* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Abnormal eye development associated with Cat4a, a
Survey
Document related concepts
Transcript
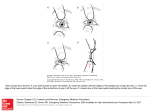
Abnormal Eye Development Associated with Cat4a, a Dominant Mouse Cataract Mutation o n Chromosome 8 Patricia A. Grimes,1 Brigitte Koeberlein,1 Jack Favor,2 Angelika Neuhduser-Klaus,2 Dwight Stambolian1 and PURPOSE. Cat4a, one of four mutant alleles at the mouse Cat4 locus, causes central corneal opacity and anterior polar cataract in heterozygotes and microphthalmia in homozygotes. The Cat4 locus has been mapped to chromosome 8, 31 cM from the centromere. In this study ocular development of Cat4a mutant mice was investigated to characterize the defects in eye morphogenesis. METHODS. Serial sections from eyes of wild-type, heterozygous, and homozygous littermates were examined by means of light microscopy at selected intervals from embryonic day 11 to postnatal day 1. Eyes of adult heterozygous and homozygous mice also were evaluated histologically. RESULTS. Failure of separation of the lens vesicle from the surface ectoderm was the earliest structural defect observed. In heterozygous embryos, the abnormality was limited to persistent connection of the anterior pole of the lens to the cornea. Adult heterozygotes had defects in the central corneal stroma and endothelium and anterior polar cataracts with or without keratolenticular adhesion. In homozygous embryos, the persistent connection of lens to surface ectoderm was associated with aborted lens development, failure of closure of the optic fissure, and impairment of growth of the eyecup. Microphthalmic eyes of adult homozygous mice had a poorly developed cornea, and the anterior chamber and vitreous compartment were absent. An extensively folded retina and remnants of a degenerated lens filled the interior of the globe. CONCLUSIONS. A developmental defect inhibits separation of the lens vesicle from surface ectoderm in mice heterozygous or homozygous for the Cat4a mutation. In homozygotes subsequent lens and eye morphogenesis are also severely affected. Cat4a shows phenotypical similarity to several other independent mouse mutations including Small eye, a mutation of the Pax6 gene. Cat4 may be one of several genes involved in a common developmental path and may be part of the fVo;6-regulated gene cascade governing eye morphogenesis. (Invest Ophthalmol Vis Set 1998;39:1863-1869) M ouse mutants with congenital ocular abnormalities are an important resource for identification and investigation of genes involved in normal eye development. Characterization of such genes in lower animals can aid in isolation of their human homologues and increase understanding of congenital ocular defects in humans. The mouse Cat4 locus includes four independent mutant alleles, all of which are expressed in heterozygous carriers as anterior polar cataract and central corneal opacity.1 Three of these mutations (Cat4b, Cat4c, and Cat4d) are homozygous lethal, and heterozygotes display moderate microphthalmia in addition to cornea and lens abnormalities. Carriers of the re- maining allele (Cat4a) have eyes of normal size whereas homozygotes, which are viable and fertile, show severe microphthalmia with closed eyelids. Although in an earlier study we suggested linkage of Cat4 to chromosome 2, 2 more extensive mapping has localized Cat4 to the central region of chromosome 8 at position cM31 3 No other known mutations resulting in cataract or microphthalmia map to chromosome 8, and Cat4 mutants have no visible abnormalities other than eye defects. In this histologic study, we describe the abnormalities of ocular development in heterozygous and homozygous mice expressing the Cat4a mutant allele. METHODS From the department of Ophthalmology and Scheie Eye Institute, University of Pennsylvania School of Medicine, Philadelphia; and the 2 GSF-Institut fiir Saugetiergenetik, Neuherberg, Germany. Supported by Research Grant EY10321 from the National Institutes of Health, Bethesda, Maryland; the Nina and Paul Mackall Trust, and an unrestricted grant from Research to Prevent Blindness, New York, New York. Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 1995. Submitted for publication November 26, 1997; revised April 13, 1998; accepted May 6, 1998. Proprietary interest category: N. Reprint requests: Patricia A. Grimes, D-603 Richards Building, University of Pennsylvania, Philadelphia, PA 19104-6075. Investigative Ophthalmology & Visual Science, September 1998, Vol. 39, No. 10 Copyright © Association for Research in Vision and Ophthalmology Downloaded From: http://iovs.arvojournals.org/ on 06/18/2017 The animals used in this study were descendants of congenic C5H-Cat4a/+ mice maintained in Neuherberg. Progeny of mutant animals were examined at intervals between embryonic day (E)ll and birth. The day of vaginal plug detection was designated E0. The heterozygous phenotype was characterized at E l l , E13, E15, and E17 in litters of obligate heterozygotes derived from mating homozygous to wild-type mice. The homozygous phenotype was identified by comparison with the established heterozygous phenotype at the same intervals in litters resulting from homozygote X heterozygote or heterozygote X heterozygote mating. Pregnant females were killed with an overdose of carbon dioxide. Embryos were fixed in Carnoy's 1863 1864 Grimes et al. IOVS, September 1998, Vol. 39, No. 10 solution (3 parts 95% ethanohl part glacial acetic acid) for 24 hours and then transferred to 70% ethanol. The heads were embedded in glycol methacrylate (Historesin; Leica Instruments, Heidelberg, Germany) and sectioned coronally at 3 5 jLtm to 6 jam. Serial sections through the eyes were collected and stained with toluidine blue. The eyes of six heterozygous and four homozygous adult mice (4-6 months old) were also examined, fixed in 4% paraformaldehyde for 24 hours, and similarly processed. All experimental procedures in this study conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. RESULTS External E x a m i n a t i o n o f E m b r y o s On gross examination, heterozygous embryos did not differ from normal littermates during gestation, but homozygous embryos could be unambiguously identified from El3 onward by inspecting the eyes (Fig. 1). In homozygous mutants at El3 the pigmented anterior margin of the optic cup was abnormally heart shaped with a narrow ventral gap at the site of the optic fissure (Fig. IC). At later stages of development, extension of the dorsal and lateral margins of the optic cup became more prominent, until by El7 the pupillary area was reduced to a narrow Y-shaped opening (Fig. ID). The diameter of the optic cup was consistently smaller than normal in the homozygous embryos. Early D e v e l o p m e n t : E l l t o E13 At El 1, the earliest stage examined, the lens vesicle was normally separated from surface ectoderm (Fig. 2A). In all heterozygous and homozygous mutant embryos, however, a cellular strand connected the surface ectoderm and the anterior wall of the lens vesicle (Fig. 2B, 2C). In homozygous embryos, the lens vesicle and the optic cup were also notably smaller than normal, and the ventral wall of the optic cup was poorly developed. At El3, the optic cup and lens of normal (Fig. 3A) and heterozygous embryos (Fig. 3B) were similar in size and differentiation despite a persistent connection between corneal and lens epithelium in the heterozygotes. More severe abnormalities were evident in homozygous embryos (Fig. 3C, 3D). A small mass of primary lens fibers formed from the posterior wall of the lens vesicle, but neither an organized anterior lens epithelium nor an equatorial bow region was present. The small optic cup had defects consistent with those observed in intact embryos (Fig. IC). An abnormally extended and folded region of the dorsal margin of the optic cup was bordered laterally by shortened and blunted regions (Fig. 3C, 3D), and the optic fissure, closed at this stage in normal and heterozygous embryos, persisted as a narrow cleft in the ventral optic cup (Fig. 3C). Late Development a n d Adult Phenotype of Heterozygous Mutants Abnormalities of heterozygous eyes in subsequent development were limited to the site of contact between lens and cornea. At El5 and El7, a full-thickness defect extended through the otherwise normally formed corneal stroma and endothelium. In some eyes the defect was filled with cells connected to and resembling the corneal epithelium with only Downloaded From: http://iovs.arvojournals.org/ on 06/18/2017 FIGURE 1. External phenotype of fixed heterozygous (+/—) and homozygous (—/—) embryos at embryonic day (E)13 (A, C) and E17 (B, D). Eyelids were removed from El7 specimens before photography; all lenses are opaque because of fixation. Heterozygous embryos (A, B) show no visible