* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hemoglobin and the Movement of Oxygen
Survey
Document related concepts
Transcript
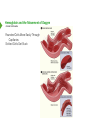
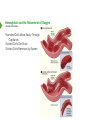
Hemoglobin and the Movement of Oxygen Hemoglobin and the Movement of Oxygen • Introduction Hemoglobin and the Movement of Oxygen • Introduction Animals Have Widely Varying Needs for Oxygen Demand for Oxygen Can Change in Seconds Basal Needs are Significant - Diffusion not Enough Hemoglobin and the Movement of Oxygen • Introduction Animals Have Widely Varying Needs for Oxygen Demand for Oxygen Can Change in Seconds Basal Needs are Significant - Diffusion not Enough Exercise, Fight/Flight Add to the Need Hemoglobin and the Movement of Oxygen • Introduction Animals Have Widely Varying Needs for Oxygen Demand for Oxygen Can Change in Seconds Basal Needs are Significant - Diffusion not Enough Exercise, Fight/Flight Add to the Need ATP Energy Produced Aerobically 15 Times More Efficiently Than Anaerobically Hemoglobin and the Movement of Oxygen • Introduction Animals Have Widely Varying Needs for Oxygen Demand for Oxygen Can Change in Seconds Basal Needs are Significant - Diffusion not Enough Exercise, Fight/Flight Add to the Need ATP Energy Produced Aerobically 15 Times More Efficiently Than Anaerobically Respiration versus Fermentation Hemoglobin and the Movement of Oxygen • Introduction Animals Have Widely Varying Needs for Oxygen Demand for Oxygen Can Change in Seconds Basal Needs are Significant - Diffusion not Enough Exercise, Fight/Flight Add to the Need ATP Energy Produced Aerobically 15 Times More Efficiently Than Anaerobically Respiration versus Fermentation Efficient, Adaptable Oxygen Delivery is Necessary Quaternary Structure • Interaction of multiple protein subunits Quaternary Structure • Interaction of multiple protein subunits Hemoglobin - 4 Subunits (α2β2), 1 Heme Each, 1 O2 Each, 1 “Donut Hole” Myoglobin - 1 Subunit, 1 Heme, 1 O2 Each Quaternary Structure • Interaction of multiple protein subunits Hemoglobin - 4 Subunits (α2β2), 1 Heme Each, 1 O2 Each, 1 “Donut Hole” Myoglobin - 1 Subunit, 1 Heme, 1 O2 Each Quaternary Structure • Interaction of multiple protein subunits Hemoglobin - 4 Subunits (α2β2), 1 Heme Each, 1 O2 Each, 1 “Donut Hole” Myoglobin - 1 Subunit, 1 Heme, 1 O2 Each Quaternary Structure • Interaction of multiple protein subunits Hemoglobin - 4 Subunits (α2β2), 1 Heme Each, 1 O2 Each, 1 “Donut Hole” Myoglobin - 1 Subunit, 1 Heme, 1 O2 Each Quaternary Structure • Interaction of multiple protein subunits Hemoglobin - 4 Subunits (α2β2), 1 Heme Each, 1 O2 Each, 1 “Donut Hole” Myoglobin - 1 Subunit, 1 Heme, 1 O2 Each Quaternary Structure • Interaction of multiple protein subunits Hemoglobin - 4 Subunits (α2β2), 1 Heme Each, 1 O2 Each, 1 “Donut Hole” Myoglobin - 1 Subunit, 1 Heme, 1 O2 Each Quaternary Structure • Interaction of multiple protein subunits Hemoglobin - 4 Subunits (α2β2), 1 Heme Each, 1 O2 Each, 1 “Donut Hole” Myoglobin - 1 Subunit, 1 Heme, 1 O2 Each Quaternary Structure • Interaction of multiple protein subunits Hemoglobin - 4 Subunits (α2β2), 1 Heme Each, 1 O2 Each, 1 “Donut Hole” Myoglobin - 1 Subunit, 1 Heme, 1 O2 Each Quaternary Structure • Interaction of multiple protein subunits Hemoglobin - 4 Subunits (α2β2), 1 Heme Each, 1 O2 Each, 1 “Donut Hole” Myoglobin - 1 Subunit, 1 Heme, 1 O2 Each Hemoglobin and the Movement of Oxygen • Structure and Function Hemoglobin and the Movement of Oxygen • Structure and Function Heme Prosthetic Group Ferrous Iron - Methemoglobin Won’t Work Only Fe++ Binds Oxygen Hemoglobin and the Movement of Oxygen • Structure and Function Hemoglobin and the Movement of Oxygen • Structure and Function Edge-on View Hemoglobin and the Movement of Oxygen • Structure and Function Edge-on View Attached to Remainder of Global Subunit Hemoglobin and the Movement of Oxygen • Structure and Function Edge-on View Attached to Remainder of Global Subunit Hemoglobin and the Movement of Oxygen • Structure and Function Edge-on View Attached to Remainder of Global Subunit Histidine’s Movement Changes Global Unit’s Shape Hemoglobin and the Movement of Oxygen • Cooperativity Exiting Lungs Hemoglobin and the Movement of Oxygen • Cooperativity Entering Lungs Exiting Lungs Hemoglobin and the Movement of Oxygen • Cooperativity No Oxygen (T-State) Entering Lungs Exiting Lungs Hemoglobin and the Movement of Oxygen • Cooperativity No Oxygen (T-State) Exiting Lungs Entering Lungs Oxygen Bound Hemoglobin and the Movement of Oxygen • Cooperativity Affected by O2 Binding (R-State) No Oxygen (T-State) Exiting Lungs Entering Lungs Oxygen Bound Hemoglobin and the Movement of Oxygen • Cooperativity Affected by O2 Binding (R-State) No Oxygen (T-State) Exiting Lungs Entering Lungs Oxygen Bound Affected by O2 Binding R-State Hemoglobin and the Movement of Oxygen • Cooperativity Affected by O2 Binding (R-State) Fully Oxygenated (R-State) No Oxygen (T-State) Exiting Lungs Entering Lungs Oxygen Bound Affected by O2 Binding R-State Hemoglobin and the Movement of Oxygen • Cooperativity Affected by O2 Binding (R-State) Fully Oxygenated (R-State) No Oxygen (T-State) Exiting Lungs Entering Lungs Oxygen Bound Affected by O2 Binding R-State Binding of the first O2 favors binding of second, etc. - Cooperatively Cooperatively Important as Hemoglobin Rapidly Passes Through Lungs Hemoglobin and the Movement of Oxygen • Cooperativity Hemoglobin and the Movement of Oxygen • Cooperativity At Low O2, Myoglobin Holds More than Hemoglobin Hemoglobin and the Movement of Oxygen • Cooperativity At Low O2, Myoglobin Holds More than Hemoglobin Hemoglobin and the Movement of Oxygen • Cooperativity At Low O2, Myoglobin Holds More than Hemoglobin Hemoglobin and the Movement of Oxygen • Cooperativity At Low O2, Myoglobin Holds More than Hemoglobin At High O2, Both Hold 100% Hemoglobin and the Movement of Oxygen At High O2, Both Hold 100% • Cooperativity At Low O2, Myoglobin Holds More than Hemoglobin Hyperbolic Binding Curve Hemoglobin and the Movement of Oxygen At High O2, Both Hold 100% • Cooperativity At Low O2, Myoglobin Holds More than Hemoglobin Sigmoidal Binding Curve Hyperbolic Binding Curve Hemoglobin and the Movement of Oxygen At High O2, Both Hold 100% • Cooperativity At Low O2, Myoglobin Holds More than Hemoglobin As Curves Move to Right Less Affinity for Oxygen Hemoglobin and the Movement of Oxygen At High O2, Both Hold 100% • Cooperativity At Low O2, Myoglobin Holds More than Hemoglobin As Curves Move to Right Less Affinity for Oxygen Myoglobin Better for Storing Oxygen Hemoglobin Better at Delivering Oxygen Hemoglobin and the Movement of Oxygen • Bohr Effect Hemoglobin and the Movement of Oxygen • Bohr Effect Less Oxygen Bound at Same Pressure Hemoglobin and the Movement of Oxygen • Bohr Effect Less Oxygen Bound at Same Pressure More O2 Required To Have Same Fraction Bound Hemoglobin and the Movement of Oxygen • Bohr Effect More Affinity Hemoglobin and the Movement of Oxygen • Bohr Effect Less Affinity More Affinity Hemoglobin and the Movement of Oxygen • Bohr Effect Protons Can