* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download HYDROGEN BONDING AND OTHER MOLECULAR
Survey
Document related concepts
Transcript
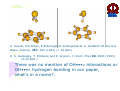
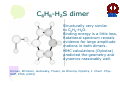
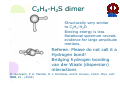
HYDROGEN BONDING AND OTHER MOLECULAR INTERACTIONS: BACKGROUND AND INTRODUCTION E. Arunan Inorganic and Physical Chemistry Department Indian Institute of Science Bangalore. 560 012 INDIA IUPAC WORKSHOP September 6, 2005 Pisa, ITLAY Background and Introduction: personal view* • Rotational spectrum of two important “hydrogen bonded dimers” revealed accurate structural information (1993). –C6H6-H2O –(C6H6)2 *Key word of ‘hydrogen bonding’ in SciFinder gave 346,465 references on 30 August 2005. Our work on these two dimers would not be among them S. Suzuki, P.G.Green, R.E.Bumgarner,S.Dasgupta,W. A. Goddard III and G.A. Blake, Science, 257, 942 (1992) (> 20 GHz) H. S. Gutwosky, T. Emilsson and E. Arunan, J. Chem. Phys 99, 4883 (1993) (3-20 GHz.) There was no mention of OH•••π interactions or OH•••π hydrogen bonding in our paper, what’s in a name?. Benzene dimer, c.mc.m distance 4.96Å Distance very close to what is found in crystal! No mention of CH•••π interactions Arunan and Gutowsky J. Chem. Phys. 98, 4294 (1993) C6H6-H2S dimer Structurally very similar to C6H6-H2O. Binding energy is a little less. Rotational spectrum reveals evidence for large amplitude motions in both dimers. MMC calculations (Dykstra) predicted the geometry and dynamics reasonably well. Arunan, Emilsson, Gutowsky, Fraser, de Oliveira, Dykstra, J. Chem. Phys. 117, 9766 (2002) C2H4-H2S dimer Structurally very similar to C2H4-H2O Binding energy is less Rotational spectrum reveals evidence for large amplitude motions. Referee: Please do not call it a Hydrogen bond! Bridging hydrogen bonding van der Waals (dispersion) interactions M. Goswami, P. K. Mandal, D. J. Ramdass, and E. Arunan, Chem. Phys. Lett. 393, 22 , (2004) C6H6-H2S dimer • Tauer, Derrick and Sherril, J. Phys. Chem. A. 109, 191 (2005) reported ab initio calculations on this dimer as a model for sulfur-π interaction. • High level restricted ab initio calculations resulted in ‘H bonded’ geometry, as the global minimum. • S down geometry is lower in energy than the sum of two monomers. • No frequency calculations to characterize the stationary point. • We found it to be a saddle point of order 2. Ab initio results Ab initio conclusion Where is sulfur-π interaction here? Why not SH-π interaction? or S-H•••π hydrogen bond? Badger and Bauer, J. Chem. Phys. 5, 839 (1937) • First paper to talk about the lengthening of X-H bond and red-shift in X-H frequencies* • “Recently, there has been considerable interest shown in a peculiar form of linkage sometimes known as the “hydrogen bond” in which as hydrogen atom, usually belonging to an hydroxyl or amino group, appears to serve as a connecting bridge between two electronegative atoms.” * We will have talks on blue shifting H bonds by Hobza and Herrebout Badger and Bauer (1937)contd. • “In order to establish such criteria one must of course decide what is meant by a hydrogen bond. Shall the term be reserved for certain cases in which O-O or other inter-nuclear distance concerned and energy required to break the bond lie within rather narrow limits, or shall it be extended to include a great variety of weaker interactions such as are responsible for the low frequency of vibration of the O-H group in single molecules of the acids and in ortho-chlorophenol, and for a part of the heats of vaporization of HCN and HCl? These latter, of course, merge into the group of interactions known as van der Waals forces.” • Today these are accepted as genuine intra- and inter-molecular hydrogen bonds S. J. Grabowski, W. A. Sokalski, and J. Leszcynski, J. Phys. Chem.A. 108, 5823 (2004) • Nature of X-H•••H-Y dihydrogen bonds and X-H•••σ interactions “A complete analysis (AIM, energy decomposition, CBS limit…) of the different parameters of the complexes shows that the stronger complexes may be classified as H bonded and the weaker complexes may be classified as van der Waals” P. Munshi and T. N. Guru Row, J. Phys. Chem. A. 109, 659 (2005) • Analysis of weak C-H•••O and C-H•••π interactions in substituted coumarins by charge density analysis “Based on the set of criteria (Popelier) defined using the AIM theory it has become possible