defects. The eyes were indistinguishable from those of normal littermates. In homozygous embryos (C, D), abnormal eye development is clearly evident. At El3 (C), the anterior margin of the eyecup shows a distinctive heart shape. At El 7 (D), extension of the eyecup margin almost completely occludes the lens, leaving only a narrow Y-shaped opening. Scale bar, 1 mm. a small protrusion of the anterior lens pole contacting the internal corneal surface (Fig. 4A). More commonly, the stromal defect was filled with a large conical protrusion of the lens pole (Fig. 4B), and sometimes (El7 only) lens fiber material extruded through the cornea into the conjunctival sac (Fig. 4C). These patterns show that the strand of cells connecting the lens to the surface ectoderm at earlier developmental stages may differentiate into corneal epithelium or lens epithelium. Lenses with a prominent extension into the cornea, in particular those that lost lens fiber material into the conjunctival sac, were smaller at El7 than those attached only to the inner corneal layers. The defect in the surface epithelium and loss of lens mass associated with extrusion of fiber material through the cornea seemed to be repaired before birth, because in all newborn and adult heterozygotes examined, corneal epithelium always covered the central stromal defect, and the lenses were of approximately normal size. IOVS, September 1998, Vol. 39, No. 10 Eye Development in a Mouse Cataract Mutation 1865 FIGURE 2. Eye structure of normal ( + / + ) , heterozygous (+/—), and homozygous (—/—) embryos at embryonic day 11. The lens vesicle, completely separated from surface ectoderm in normal embryos (A), remains joined to the surface by a strand of cells (between arrows) in heterozygous (B) and homozygous (C) embryos. In homozygous embryos, the lens vesicle is small, poorly developed, and lies deep within the eyecup. The eyecup also is small, and differentiation of its ventral portion is retarded. Toluidine blue; scale bar, 100 jixm. The patterns of lens-corneal attachment evident in El7 heterozygotes presaged the abnormalities observed in mature eyes. The lens remained attached to the cornea in 75% of the adult eyes examined, and in most instances of persistent attachment a small projection of lens epithelium and capsule was entrapped in the central corneal stromal defect (Fig. 4D). Lens tissue incorporated in the cornea was continuous with a multilayered plaque of epithelial cells and capsulelike material overlying an area of anterior cortical fiber disintegration. The structure of the remaining lens was usually normal, but in a few eyes, swelling and disorganization of posterior cortical fibers were evident. Sometimes the lens was only superficially attached to the cornea at the level of the gap in Descemet's membrane and corneal endothelium. In these cases, the defect in the corneal stroma was filled with a downward growth of corneal epithelium or fibrous scar tissue. The lens was attached by reflection of Descemet's membrane and corneal endothelium from the lateral margin of the corneal defect onto the intact lens capsule, by fine fibrous connections between the corneal stromal scar and the lens capsule, or by both. In eyes in which the lens was separated from the cornea, the structure of the central corneal lesion showed that the original attachment of the lens and cornea had been of the more superficial type. The stromal defects, which showed no entrapped lens tissue, were filled with a plug of corneal epithelium or fibrous scar tissue (Fig. 4E), and the lens capsule was intact over the region of the anterior polar cataract (Fig. 4F). Late Development and Adult Phenotype of Homozygous Mutants The lens and eyecup of homozygotes were markedly abnormal at El5 and El7. Although both structures enlarged with increased gestational age, they remained smaller than those of age-matched heterozygous or normal embryos. A thick column of closely packed cells originating from the corneal epithelium joined the lens as a disorganized cluster of abnormal lens epithelium. By El7 the lens epithelium surrounded the lens fiber mass and extended from it in multilayered cords or tubes (Fig. 5A, 5B). In sections stained with the periodic acid-Schiff Downloaded From: http://iovs.arvojournals.org/ on 06/18/2017 method, a thin lens capsule surrounded the ectopic cords of epithelial cells (not shown). No organized region of lens