Bind to Hemoglobin Protons Change Hemoglobin’s Shape Reshaped Hemoglobin Loses Oxygen Hemoglobin and the Movement of Oxygen • Bohr Effect Protons Can Bind to Hemoglobin Protons Change Hemoglobin’s Shape Reshaped Hemoglobin Loses Oxygen Rapidly Metabolizing Tissues Release Protons Hemoglobin and the Movement of Oxygen • Bohr Effect Protons Can Bind to Hemoglobin Protons Change Hemoglobin’s Shape Reshaped Hemoglobin Loses Oxygen Rapidly Metabolizing Tissues Release Protons Rapidly Metabolizing Tissues Get More Oxygen From Hemoglobin Hemoglobin and the Movement of Oxygen • Bohr Effect & CO2 Hemoglobin and the Movement of Oxygen • Bohr Effect & CO2 Hemoglobin and the Movement of Oxygen • Bohr Effect & CO2 Acid Favors Release of O2 From Hemoglobin CO2 Favors Release of O2 From Hemoglobin Hemoglobin and the Movement of Oxygen • Bohr Effect & CO2 Acid Favors Release of O2 From Hemoglobin CO2 Favors Release of O2 From Hemoglobin Acid and CO2 are Released by Rapidly Metabolizing Tissues Hemoglobin and the Movement of Oxygen • 2,3 BPG 2,3 Bisphosphoglycerate Hemoglobin and the Movement of Oxygen • 2,3 BPG Byproduct of Glycolysis Exercising Muscle Cells Rapid Use Glycolysis Exercising Muscle Cells Produce Acid, CO2, and 2,3 BPG 2,3 Bisphosphoglycerate Hemoglobin and the Movement of Oxygen • 2,3 BPG Byproduct of Glycolysis Exercising Muscle Cells Rapid Use Glycolysis Exercising Muscle Cells Produce Acid, CO2, and 2,3 BPG Binds in Hole of Donut 2,3 Bisphosphoglycerate Hemoglobin and the Movement of Oxygen • 2,3 BPG Byproduct of Glycolysis Exercising Muscle Cells Rapid Use Glycolysis Exercising Muscle Cells Produce Acid, CO2, and 2,3 BPG Binds in Hole of Donut Locks Hemoglobin in T-State 2,3 Bisphosphoglycerate Hemoglobin and the Movement of Oxygen • 2,3 BPG and Oxygen Binding Hemoglobin and the Movement of Oxygen • 2,3 BPG and Oxygen Binding Slow Metabolic Rate Hemoglobin and the Movement of Oxygen • 2,3 BPG and Oxygen Binding Slow Metabolic Rate Fast Metabolic Rate Hemoglobin and the Movement of Oxygen • 2,3 BPG and Oxygen Binding Hemoglobin and the Movement of Oxygen • 2,3 BPG and Oxygen Binding Rapidly Metabolizing Cells Produce Acid Rapidly Metabolizing Cells Release CO2 Hemoglobin and the Movement of Oxygen • 2,3 BPG and Oxygen Binding Rapidly Metabolizing Cells Produce Acid Rapidly Metabolizing Cells Release CO2 Rapidly Metabolizing Cells Release 2,3 BPG Hemoglobin and the Movement of Oxygen • 2,3 BPG and Oxygen Binding Rapidly Metabolizing Cells Produce Acid Rapidly Metabolizing Cells Release CO2 Rapidly Metabolizing Cells Release 2,3 BPG All Favor O2 Release from Hemoglobin Hemoglobin and the Movement of Oxygen • 2,3 BPG and Oxygen Binding Rapidly Metabolizing Cells Produce Acid Rapidly Metabolizing Cells Release CO2 Rapidly Metabolizing Cells Release 2,3 BPG All Favor O2 Release from Hemoglobin So Hemoglobin and the Movement of Oxygen • 2,3 BPG and Oxygen Binding Rapidly Metabolizing Cells Produce Acid Rapidly Metabolizing Cells Release CO2 Rapidly Metabolizing Cells Release 2,3 BPG All Favor O2 Release from Hemoglobin So Rapidly Metabolizing Cells Get More O2 Hemoglobin and the Movement of Oxygen • 2,3 BPG and Smoking 2,3 Bisphosphoglycerate Hemoglobin and the Movement of Oxygen • 2,3 BPG and Smoking 2,3 BPG Big Concern for Smokers Blood of Smokers has High Levels of 2,3 BPG Hemoglobin Gets Locked in T-state in Passage Through Lungs 2,3 Bisphosphoglycerate Hemoglobin and the Movement of Oxygen • 2,3 BPG and Smoking 2,3 BPG Big Concern for Smokers Blood of Smokers has High Levels of 2,3 BPG Hemoglobin Gets