* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
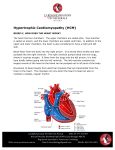
Download Consensus on Hypertrophic Cardiomyopathy.
Remote ischemic conditioning wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Coronary artery disease wikipedia , lookup
Myocardial infarction wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
1 CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY Consensus on Hypertrophic Cardiomyopathy. SAC Argentine Consensus 1. INTRODUCTION AND OVERVIEW Organizing Committee Director Dr. J. Horacio Casabé Editorial Staff Drs. Rafael Acunzo, Adrián Fernández, José Gabay, Néstor Galizio, Alejandro Hita, Gustavo Ontiveros, Roberto Peidro By the SAC Area of Standardizations and Consensus Dr. Eduardo Alberto Sampó Work Commissions Introduction and overview, definition and nomenclature, physiopathology Dr. J. Horacio Casabé Genetics Drs. Gustavo Ontiveros, Jorge Scaglione, and Alejandra Guerchicoff Electrocardiogram Drs. Rafael S. Acunzo, Isabel V. Konopka, and M. Cristina Sacheri Echocardiography Drs. Alejandro Hita, Sergio Baratta, Jorge Lax, and Eduardo Guevara Natural history and medical treatment Dr. J. Horacio Casabé Surgical treatment Drs. Roberto R. Favaloro and J. Horacio Casabé Percutaneous ablation Drs. José Manuel Gabay and Antonio Pocoví Pacemaker Dr. Néstor Galizio Assessment of risk of sudden death Dr. Adrián Fernández End-stage phase of HCM Dr. Adrián Fernández Exercise and HCM Dr. Roberto Peidro Advisory Committee Dr. Gabriela Hecht Dr. Tomás F. Cianciulli Dr. Augusto Torino The hypertrophic cardiomyopathy (HCM) is a condition that was anatomopathologically described by the French, and clinically described by Brock & Teare in England fifty years ago. (1, 2) It occurs in 1 every 500 births (3). Two main aspects stand out in its natural history: production of symptoms, which are at times incapacitating and sudden death (SD), mainly among young people, (4) although most patients have normal life expectancy. (5) This disease has triggered intense research in recent years. What is even more important is that we currently have therapies that abort life-threatening ventricular arrhythmias causing SD (6), and allow for better assessment and stratification. The purpose of this Consensus of the Argentine Society of Cardiology was to review existing evidences and set guidelines for clinicians, cardiologists, and subspecialists in the diagnosis and treatment of this multifaceted disease. To do this, the SAC convened a group of specialists in heart diseases, and a group of molecular biologists with vast experience in this disease to develop a series of updated guidelines and practices based on their personal experience and on the evidences from literature. Given the relatively low prevalence of HCM, it is necessary to point out that (3) as opposed to other areas in cardiology, there are no large-scale randomized trials (level of evidence A), and therefore the guidelines were based mainly on unique randomized trials, or on major nonrandomized or retrospective cohort studies (level of evidence B), or otherwise on consensus or experts opinions (level of evidence C). In this regard, it should be noted that in 2003, the European Society of Cardiology, together with the American College of Cardiology, published an Expert Consensus document in which all aspects of this disease (7) were fully developed, and which is fundamental for our own Consensus. However, it should be pointed out that there have been important works after its publication that influenced on the conclusions of our Consensus. (6) In the light of the above mentioned, we believe that the opinions expressed in this Consensus will stay in effect for several years. 2. DEFINITION, NOMENCLATURE, AND CLASSIFICATION HCM is defined as a non-dilated left ventricular hypertrophy in the absence of any other systemic or cardiac disease that causes it (for instance, aortic valve 2 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 stenosis or systemic hypertension). (8) Within the classification of primary cardiomyopathies (genetic, mixed, or acquired), this is the most common genetic primary cardiomyopathy. (8) Upon its description, it has been called a variety of names, the most outstanding ones being asymmetrical septal hypertrophy, idiopathic hypertrophic subaortic stenosis, dynamic subaortic stenosis, and hypertrophic obstructive cardiomyopathy. The World Health Organization (WHO) has adopted the term hypertrophic cardiomyopathy, which is the most accurate to describe this primary hypertrophy that may occur with or without left ventricular outflow tract obstruction. (9) From a clinical point of view, it is important to classify HCM