to distinguish between a hydrogen bond (C-H•••O) and van der Waals interaction (C-H•••π)” (key criterion being the mutual penetration of H and the acceptor; it depends on the van der Waals radii of atoms used) So, every one agrees that we have hydrogen bonding and van der Waals interactions. So what is van der Waals interaction? van der Waals interactions? • A van der Waals complex is a collection of two or more atoms or molecules held together by van der Waals or dispersion forces. – E. R. Bernstein, Ann. Rev. Phys. Chem. 46, 205 (1995) van der Waals interactions? • The isotropic terms are exchange repulsion and dispersion, the sum of which is often called ‘van der Waals interaction’ – G. Desiraju and T. Steiner p 17 of their book The Weak Hydrogen Bond (1997) van der Waals interactions? • The standard picture – size repulsion and induction plus dispersion attraction – is commonly referred as the van der Waals interaction. – V. Aquilanti, E. Cornicchi, M. M. Teixidor, N. Saendig, F. Pirani, and D. Capelletti Angew. Chemie. Int. Ed. 44, 2356 (2005) van der Waals interactions? • van der Waals binding relies on the long-range weak attraction between permanent and induced electric dipole (and higher) moments as well as instantaneous asymmetric charge distributions in atoms and molecules (dispersion interaction) – Koperski van der Waals complexes in supersonic beams, Wiley-VCH 2003 • The forces of attraction and repulsion between molecules are called van der Waals forces. – A. J. Stone The Theory of Intermolecular Forces, Clarendon Press, Oxford 1996 • If one considers van der Waals equations as the origin for van der Waals interactions, it appears obvious that all intermolecular interactions, including hydrogen bonding, should be classified as van der Waals interactions. van der Waals interactions could be further classified as hydrogen bonding, halogen bonding etc… – E. Arunan, written for ISRAPS Bulletin (2005) • But, what about directionality? H bond vs van der Waals! C-H•••O interaction • Directionality, isotropic vs anisotropic potentials! • H2O-CH4 complex. Early ab initio studies1 predicted C-H•••O ‘hydrogen bonds’ (V-O) • PNFTMW spectroscopic studies2 showed the structure to be O-H•••∆, one of the tetrahedral planes in CH4 (F-H) • Advanced ab initio studies3 found this geometry to be the global minimum. Novoa et al. J. Chem. Phys. 95, 5179 (1991) 2 Suenram et al. J. Chem. Phys., 101, 7230 (1994) 3 Szczesniak et al. J. Chem. Phys., 98, 3078 (1993) 1 Some points to ponder about! • The OH•••∆ interaction is like a typical hydrogen bond as the electron density in CH4 is maximum in that region. • The dispersion energy is responsible for the overall anisotropy of the potential energy surface (in CH4-H2O complex) and obtaining the right global minimum. Without dispersion V-O is more stable than F-H. • Authors note that F-H minimum becomes deeper because of the smaller steric hindrance! • Desiraju and Steiner have shown that C-H•••O contacts are predominantly linear i.e. H bonds • Legon will be giving a talk about non linear H bonds in the gas phase. IUPAC Task Group • • • • • • • • • • • • • • Ibon Alkorta (Madrid, Spain) Elangannan Arunan (Bangalore, India) David C. Clary (Oxford, UK) Robert H. Crabtree (Yale, USA) Joseph J. Dannenberg (CUNY, USA) Gautam R. Desiraju (Hyderabad, India) Henrik G. Kjaergaard (Otago, New Zealand) Roger A. Klein (Bonn, Germany) Karl Kleinermanns (Düsseldorf, Germany) Anthony C. Legon (Exeter, UK) Benedetta Mennucci (Pisa, Italy) David J. Nesbitt, (Colarado, USA) Joanna Sadlej (Warsaw,Poland) Steve Scheiner (Utah, USA) Aim of the project • This project aims 1) to take a comprehensive look at intermolecular interactions and classify them and 2) to give a modern definition of the hydrogen bond, taking in to account all current experimental and theoretical information, and including hydrogen bonded systems both in gaseous and condensed phases as well as in chemical and biological systems. Workshop • 22 talks in 3 days • 11 Task group members • 11 Contributed speakers • 17 participants • On 9 September 2005, Plenary Discussion and IUPAC Task group meeting and a position paper. Acknowledgements IUPAC • Bryan Henry, Vice-president • Fabienne Meyers, Publishing • Enid Weatherwax, Administrative • Linda Yapp, Accounting Local Organizers • Roger Klein and Benedetta Mennucci Thank you all for being here! Let us begin