Locked in T-state in Passage Through Lungs Oxygen Carrying Capacity of Blood Reduced 2,3 Bisphosphoglycerate Hemoglobin and the Movement of Oxygen • 2,3 BPG and Smoking 2,3 BPG Big Concern for Smokers Blood of Smokers has High Levels of 2,3 BPG Hemoglobin Gets Locked in T-state in Passage Through Lungs Oxygen Carrying Capacity of Blood Reduced Carbon Monoxide Levels Also Higher in Smokers 2,3 Bisphosphoglycerate Hemoglobin and the Movement of Oxygen • Movement of CO2 Hemoglobin and the Movement of Oxygen • Movement of CO2 Rapidly Metabolizing Tissue Hemoglobin and the Movement of Oxygen • Movement of CO2 HbO + H+ Rapidly Metabolizing Tissue O2 Blood Hemoglobin and the Movement of Oxygen • Movement of CO2 HbO + H+ Rapidly Metabolizing Tissue O2 Blood H+:Hb-CO2 Hemoglobin and the Movement of Oxygen • Movement of CO2 HbO + H+ Rapidly Metabolizing Tissue O2 Blood H+:Hb-CO2 Some Hemoglobin and the Movement of Oxygen • Movement of CO2 HbO + H+ Rapidly Metabolizing Tissue Blood H+:Hb-CO2 Some H2O Blood O2 Remainder Hemoglobin and the Movement of Oxygen • Movement of CO2 HbO + H+ Rapidly Metabolizing Tissue O2 Blood H+:Hb-CO2 Some H2O Blood HbO H+:Hb-CO2 Remainder Hemoglobin and the Movement of Oxygen • Movement of CO2 HbO + H+ Rapidly Metabolizing Tissue O2 Blood H+:Hb-CO2 Some H2O Remainder Blood HbO Lungs H+:Hb-CO2 O2 + H+:Hb-CO2 Hemoglobin and the Movement of Oxygen • Movement of CO2 HbO + H+ Rapidly Metabolizing Tissue O2 Blood H+:Hb-CO2 Some H2O Remainder Blood HbO HbO Lungs H+:Hb-CO2 O2 + H+:Hb-CO2 Hemoglobin and the Movement of Oxygen • Movement of CO2 HbO + H+ Rapidly Metabolizing Tissue O2 Blood H+:Hb-CO2 Some H2O Remainder Blood HbO HbO Lungs H+:Hb-CO2 O2 + H+:Hb-CO2 Hemoglobin and the Movement of Oxygen • Movement of CO2 HbO + H+ Rapidly Metabolizing Tissue O2 Blood H+:Hb-CO2 Some H2O Remainder Blood HbO H+:Hb-CO2 HbO H2O + CO2 O2 + H+:Hb-CO2 Lungs Hemoglobin and the Movement of Oxygen • Movement of CO2 HbO + H+ Rapidly Metabolizing Tissue O2 Blood H+:Hb-CO2 Some H2O Remainder Blood Exhaled HbO H+:Hb-CO2 HbO H2O + CO2 O2 + H+:Hb-CO2 Lungs Hemoglobin and the Movement of Oxygen • Carbon Monoxide and Heme Hemoglobin and the Movement of Oxygen • Carbon Monoxide and Heme An Additional Histidine is Present at the Heme Iron Site Reduces Affinity to CO, but Does Not Eliminate it Carbon Monoxide in Cigarette Smoke Hemoglobin and the Movement of Oxygen • Carbon Monoxide and Heme An Additional Histidine is Present at the Heme Iron Site Reduces Affinity to CO, but Does Not Eliminate it Carbon Monoxide in Cigarette Smoke Note That CO2 Does Not Bind to Heme, nor do Protons Hemoglobin and the Movement of Oxygen • Fetal Hemoglobin Hemoglobin and the Movement of Oxygen • Fetal Hemoglobin The Body Makes Different Globins Over Time Most Variations Centered on Birth Fetal Hemoglobin Mostly α2γ2 Hemoglobin and the Movement of Oxygen • Fetal Hemoglobin The Body Makes Different Globins Over Time Most Variations Centered on Birth Fetal Hemoglobin Mostly α2γ2 High Most of Life Hemoglobin and the Movement of Oxygen • Fetal Hemoglobin The Body Makes Different Globins Over Time Most Variations Centered on Birth Fetal Hemoglobin Mostly α2γ2 High Most of Life Highest in Fetus Hemoglobin and the Movement of Oxygen • Fetal Hemoglobin The Body Makes Different Globins Over Time Most Variations Centered on Birth Fetal Hemoglobin Mostly α2γ2 High Most of Life Highest in Fetus At Adult Levels by 24 Weeks Hemoglobin and the Movement of Oxygen • Fetal Hemoglobin Fetal Hemoglobin Can’t Bind to 2,3 BPG Mostly Remains in R-state Increasing