hemodynamically as: 1. Obstructive: the left ventricular outflow tract obstruction (LVOTO) may be persistent during rest, latent (provocable), or labile (variable). The two types of obstruction include subaortic obstruction (most frequent) and mid-ventricular obstruction (approximately 5%). Subaortic obstruction is due to systolic anterior motion (SAM) of the anterior or posterior leaflet of the mitral valve, the tendinous cords or both; the mitral valve is swept towards the septum as a result of dragging (Venturi effect), causing mitral regurgitation. Mid-ventricular obstruction is due to an anomalous insertion of the anterior papillary muscle or to excessive hypertrophy of the mid-ventricular septum or papillary muscle, with abnormal alignment. Both obstructions may coexist. (1012) 2. Non-obstructive: the obstruction is neither at rest nor provocable with Valsalva or exercise. They are divided into those with preserved left ventricular systolic function (LVSF) (or supranormal), and those with altered LVSF (end-stage). (11) Recent evidence has shown that about 70% of the HCM patients have either resting or latent LVOTO, (13) although the real meaning of this finding for therapy management is still unclear. What is known for certain is that patients with significant obstructions (> 30 mm Hg) have increasing progression of severe symptoms, heart failure, and death, mainly when they are not very symptomatic. (14, 15) pler imaging in patients relatives with that condition who do not phenotypically have the disease. As myocardial fibrosis increases, LV stiffness increases, and atrial pressure also increases in order to complete the ventricular filling. This may lead to high capillary wedge pressure, and cause dyspnea. (7, 1619) Left ventricular outflow tract obstruction Patients with dynamic obstruction may have symptoms that improve with the obstruction relief due to medication, surgical myomectomy, or septal branch ablation. (10, 12) As already mentioned, the prognosis of patients with significant LVOTO is worse than that of patients without that obstruction, probably due to the chronic damage that implies greater parietal stress, myocardial ischemia, necrosis, and replacement fibrosis. (14, 20, 21) Myocardial ischemia In this disease, myocardial ischemia is sometimes evidenced by typical or atypical angina, presence of permanent or reversible perfusion defects, (22) impairment of coronary reserve (23, 24), and fibrosis areas in the pathologic anatomy. (25) In HCM, there is an imbalance between oxygen supply and demand: on the one hand, there are anatomic abnormalities (intimal hypertrophy of arterioles) (26) and functional abnormalities (23, 24) due to microvasculature narrowing; on the other hand, there is significant hypertrophy and muscular mass increase, which are typical of this disease. It is important to remember that arteriosclerotic ischemic heart disease may complicate clinical outcomes, thus worsening the prognosis. (27) Mitral regurgitation As mentioned above, (generally mild to moderate) mitral regurgitation is mainly due to distortion of the mitral apparatus as a result of SAM and Venturi suction effect: the direction of the regurgitant jet is lateral or posterior, especially during mid and late systole. In general, severity of regurgitation is directly proportional to subaortic gradient. (28) When the jet is central, anterior, or multiple, intrinsic anomalies of the mitral valve may occur (myxomatous degeneration, valve fibrosis, anomalous insertion). (29) The physiopathology of HCM is complex and multifactorial; one or more mechanisms may predominate in each patient to produce the same symptom. These mechanisms include: diastolic dysfunction, LVOTO, myocardial ischemia, mitral regurgitation, atrial fibrillation, and autonomic dysfunction. Atrial fibrillation Atrial fibrillation (AF) is the most common sustained arrhythmia in HCM, and is independently associated with progressive heart failure, higher mortality rate due to cardiac failure, and fatal and non-fatal stroke. It may be poorly tolerated due to secondary diastolic shortening at rapid ventricular rate and/or to lack of atrial contraction to ventricular filling. (30, 31) Diastolic dysfunction All HCM patients have some degree of diastolic dysfunction; in fact, this can be diagnosed by tissue Dop- Autonomic dysfunction About 25% of HCM patients have an inadequate response to exercise, which is manifested by the impos- 3. PHYSIOPATHOLOGY CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY sibility to increase the BP above 20 mm Hg, or by the BP fall. This response is due to systemic vasodilation during exercise, and it occurs in spite of an adequate minute volume to the