fiber formation from epithelial cells was present. Where the epithelial covering of the lens was incomplete, lens fibers bulged from the surface or erupted into the vitreous cavity (Fig. 5B). The eyecup extended anteriorly to occlude the pupillary area and enclose the lens. A layer of condensed mesenchyme extending from the developing choroid fused with the overlying corneal stroma, and no anterior chamber formed. The optic fissure remained partially open (Fig. 5A), but differentiation of the neural retina and pigment epithelium otherwise appeared normal. Adult homozygous eyes consistently were very small and located deep in the orbit behind permanently closed lids. The average diameter of the fixed globes was 1 mm, approximately one third the diameter of normal adult eyes and approximately the same size as the eyes of homozygous embryos at El7. The microphthalmic eyes were characterized by a poorly developed cornea overlying an almost completely closed eyecup (Fig. 5C). Neither corneal endothelium nor anterior chamber was present. In all eyes, a strand of corneal epithelial tissue penetrated the disorganized corneal stroma and subadjacent densely pigmented uveal tissue to fuse with remnants of the lens lying within the eyecup. Lens elements in the adult eye consisted only of isolated or clustered bladder cells sometimes surrounded by lens capsule. The ectopic strands of lens epithelial cells prominent in developing eyes at El7 were not seen in the adult eyes. The remainder of the eye was filled with an extensively folded but well-differentiated retina. Retinal pigmented epithelium and choroid were normal in appearance except for colobomata near the optic nerve. DISCUSSION The Cat4a mutation caused a developmental failure of lens vesicle separation from surface ectoderm. In heterozygous embryos, subsequent growth and differentiation of the lens and other ocular structures was not inhibited despite persis- 1866 Grimes et al. IOVS, September 1998, Vol. 39, No. 10 FIGURE 3. Eye structure of normal ( + / + ) , heterozygous (+/—), and homozygous (—/—) embryos at embryonic day 13. The lens and optic cup of normal (A) and heterozygous (B) embryos are similar in size and organization except for the connection of corneal and lens epithelium (arrows) in the heterozygous mutants. In homozygous embryos (C), the lens consists of a small ball of primary lens fibers capped anteriorly by a long cellular stalk (arrows) continuous with the surface ectoderm. There is no organized anterior lens epithelium or zone of differentiating fibers. The dorsal margin of the optic cup is shortened and blunted in this plane of section, and the open optic fissure is visible as a narrow cleft (arrowheads) in the ventral wall of the optic cup. In a deeper plane of section through the same homozygous eye (D), the optic cup extends anteriorly to encompass the lens; a prominent fold (arrowhead) in the dorsal arm of the optic cup indicates that the eye is not enlarging normally. Toluidine blue; scale bar, 100 /xm. tent ectodermal connection. The resultant abnormalities of the mature eye, including defects in the central corneal stroma and endothelium and anterior polar cataracts with or without keratolenticular adhesion, arose directly from the preexisting epithelial connection between the lens and surface ectoderm. In homozygous embryos, defective lens vesicle separation led to abortive lens development, failure of closure of the optic fissure, and inhibited growth of the optic cup that resulted in microphthalmia. Lens vesicle-ectoderm separation in mouse eye morphogenesis involves localized cell death in the epithelial layer,4 but the mechanism triggering this event is unknown. Expression of the Cat4a mutation in the ectodermal cells or adjacent mesenchyme could inhibit necessary interactions between the ectoderm, mesenchymal cells, or extracellular matrix required for separation. In homozygous embryos, the lens vesicle not only remains attached to the surface ectoderm by a long stalk but is positioned deep in the optic cup and does not form an orga- Downloaded From: http://iovs.arvojournals.org/ on 06/18/2017 nized anterior epithelium. This defect and the ensuing abnormalities of lens development may arise from a failure of the inductive interactions between the lens and the mesenchymal and neural tissue at the margin of the eyecup that control size, shape, and orientation of the developing lens. 5 6 The abnormal expansion of the lens epithelial cell population noted in El7 homozygous embryos suggests a transient stimulation of cell proliferation, but the increased epithelial cell population at this stage could also result from a decreased rate of epithelial cell differentiation into lens fibers. The small size of the lens in homozygous embryos during development may account for the associated microphthalmia; normal lens growth is required for appropriate enlargement of other eye structures with the notable exception of the retina. 