Affinity Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Sickle Cell Anemia is a Genetic Disease Affecting Hemoglobin Multiple Forms - Mutation of Glu to Val at Position #6 Most Common Red Blood Cells Lose Rounded Shape and Form Sickles Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Sickle Cell Anemia is a Genetic Disease Affecting Hemoglobin Multiple Forms - Mutation of Glu to Val at Position #6 Most Common Red Blood Cells Lose Rounded Shape and Form Sickles Shape Change Happens in Low O2 Conditions - Exercise Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Sickle Cell Anemia is a Genetic Disease Affecting Hemoglobin Multiple Forms - Mutation of Glu to Val at Position #6 Most Common Red Blood Cells Lose Rounded Shape and Form Sickles Shape Change Happens in Low O2 Conditions - Exercise Change Caused by Polymerization of Hemoglobin Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Sickle Cell Anemia is a Genetic Disease Affecting Hemoglobin Multiple Forms - Mutation of Glu to Val at Position #6 Most Common Red Blood Cells Lose Rounded Shape and Form Sickles Shape Change Happens in Low O2 Conditions - Exercise Change Caused by Polymerization of Hemoglobin Sickled Cells Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Rounded Cells Move Easily Through Capillaries Sickled Cells Get Stuck Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Rounded Cells Move Easily Through Capillaries Sickled Cells Get Stuck Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Rounded Cells Move Easily Through Capillaries Sickled Cells Get Stuck Sickled Cells Removed by Spleen Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Why is Sickle Cell Anemia so Widespread? Why Has it Not Been Selected Against? Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Why is Sickle Cell Anemia so Widespread? Why Has it Not Been Selected Against? Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Why is Sickle Cell Anemia so Widespread? Why Has it Not Been Selected Against? Greatest Incidence of Sickle Cell Anemia Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Why is Sickle Cell Anemia so Widespread? Why Has it Not Been Selected Against? Greatest Incidence of Sickle Cell Anemia Greatest Incidence of Malaria Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Why is Sickle Cell Anemia so Widespread? Why Has it Not Been Selected Against? Greatest Incidence of Sickle Cell Anemia Greatest Incidence of Malaria Hemoglobin and the Movement of Oxygen • Sickle Cell Anemia Benefit of Sickle Cell Mutation for Heterozygotes No Benefit to Homozygous Recessive or Dominant Hemoglobin and the Movement of Oxygen • Summary Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Changing Hemoglobin’s Shape Converts Hemoglobin from T-state to R-state Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Changing Hemoglobin’s Shape Converts Hemoglobin from T-state to R-state R-state Binds Oxygen Better. T-state Releases O2 Better Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Changing Hemoglobin’s Shape Converts Hemoglobin from T-state to R-state R-state Binds Oxygen Better. T-state Releases O2 Better In the Bohr Effect, Binding of CO2 and H+ Favors O2 Release Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Changing Hemoglobin’s Shape Converts Hemoglobin from T-state to R-state R-state Binds Oxygen Better. T-state Releases O2 Better In the Bohr Effect, Binding of CO2 and H+ Favors O2 Release The Bohr Effect Explains How Oxygen and CO2 Exchanged in Lungs Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Changing Hemoglobin’s Shape