effort; as we will see later, this finding is associated with a higher incidence of sudden death. (3234) 4. GENETICS So far, more than 450 mutations have been identified in the 20 genes that cause phenotypes compatible with HCM. These genes include strictly sarcomeric genes, Z-disc genes, calcium homeostasis genes, and within differential diagnoses, genes involved in metabolic processes (Table 1). (35, 36) Sarcomeric gene mutations are passed on to the offspring with autosomal dominant mode. (37) These have been identified in 36% to 60% of familial cases of HCM, and in 5% to 50% of sporadic forms of this disease. However, the percentage may be even lower, depending on the inclusion criteria used to carry out the genotype study. An additional 2% to 7% of the cases correspond to non-sarcomeric gene mutations. Mutations in ±myosin (MYH7) gene and in myosin binding protein C (MYBPC3) are the most common 3 causes of HCM, and troponin T (TNNT2) gene mutations being in the third place. (38, 39) Molecular-genetic physiopathology The mutations observed most frequently are single DNA base substitutions, which lead to amino acid change (missense) or a termination codon (nonsense). In gen MYH7 mutations, a dominant negative effect of the mutated allele on the wild allele is usually observed, whereas MYBPC3 mutations produce mainly an allelic haploinsufficiency. (40, 41) The molecular mechanisms by which mutations lead to left ventricular hypertrophy (LVH) are not exactly known yet. The two most firmly argued mechanisms do not necessarily exclude each other. One of the hypotheses is that mutations would give rise to disturbance in energy consumption by the mutated protein, with deterioration of myocyte function, and the following LVH as a compensatory mechanism. (42) Another hypothesis postulates that mutation in itself causes a structural disorder through the conformational change of the altered sarcomeric protein. (43) In both of the postulated mechanisms, changes in the ATPase activity and the Ca++ homeostasis are considered important participants of Table 1. Genes related to hypertrophic cardiomyopathy* *Adapted from Bos et al. (40) Sarcomeric genes include: those which encode thin filament proteins (troponin T, I, and C; ±-tropomyosin, actin), intermediate filament proteins (myosin binding protein C), and thick filament proteins (²-myosin, and the essential or regulatory light chains of myosin). Z-disc genes are a group of genes that encode proteins involved in the cytoarchitecture of cardiomyocytes, and in mechanosensitive signals. Their products include titin, muscle LIM protein, and telethonin. Genes involved in calcium homeostasis include the sarcoplasmic reticulum genes, which are the ones most involved in this pathology. Genes involved in metabolic processes include mitochondrial genes, and lysosomal protein genes, whose alterations are evidenced as phenocopies of HCM. 4 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 the process, as well as a number of modifying factors of the genic expression. (44) Correlation between phenotype and genotype It has been evidenced that cases of HCM with identified mutations in sarcomeric genes are associated to a higher degree of median septal LVH, whereas Zgenes are associated to sigmoid forms of LVH, and to greater functional left ventricular outflow tract obstruction. (45) In turn, phenocopies of HCM (patients with different genotypes with similar clinical presentations) due to storage diseases show significant thickness of the LV wall, and greater incidence of arrhythmias and accessory pathways, but they mainly show more extracardiac lesions than the previous groups. (36) HCM cases due to mutations in the BMH7 gene have been associated to severe forms of hypertrophy occurring early in life, with significant LVOTO and sudden death. (46) Cases due to mutations in the MYBPC3 gene have been associated to late development of the disease and good prognosis in general, whereas cases due to mutations in the TNNT2 gene have been related to mild or moderate levels of LVH, but with high risk of sudden death. (47, 48) However, such associations lack statistical significance, so it is still not possible to reliably discern phenotypes on the basis of the knowledge of the mutated gene. (49) Likewise, at present there are significant limitations to establish an adequate phenotype-genotype correlation for mutations already determined (allele-phenotype correlation), due to the functional effects of numerous genetic modulators, and to the demonstrated degrees of phenotypic plasticity in this disease. (50, 51) Conversely, it has been demonstrated that there is a direct correlation between severity of the medical