7 9 A direct effect of the homozygous mutation on development of the optic cup cannot be excluded. At least four other independent mutations result in persistent lens-ectoderm connection and microphthalmia in the IOVS, September 1998, Vol. 39, No. 10 Eye Development in a Mouse Cataract Mutation 1867 FIGURE 4. Embryonic day (E)17 and adult heterozygotes. At El7 (A, B, C), attachment between cornea and lens is always present but varies in extent as indicated in sections from three representative embryos. (A) The intact anterior pole of the lens touches the cornea at the site of a defect in the corneal endothelium. An overlying defect in the corneal stroma is filled by corneal epithelial cells (arrows). (B) A conical protrusion of the anterior lens bulges through the corneal stroma. The lens epithelium is interrupted at the tip of the protrusion, but the overlying corneal epithelium is intact. (C) Lens fibers rupture into the conjunctival sac (asterisks) through a defect in the lens epithelium and all corneal layers. In a representative adult heterozygous mouse, the lens is connected to the cornea in one eye (D) but is separated in the other eye (E, F). (D) At the cornea-lens attachment, lens cells and capsule embedded in the stromal defect are continuous with the anterior lens pole where a multilayered plaque of epithelial cells overlies an area of cortical disintegration. Corneal endothelium and Descemets membrane terminate on either side of the fusion point (arrows). (E) In the cornea of the other eye, fibrous scar tissue fills the stromal defect and extends irregularly through the gap in the corneal endothelium and Descemets membrane (arrows). (F) The separate and normally positioned lens has an anterior polar cataract similar to that seen in the attached lens. Toluidine blue; scale bar, 100 jixm. mouse. These include the dominant mutations Small eye (Pax6)H)J l and Coloboma,12,1* both located on chromosome 2, and the recessive mutations, dysgenetic lens1•'1'15 and aphakia. 16 The phenotypical similarities suggest that the affected genes function in the same essential steps of eye morphogenesis. The affected gene responsible for the Small eye phenotype in mice has been identified as Pax6, and mutations of the human PAX6 gene have been identified as a cause of congenital aniridia. 1720 Pax6 encodes a highly conserved and developmentally expressed transcription factor that has been proposed to regulate the cascade of genes that participate in eye morphogenesis. 21 In mouse development Cat4, Coloboma, dysgenetic lens, and aphakia may all be part of the Pax6 regulatory cascade. The phenotype of Cat4ci/+ mice resembles Peters' anomaly, a human congenital condition characterized by defects in the central corneal stroma, Descemet's membrane, and corneal endothelium sometimes associated with keratolenticular adhesion and cataract. 22 Identification of PAX6 mutations in two cases of Peters' anomaly11 is consistent with the presence of a Downloaded From: http://iovs.arvojournals.org/ on 06/18/2017 comparable phenotype in Small eye mice; PAX6 mutations, however, may only be a rare cause of Peters' anomaly in humans. 23 Peters' anomaly occurs with and has been grouped with Rieger's anomaly, Axenfeld's anomaly, iridogoniodysgenesis, sclerocornea, and congenital endothelial dystrophy, all developmental malformations of the anterior chamber attributed to mesenchymal dysgenesis of the iris and cornea. 22 24 These disorders show phenotypic overlap, and affected members of the same family may display one or the other malformation 24 suggesting variable expression of the same gene defect. Conversely, genetic heterogeneity is indicated in some phenotypically similar conditions, such as Rieger's syndrome with linkage to 4q25 25 and 13ql4. 