Converts Hemoglobin from T-state to R-state R-state Binds Oxygen Better. T-state Releases O2 Better In the Bohr Effect, Binding of CO2 and H+ Favors O2 Release The Bohr Effect Explains How Oxygen and CO2 Exchanged in Lungs 2,3 BPG is Produced by Rapidly Metabolizing Cells. It too Favors O2 Release Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Changing Hemoglobin’s Shape Converts Hemoglobin from T-state to R-state R-state Binds Oxygen Better. T-state Releases O2 Better In the Bohr Effect, Binding of CO2 and H+ Favors O2 Release The Bohr Effect Explains How Oxygen and CO2 Exchanged in Lungs 2,3 BPG is Produced by Rapidly Metabolizing Cells. It too Favors O2 Release Fetal Hemoglobin Can’t Bind 2,3 BPG and has Greater O2 Affinity Than Adult Hemoglobin Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Changing Hemoglobin’s Shape Converts Hemoglobin from T-state to R-state R-state Binds Oxygen Better. T-state Releases O2 Better In the Bohr Effect, Binding of CO2 and H+ Favors O2 Release The Bohr Effect Explains How Oxygen and CO2 Exchanged in Lungs 2,3 BPG is Produced by Rapidly Metabolizing Cells. It too Favors O2 Release Fetal Hemoglobin Can’t Bind 2,3 BPG and has Greater O2 Affinity Than Adult Hemoglobin Sickle Cell Anemia (SCA) is a Genetic Disease of Hemoglobin Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Changing Hemoglobin’s Shape Converts Hemoglobin from T-state to R-state R-state Binds Oxygen Better. T-state Releases O2 Better In the Bohr Effect, Binding of CO2 and H+ Favors O2 Release The Bohr Effect Explains How Oxygen and CO2 Exchanged in Lungs 2,3 BPG is Produced by Rapidly Metabolizing Cells. It too Favors O2 Release Fetal Hemoglobin Can’t Bind 2,3 BPG and has Greater O2 Affinity Than Adult Hemoglobin Sickle Cell Anemia (SCA) is a Genetic Disease of Hemoglobin In Low O2 Concentration, Red Blood Cells of SCA Sufferers Form Sickle Shapes Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Changing Hemoglobin’s Shape Converts Hemoglobin from T-state to R-state R-state Binds Oxygen Better. T-state Releases O2 Better In the Bohr Effect, Binding of CO2 and H+ Favors O2 Release The Bohr Effect Explains How Oxygen and CO2 Exchanged in Lungs 2,3 BPG is Produced by Rapidly Metabolizing Cells. It too Favors O2 Release Fetal Hemoglobin Can’t Bind 2,3 BPG and has Greater O2 Affinity Than Adult Hemoglobin Sickle Cell Anemia (SCA) is a Genetic Disease of Hemoglobin In Low O2 Concentration, Red Blood Cells of SCA Sufferers Form Sickle Shapes Sickled Cells Stick in Capillaries and Can be Fatal Hemoglobin and the Movement of Oxygen • Summary Animals Have Widely Varying O2 Needs ATP Generated Much More Efficiently in Presence of O2 Hemoglobin and Myoglobin are Related, but Have Different Functions Hemoglobin has Four Subunits and Hemes. Myoglobin has One of Each Bind of O2 by Heme’s Iron Pulls up on a Histidine and Change’s Hemoglobin’s Shape Changing Hemoglobin’s Shape Converts Hemoglobin from T-state to R-state R-state Binds Oxygen Better. T-state Releases O2 Better In the Bohr Effect, Binding of CO2 and H+ Favors O2 Release The Bohr Effect Explains How Oxygen and CO2 Exchanged in Lungs 2,3 BPG is Produced by Rapidly Metabolizing Cells. It too Favors O2 Release Fetal Hemoglobin Can’t Bind 2,3 BPG and has Greater O2 Affinity Than Adult Hemoglobin Sickle Cell Anemia (SCA) is a Genetic Disease of Hemoglobin In Low O2 Concentration, Red Blood Cells of SCA Sufferers Form Sickle Shapes Sickled Cells Stick in Capillaries and Can be Fatal People Heterozygous for the Mutated Gene Survive Malaria Better