 • How do contrast media help in differentiating between body structures? Answer • Contrast media help differentiate between body structures by artificially changing the absorption rate of a particular structure so that it can be seen clearly instead of blending in with adjacent structures. For example, barium sulfate absorbs radiation and shows up as white areas on a radiograph. Fluoroscopy • Use x-rays to observe movement within the body – Barium sulfate through the digestive tract – Iodinated compounds showing the beating of the heart Computed Tomography (CT, CAT scan) • Tomography – X-ray tube and film move in relation to one another, blurring all structures except those in focal plane. • CT uses x-rays from a tube circling the patient, analyzed by computers to create crosssectional images Checkpoint Question 4 • How does fluoroscopy differ from computed tomography and sonography? Answer • Fluoroscopy uses x-rays to show movement within the body. CT uses a combination of xrays and computers to create cross-sectional images of the body. Sonography uses highfrequency sound waves to create crosssectional still or real-time (motion) images of the body. Radiation Therapy • High-energy radiation is used to destroy cancer cells • Treatments must be planned carefully by radiologist • Most patients have some side effects Transfer of Radiographic Information • Radiographic images remain part of permanent record • Digital images saved on disk • X-ray films belong to site where study was performed • Examining physician or radiologist writes summary of the examination • Medical assistant obtains patient’s permission to have summary sent to office physician Teleradiology • Use of computed imaging and information systems • Provides new benefits in medicine • Digital images can be transmitted via telephone lines to distant locations • Allows for consultation with experts on difficult cases Dental X- Rays • What is the purpose of dental x- rays • What does the assistant giving the x- ray look for • What is the educational requirements of the person giving the x- ray • Specific shots that are taken when in the “chair” Purpose of dental x - rays • To help the dentist reach a correct diagnosis. • Help to provide the patient with proper care and treatment. What is found through x – rays? • • • • • • • • • • • Tooth Decay Periodontal disease Extra Teeth Bone cancer (cysts) Osteoporosis Root fragments( configuration) Abscesses of the teeth or gums Tooth position Tatar below the gum line Jaw joint irregularities Facial bone composition Reported problems • Table systems - mechanical system for positioning tabletops -Wires and cables may break ---- tabletop to jam • Control unit -Control levers jam (MA, KV, Time, …) • • • • • • Collimator (Levers) Light Localizer Tube PCB Control Mechanical jam of film tray Collimator loss from its x-ray tube ****** LOSS X-RAY TUBE SUSPENSION ****** Purchase Considerations Tube (focal spot of the tube determine the resolution of images) Generator (High frequency generator needs less space and smaller HV cables) Table (Fixed, floating top, tilting, or elevating which is suitable for traumatic and emergency patients). Digital radiography Image quality, storage space, Digital Imaging and Communication in Medicine (DICOM), remote control,