26 The gene involved in 4q25-linked Rieger's syndrome (RIEG/PITX2)27 and the mouse homologue (Rieg/Pitx2)z ,2H have recently been cloned and characterized as a transcription factor belonging to the class of bicoid-vel'Ated homeo proteins. A mutation of RIEG/PITX2 has also been identified in 4q25-linked autosomal dominant iris hypoplasia, a condition that does not have several of the features characteristic of the Rieger's syndrome pheno- 1868 IOVS, September 1998, Vol. 39, No. 10 G r i m e s et al. FIGURE 5. Embryonic day (E)17 and adult homozygotes. At E l 7 (A, B) n o anterior c h a m b e r is present, and the optic c u p encloses the abnormal lens. (A) In this peripheral section, a column of cells (arrows) projects from the anterior lens epithelium into the corneal stroma through a n a r r o w opening in the optic c u p . The cell column fuses with t h e corneal epithelium in adjacent sections. The lens epithelium forms a multilayered covering around t h e lens fiber mass and extends as cords of cells into the vitreous cavity. The optic fissure (arrowheads) persists as a n a r r o w cleft in the ventral optic cup. (B) A central section through the lens of the same eye shows the absence of any region of lens fiber differentiation and the disorganization of existing fibers. At gaps in the multilayered epithelial covering (arrowheads), lens fiber material erupts into the vitreous cavity. In an adult homozygous mouse (C), the cornea of the microphthalmic globe is pierced centrally by a strand of corneal epithelial cells (arrows) that connects in adjacent sections with lens remnants c o m p o s e d of bladder cells (le), scattered epithelial cells, and lens capsule. The anterior c h a m b e r is absent. Densely pigmented tissue continuous with the choroidal layer extends forward and is fused with the irregularly organized corneal stroma. The vitreous cavity is also absent, and the globe is filled with folded retina (re) that is well differentiated in some areas. Toluidine blue; scale bar, (A, B) 100 jam; (C) 200 jum. RIEG together with PAX6 and other as yet unidentype.' tified genes at loci associated with anterior c h a m b e r dysgenesis are likely to be part of a c o m m o n developmental pathway. The gene affected at the Cat4 locus seems to have an early function in eye morphogenesis, and its isolation and characterization should provide information about the genetic regulation of ocular development in the mouse and possibly in humans. No other mutations resulting in cataract or microphthalmia have b e e n m a p p e d to m o u s e c h r o m o s o m e 8, and n o likely candidate genes are evident in the region near the Cat4 locus. 3 K n o w n homology correlations b e t w e e n the m o u s e and h u m a n g e n o m e indicate that the proximal and central portions of m o u s e c h r o m o s o m e 8 are syntenic with portions of h u m a n c h r o m o s o m e s 8 ( 8 p 2 1 - p 2 3 ) , 19 ( 1 9 p l 3 1 13.2; 1 9 . 2 c e n - q l 3 , 4 ( 4 q 2 8 ~ q 3 1 ) , and 16 ( I 6 q l 3 - q 2 2 ) . 3 1 The only h u m a n cataract or eye defect m a p p e d to these regions is anterior segment ocular dysgenesis at 4 q 2 8 - q 3 1 3 2 Carriers of this autosomal dominant disorder express corneal opacities, iris adhesions, and cataract. 3 3 The region of the Rieger's synd r o m e locus at 4q25 is homologous to a region of mouse c h r o m o s o m e 3 3 4 and is unrelated to Cat4\ Rieg/Pitx2 has b e e n m a p p e d to this portion of m o u s e c h r o m o s o m e 3 2 8 At present, based on similarities in p h e n o t y p e , m o d e of inheritance, and chromosomal position, mouse Cat4 may be homologous with h u m a n anterior segment ocular dysgenesis. Additional studies of Cat4 are required to establish its role in eye Downloaded From: http://iovs.arvojournals.org/ on 06/18/2017 development and its association with congenital ocular abnormalities in humans. References 1. Favor J, Pretsch W. Genetic localization and phenotypic expression of X-linked cataract (Xcat) in Mus musculus. Genet Res. 1990;56:157-162. 2. Grimes PA, Koeberlein B, Favor J, Neuhauser-Klaus A, Stambolian D. Abnormal eye development associated with Cat4a, a dominant mouse cataract mutation on chromosome 2 [ARVO Abstract]. Invest Ophthalmol Vis Set. 1995;36(4):S798. Abstract nr 3700. 3. Favor J, Grimes P, Neuhauser-Klaus A, Pretsch W, Stambolian D. The mouse Cat4 locus maps to chromosome 8 and mutants express lens-corneal adhesion. Mamm Genome. 1997;8:403-406. 