Than Others Protein Structure Protein Structure Primary -‐ Sequence of amino acid Secondary -‐ Interaction of amino acids close in primary sequence Tertiary -‐ Interaction of amino acids distantly located Protein Structure Primary -‐ Sequence of amino acid Secondary -‐ Interaction of amino acids close in primary sequence Tertiary -‐ Interaction of amino acids distantly located Quaternary -‐ Interaction of protein subunits Metabolic Melody Hemoglobin's Moving Around (To the tune of "Santa Claus is Coming to Town") Copyright © Kevin Ahern Oh isn't it great What proteins can do Especially ones that bind to O2 Hemoglobin's moving around Inside of the lungs It picks up the bait And changes itself from T to R state Hemoglobin's moving around The proto-porphyrin system Its iron makes such a scene Arising when an O2 binds Pulling up on histidine The binding occurs Cooperatively Thanks to changes qua-ter-nar-y Hemoglobin's moving around It exits the lungs Engorged with O2 In search of a working body tissue Hemoglobin's moving around Hemoglobin's Moving Around (To the tune of "Santa Claus is Coming to Town") Copyright © Kevin Ahern Oh isn't it great What proteins can do Especially ones that bind to O2 Hemoglobin's moving around The proton concentration Is high and has a role Between the alpha betas It finds imidazole Inside of the lungs It picks up the bait And changes itself from T to R state Hemoglobin's moving around To empty their loads The globins decree "We need to bind 2,3BPG" Hemoglobin's moving around The proto-porphyrin system Its iron makes such a scene Arising when an O2 binds Pulling up on histidine The stage is thus set For grabbing a few Cellular dumps of CO2 Hemoglobin's moving around The binding occurs Cooperatively Thanks to changes qua-ter-nar-y Hemoglobin's moving around And then inside the lungs it Discovers ox-y-gen And dumps the CO2 off To start all o'er again It exits the lungs Engorged with O2 In search of a working body tissue Hemoglobin's moving around So see how this works You better expect To have to describe the Bohr effect Hemoglobin's moving around Metabolic Melody The Bloody Things (To the tune of “Coke ® It's The Real Thing”) Copyright © Kevin Ahern The Bloody Things (To the tune of “Coke ® It's The Real Thing”) Copyright © Kevin Ahern I’m gonna put some oxygens beside my porphyrin rings To nudge the irons up a notch and yank on histidines The globins’ shapes will change a bit, oh what a sight to see The way they bind to oxygen co-op-er-AH-tive-ly And as I exit from the lungs to swim in the bloodstream Metabolizing cells they all express their needs to me To them I give up oxygen and change from R to T While my amines, they hang onto the protons readily The Bloody Things (To the tune of “Coke ® It's The Real Thing”) Copyright © Kevin Ahern I’m gonna put some oxygens beside my porphyrin rings To nudge the irons up a notch and yank on histidines The globins’ shapes will change a bit, oh what a sight to see The way they bind to oxygen co-op-er-AH-tive-ly But that's not all the tricks I know, there's more that’s up my sleeve Like gaps between sub-U-nits that hold 2,3-BPG When near met-a-bo-LI-zing cells, I bind things that diffuse The protons and bicarbonates from lowly cee oh twos And as I exit from the lungs to swim in the bloodstream Metabolizing cells they all express their needs to me To them I give up oxygen and change from R to T While my amines, they hang onto the protons readily CHORUS That’s the way it is When your cells are at play Go say hip hip hooray For the bloody things