condition and compound heterozygote mutations or double mutations, since these show more aggressive progress of the disease than those who are simple heterocygotes. It is estimated that 5% of HCM patients have these compound genetic forms, and therefore stand for a subpopulation with increased risk for the occurrence of an adverse clinical event. (52) Genetic modifiers Both genetic and environmental factors can act as disease modifiers. The modulator effect of the phenotype expression on a mutation determined by sex has been demonstrated, as well as by numerous genetic polymorphisms, mainly those from the genes involved in the renin-angiotensin system. (53) Moreover, environmental and acquired factors, such as diet, physical exercise, and blood pressure level of each individual, also exert modulating effects on the genetic mutations that determine the phenotype expression of the disease. The extent of these factors still has to be properly characterized, both as possible clinical event prediction markers and as markers of disease modulation. (54) Genetic testing Genetic diagnosis aims at detecting disorders at molecular-genetic level, with clear clinical objectives. Among the current techniques, the DNA direct sequencing technique is the most used for that purpose. Undoubtfully, this technique has become the most appropriate method to make HCM diagnosis. This way, it is possible to confirm the correct etiology in patients with echocardiographic diagnosis of HCM, as well as to identify a vast population of patients who have phenocopies of HCM in spite of being classified within this category. (36, 55) Through genetic analysis, it is also possible to identify relatives who are asymptomatic mutation carriers. It is estimated that up to 30% of the relatives may be silent carriers. (56) Through presymptomatic diagnosis, it is possible to plan and optimize a closer echocardiographic follow-up in carriers rather than in non-carriers of mutations, and thus make an early diagnosis of the disease. (57) To date, the possibility to stratify the risk for the occurrence of unfavorable clinic events through these techniques is still uncertain. It is possible that the inclusion of new genetic markers will make it feasible in the near future. (50) It should be mentioned that, in our sphere, there is still a long way to go regarding basic research for the realization of genetic analysis to be included in health care practice for HCM patients. In addition, several considerations will have to be resolved regarding the clinical and analytical validation of the method before deciding to perform the analysis in some of the international centers that offer it as a service, in order to interpret the results correctly. (58) Genetic analysis. Conclusions In our sphere, genetic analysis is carried out in the research field, but with no diagnostic purposes, until it is clinicallyvalidated. Level of evidence C. In international centers, genetic analysis with diagnostic purposes is recommended with due considerations to its limitations. Level of evidence C. There is no consensus regarding recommendations for genetic analysis with prognostic purposes. 5. DIAGNOSIS A. Electrocardiogram (10) A variety of electrocardiographic abnormalities can be found in HCM; these abnormalities are determined by the extent, degree, and distribution of the myocardial hypertrophy involved, the presence of fibrosis and/or necrosis of the cardiac muscle, and the occurrence of intraventricular conduction disorders. (20, 5962) About 95% of the patients who carry hypertrophic cardiomyopathy show ECG alterations that are not 5 CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY diagnostic of the condition. (20) Common abnormalities involve ST segment and T wave, and about 50% of the patients show signs of left ventricular enlargement. (59, 60) One of the most significant characteristics of the ECG in young patients with hypertrophic cardiomyopathy is the presence of abnormal deep narrow Qwaves; theyre present in 30% of the cases, and sometimes precede the hypertrophy detected in the ECG. (63) These Q-waves are usually present in the derivations that face the inferior and lateral walls of the left ventricle. An example is shown in Figure 1. As opposed to a myocardial necrosis due to arteriosclerotic coronary artery disease, Q-waves of HCM occur due to the increase of electric forces generated in hypertrophic areas. Their direction and magnitude will be related to the vector resulting from the location of areas with major ventricular hypertrophy, and the changes that they cause in cardiac geometry. (63, 64) Recognizing these characteristics in Q-waves is very important, since they are