* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 21
Survey
Document related concepts
Transcript
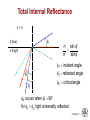
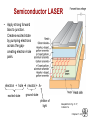
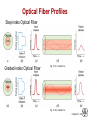
Chapter 21: Optical Properties ISSUES TO ADDRESS... • What happens when light shines on a material? • Why do materials have characteristic colors? • Why are some materials transparent and others not? • Optical applications: -- luminescence -- photoconductivity -- solar cell -- optical communications fibers Chapter 21 - 1 Optical Properties Light has both particulate and wavelike properties – Photons - with mass E h hc E energy wavelength frequency h Planck' s constant (6.62 x10 34 J s) c speed of light (3.00 x 108 m/s) Chapter 21 - 2 Refractive Index, n • Transmitted light distorts electron clouds. no transmitted light + transmitted light + electron cloud distorts • Light is slower in a material vs vacuum. c (velocity of light in vacuum) n = refractive index v (velocity of light in medium) --Adding large, heavy ions (e.g., lead can decrease the speed of light. --Light can be "bent" • Note: n = f () Typical glasses ca. Plastics PbO (Litharge) Diamond 1.5 -1.7 1.3 -1.6 2.67 2.41 Selected values from Table 21.1, Callister 7e. Chapter 21 - 3 Total Internal Reflectance n > n’ 1' n’(low) n (high) n sin n sin i incident angle i refracted angle c 1 c critical angle c occurs when i 90 for i c light is internally reflected Chapter 21 - 4 Example: Diamond in air n sin 2.41 sin 90 1 n sin 1 sin c sin c sin c 1 2.41 c 24.5 • Fiber optic cables are clad in low n material for this reason. Chapter 21 - 5 Light Interaction with Solids • Incident light is either reflected, absorbed, or transmitted: Io IT I A IR IS Reflected: IR Absorbed: IA Transmitted: IT Incident: I0 Scattered: IS • Optical classification of materials: Transparent Translucent Opaque single crystal polycrystalline dense Adapted from Fig. 21.10, Callister 6e. (Fig. 21.10 is by J. Telford, with specimen preparation by P.A. Lessing.) polycrystalline porous Chapter 21 - 6 Optical Properties of Metals: Absorption • Absorption of photons by electron transition: Energy of electron unfilled states E = h required! Io Planck’s constant (6.63 x 10-34 J/s) filled states freq. of incident light Adapted from Fig. 21.4(a), Callister 7e. • Metals have a fine succession of energy states. • Near-surface electrons absorb visible light. Chapter 21 - 7 Light Absorption I t e I0 linear absorption coefficient [] cm1 t sample thickness I ln t I0 Chapter 21 - 8 Optical Properties of Metals: Reflection • Electron transition emits a photon. Energy of electron IR re-emitted photon from material surface unfilled states “conducting” electron E filled states Adapted from Fig. 21.4(b), Callister 7e. • Reflectivity = IR/Io is between 0.90 and 0.95. • Reflected light is same frequency as incident. • Metals appear reflective (shiny)! Chapter 21 - 9 Reflectivity, R • Reflection – Metals reflect almost all light – Copper & gold absorb in blue & green => gold color 2 n 1 R reflectivi ty n 1 2 • Example: Diamond 2.41 1 R 0.17 2.41 1 17% of light is reflected Chapter 21 - 10 Scattering • In semicrystalline or polycrystalline materials • Semicrystalline – density of crystals higher than amorphous materials speed of light is lower - causes light to scatter - can cause significant loss of light • Common in polymers – Ex: LDPE milk cartons – cloudy – Polystyrene – clear – essentially no crystals Chapter 21 - 11 Selected Absorption: Semiconductors • Absorption by electron transition occurs if h > Egap Energy of electron blue light: h = 3.1 eV unfilled states red light: h = 1.7 eV incident photon energy h Io Egap filled states Adapted from Fig. 21.5(a), Callister 7e. • If Egap < 1.8 eV, full absorption; color is black (Si, GaAs) • If Egap > 3.1 eV, no absorption; colorless (diamond) • If Egap in between, partial absorption; material has a color. Chapter 21 - 12 Wavelength vs. Band Gap Example: What is the minimum wavelength absorbed by Ge? Eg = 0.67 eV hc (6.62 x 1034 J s)(3 x 10 8 m/s) c 1.85 m 19 Eg (0.67eV)(1.60 x 10 J/eV) note : for Si Eg 1.1 eV c 1.13 m If donor (or acceptor) states also available this provides other absorption frequencies Chapter 21 - 13 Color of Nonmetals • Color determined by sum of frequencies of -- transmitted light, -- re-emitted light from electron transitions. • Ex: Cadmium Sulfide (CdS) -- Egap = 2.4 eV, -- absorbs higher energy visible light (blue, violet), -- Red/yellow/orange is transmitted and gives it color. -- Sapphire is colorless (i.e., Egap > 3.1eV) -- adding Cr2O3 : • • • • • alters the band gap blue light is absorbed yellow/green is absorbed red is transmitted Result: Ruby is deep red in color. Transmittance (%) • Ex: Ruby = Sapphire (Al2O3) + (0.5 to 2) at% Cr2O3 80 sapphire 70 ruby 60 50 40 0.3 wavelength, (= c/)(m) 0.5 0.7 0.9 Adapted from Fig. 21.9, Callister 7e. (Fig. 21.9 adapted from "The Optical Properties of Materials" by