4. Cohen AL Electron microscopic observations of the developing mouse eye, I: basement membranes during early development and lens formation. Dev Biol. 1961;3:297-316. 5. Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol. 1965; 12:79-92. 6. Coulombre JL, Coulombre Aj. Lens development, IV: Size, shape and orientation. Invest Ophthalmol. 1969;8:251-257. 7. Coulombre AJ, Coulombre JL. Lens development, I: Role of the lens in eye growth. J Exp Zool. 1964;156:39-48. 8. Breitman ML, Bryce DM, Giddens E, et al. Analysis of lens cell fate and eye morphogenesis in transgenic mice ablated for cells of the lens lineage. Development. 1989;106:457-463. 9. Klein KL, Klintworth GK, Bernstein A, Breitman ML. Embryology and morphology of microphthalmia in transgenic mice expressing IOVS, September 1998, Vol. 39, No. 10 10. 11. 12. 13. 14. 15. 16. 17. 18. 19- 20. 21. 22. a gamma F-crystallin/diphtheria toxin A hybrid gene. Lab Invest. 1992;67:31-41. Hogan BLM, Hirst EM, Horsburgh G, Hetherington CM. Small eye (Sey): a mouse model for the genetic analysis of craniofacial abnormalities. Development. 1988;103:115-119. Hanson IM, Fletcher JM, Jordan T, et al. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters' anomaly. Nat Genet. 1994;6:168-173Theiler K, Varnum DS. Development of coloboma (Cm/+), a mutation with anterior lens adhesion. Anat Embryol. 1981; 162:121 -126. Hess EJ, Collins KA, Copeland NG, Jenkins NA, Wilson MC. Deletion map of the coloboma (Cm) locus on mouse chromosome 2. Genomics. 1994;21:257-261. Sanyal S, Hawkins RK. Dysgenetic lens idyl): a new gene in the mouse. Invest Ophthalmol Vis Sci. 1979;18:642-645. Sanyal S, van Nie R, De Moes J, Hawkins RK. Map position of dysgenetic lens (dyt) locus on chromosome 4 in the mouse. Genet ResCamb. 1986;48:199-200. Zwaan J. Immunofluorescent studies on aphakia, a mutation of a gene involved in the control of lens differentiation in the mouse embryo. Devel Biol. 1975;44:306-312. Ton CCT, Hirvonen H, Miwa H, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059-1074. Hill RE, Favor J, Hogan BLM, et al. Mouse Small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522-525. Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992;2:232-239. Jordan T, Hanson I, Zaletayev D, et al. The human PAX6 gene is mutated in two patients with aniridia. Nat Genet. 1992; 1:328 -332. Haider G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788-1792. Waring GO, Rodrigues MM, Laibson PR. Anterior chamber cleavage syndrome. A stepladder classification. Surv Ophthalmol. 1975; 20:3-27. Downloaded From: http://iovs.arvojournals.org/ on 06/18/2017 Eye Development in a Mouse Cataract Mutation 1869 23. Churchill A, Anwar R, Markham AF. PAX6, aniridia and Peters' anomaly [ARVO Abstract]. Invest Ophthalmol Vis Sci. 1997;38(4): S25. Abstract nr 112. 24. Holmstrom GE, Reardon WP, Baraitser M, Elston JS, Taylor DS. Heterogeneity in dominant anterior segment malformations. Br J Ophthalmol. 1991;75:591-597. 25. Murray JC, Bennet SR, Kwitek AE, et al. Linkage of Rieger syndrome to the region of the epidermal growth factor gene on chromosome 4. Nat Genet. 1992;2:46-49. 26. Philips JC, Del Bono EA, Haines JL, et al. A second locus for Rieger syndrome maps to chromosome 13ql4. Am J Hum Genet. 1996; 59:613-619. 27. Semina EV, Reiter R, Leysens NJ, et al. Cloning and characterization of a novel bicoid-related transcription factor gene. RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392-399. 28. Gage P, Camper SA. Pituitary homeobox2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Hum Mol Genet. 1997;6:457-464. 29- Heon E, Sheth BP, Kalenak JW, et al. Linkage of autosomal dominant iris hypoplasia to the region of the Rieger syndrome locus (4q25). Hum Mol Genet. 1995;4:1435-1439. 30. Alward WLM, Semina EV, Kalenak JW, et al. Autosomal dominant iris hypoplasia is caused by a mutation in the Rieger syndrome (RIEG/PITX2) gene. Am J Ophthalmol. 1998;125:98-100. 31. Ceci JD. Mouse chromosome 8. Mamm Genome. 1996;6(suppl): S151-S169. 32. Ferrell RE, Hittner HM, Kretzer FL, Antoszyk JH. Anterior segment mesenchymal dysgenesis: probable linkage to the MNS blood group on chromosome 4. Am J Hum Genet. 1982;34: 245-249. 33. Hittner HM, Kretzer FL, Antoszyk JH, Ferrell RE, Mehta RS. Variable expressivity of autosomal dominant anterior segment mesenchymal dysgenesis in six generations. Am J Ophthalmol. 1982;93:5770. 34. DeBry RW, Seldin MF. Mouse/human homology relationships. Genomics. 1996;33:337-351.