A. Javan, Scientific American, 1967.) Chapter 21 - 14 Luminescence • Luminescence – emission of light by a material – material absorbs light at one frequency & emits at another (lower) frequency. Conduction band Eg trapped states Eemission activator level Valence band How stable is the trapped state? • If very stable (long-lived = >10-8 s) = phosphorescence • If less stable (<10-8 s) = fluorescence Example: glow in the dark toys. Charge them up by exposing them to the light. Reemit over time. -phosphorescence Chapter 21 - 15 Photoluminescence Hg uv electrode electrode • Arc between electrodes excites mercury in lamp to higher energy level. • electron falls back emitting UV light (i.e., suntan lamp). • Line inner surface with material that absorbs UV, emits visible Ca10F2P6O24 with 20% of F - replaced by Cl • Adjust color by doping with metal cations Sb3+ blue Mn2+ orange-red Chapter 21 - 16 Cathodoluminescence • Used in T.V. set – Bombard phosphor with electrons – Excite phosphor to high state – Relaxed by emitting photon (visible) ZnS (Ag+ & Cl-) (Zn, Cd) S + (Cu++Al3+) Y2O2S + 3% Eu blue green red • Note: light emitted is random in phase & direction – i.e., noncoherent Chapter 21 - 17 LASER Light • Is non-coherent light a problem? – diverges – can’t keep tightly columnated • How could we get all the light in phase? (coherent) – LASERS • • • • • Light Amplification by Stimulated Emission of Radiation • Involves a process called population inversion of energy states Chapter 21 - 18 Population Inversion • What if we could increase most species to the excited state? Fig. 21.14, Callister 7e. Chapter 21 - 19 LASER Light Production • “pump” the lasing material to the excited state – e.g., by flash lamp (non-coherent lamp). Fig. 21.13, Callister 7e. – If we let this just decay we get no coherence. Chapter 21 - 20 LASER Cavity “Tuned” cavity: • Stimulated Emission – One photon induces the emission of another photon, in phase with the first. – cascades producing very intense burst of coherent radiation. • i.e., Pulsed laser Fig. 21.15, Callister 7e. Chapter 21 - 21 Continuous Wave LASER • Can also use materials such as CO2 or yttriumaluminum-garret (YAG) for LASERS • Set up standing wave in laser cavity – – tune frequency by adjusting mirror spacing. • Uses of CW lasers 1. 2. 3. 4. 5. 6. Welding Drilling Cutting – laser carved wood, eye surgery Surface treatment Scribing – ceramics, etc. Photolithography – Excimer laser Chapter 21 - 22 Semiconductor LASER • Apply strong forward bias to junction. Creates excited state by pumping electrons across the gapcreating electron-hole pairs. electron + hole neutral + h excited state ground state photon of light Adapted from Fig. 21.17, Callister 7e. Chapter 21 - 23 Uses of Semiconductor LASERs • #1 use = compact disk player – Color? - red • Banks of these semiconductor lasers are used as flash lamps to pump other lasers • Communications – Fibers often turned to a specific frequency (typically in the blue) – only recently was this a attainable Chapter 21 - 24 Applications of Materials Science • New materials must be developed to make new & improved optical devices. – Organic Light Emitting Diodes (OLEDs) – White light semiconductor sources Fig. 21.12, Callister 7e. Reproduced by arrangement with Silicon Chip magazine.) • New semiconductors • Materials scientists (& many others) use lasers as tools. • Solar cells Chapter 21 - 25 Solar Cells • p-n junction: • Operation: P-doped Si conductance Si electron Si P Si Si B creation of hole-electron pair - - - + + + + • Solar powered weather station: Si Si light n-type Si p-n junction p-type Si n-type Si p-n junction P-type Si hole -- incident photon produces hole-elec. pair. -- typically 0.5 V potential. -- current increases w/light intensity. Si Si B-doped Si polycrystalline Si Los Alamos High School weather station (photo courtesy P.M. Anderson) Chapter 21 - 26 Optical Fibers • prepare preform as indicated in Chapter 13 • preform drawn to 125 m or less capillary fibers • plastic cladding applied 60 m Fig. 21.20, Callister 7e. Fig. 21.18, Callister 7e. Chapter 21 - 27 Optical Fiber Profiles Step-index Optical Fiber Graded-index Optical Fiber Fig. 21.21, Callister 7e. Fig. 21.22, Callister 7e. Chapter 21 - 28 SUMMARY • When light (radiation) shines on a material, it may be: -- reflected, absorbed and/or transmitted. • Optical classification: -- transparent, translucent, opaque • Metals: -- fine succession of energy states causes absorption and reflection. • Non-Metals: -- may have full (Egap < 1.8eV) , no (Egap > 3.1eV), or partial absorption (1.8eV < Egap = 3.1eV). -- color is determined by light wavelengths that are transmitted or re-emitted from electron transitions. -- color may be changed by adding impurities which change the band gap magnitude (e.g., Ruby) • Refraction: -- speed of transmitted light varies among materials. Chapter 21 - 29 ANNOUNCEMENTS Reading: Core Problems: Self-help Problems: Chapter 21 - 30