often confused with the presence of myocardial necrosis. (65) Instead, wide Q-waves are related to greater disorganization of the hypertrophic muscle or to its fibrosis and/or necrosis. (66) While myofibrillar disorganization and interstitial fibrosis involve mainly the common ventricular myocardium, they may also affect the specialized conduction system with the presence of a variety of intraventricular conduction disorders. (61, 62) For first-grade relatives of HCM patients, the probability to be carriers of this disease is 50%; in this context, the presence of abnormalities, whether in the ECG and/or in the echocardiogram, have even more clinical significance than in general population. In 1997, a group of specialists headed by McKenna posed a series of echocardiographic, electrocardiographic, and clinical criteria to diagnose the disease when this is familial (with two or more affected members). (67) Electrocardiographic criteria include: Major criteria 1. Signs of left ventricular enlargement with changes in ventricular repolarization (Romhilt - Estes score ≥ 5) (Table 2). 2. Negative Q-waves with amplitude ≥ 3 mm in derivations I, aVL with an angle between electric axes of QRS complex and of T-wave ≥ 30 degrees, of V3 to V6 or ≥ 3 mm, and in derivations II, III, and aVF ≥ 5 mm. 3. Abnormal Q-waves (duration above 40 msec or Rwave amplitude > 25% of its voltage) in at least two derivations. (68) Minor criteria 1. Total bundle branch block or intraventricular conduction disorders in derivations that explore the left ventricle. Fig. 1. ECG of a patient with HCM. Table 2. Romhilt-Estes score 1. S wave in V1 or V2, or R wave in V5 or V6 > 30 mm 3 points 2. Secondary ST segment changes 3 points 3. Left atrial enlargement 3 points 4. Left deviation of the QRS axis* 2 points 5. Onset of the intrinsecoid deflection 0.05 sec 1 points * In the absence of anterior hemiblock. Left ventricular hypertrophy is diagnosed when the total score is ≥ 5. 2. Minor abnormalities of ventricular repolarization in left precordial derivations. 3. Deep S wave in V2 (>25 mm). The main ECG abnormalities in patients with hypertrophic cardiomyopathy are included in Table 3. In the overall population, these ECG findings are relevant for HCM diagnosis, in the absence of any other cause that justifies them. In the European guidelines, performing a resting electrocardiogram has a class I recommendation, with level of evidence B, for the exclusion of cardiovascular disease that may cause sudden death in individuals who are going to practice either recreational or competitive physical activity. In contrast, in the United States, performing an electrocardiogram is not yet recommended as precompetitive screening, not even in high-performance athletes. (70) Apical HCM is a particular form of hypertrophic cardiomyopathy; its typical ECG (Figure 2) shows the absence of Q wave in I, aVL, and V5 and V6; the presence of high R waves in V2 derivation; a rectified ST segment elevation mainly in V2 derivation; and huge and pseudo-ischemic negative T waves in the anterolateral wall. These ECG abnormalities, typical of apical HCM, are not present in all cases, especially during the early and late stages of the condition, in which diagnosis is difficult and usually mistaken for other conditions. (59, 71) 6 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 Table 3. ECG abnormalities in patients with HCM P wave 1 Left atrial enlargement: negative portion of P wave in V1 ≥ 0.1 mV in amplitude with a duration ≥ 0.04 sec. 2 Right atrial enlargement: P wave amplitude in II, III, or V1 ≥ 0.25 mV QRS complex 1 Right axis deviation of the QRS complex in the frontal plane ≥ 1200, or left deviation from -30º to -90º 2 Voltage increase: - Of R wave in the frontal plane≥ 2 mV or in V5 and V6 ≥ 3 mV - Of S wave in V1 or V2 ≥ 3 mV - R or R in V1 e≥ 0.5 mV - R/S relation ≥ 1 Q wave (except in aVR) 1 Duration ≥ 0.04 sec 2 Q/R relation ≥ 25% 3 Amplitude ≥ 3 mm in two contiguous derivations 4 QS pattern in two or more derivations 5 Absence of the normal Q wave QRS complex duration Left or right bundle branch block with a duration ≥ 0.12 sec. Ventricular repolarization 1 ST segment - ST segment elevation or depression in two or more contiguous derivations 2 T wave - Flat or inverted in more than two derivations, except in children - Amplitude ≥ 10 mm 3 QTc interval - Duration > 0.44 sec. in men or > 0.46 sec. in women Rhythm and conduction disorders - Early ventricular extrasystoles or complex ventricular arrhythmias - Supraventricular tachycardia, flutter and/or atrial fibrillation - Short PR interval (0.12 sec) with or without delta wave - Resting sinus bradycardia (rate ≤ 40 bpm) - First-degree AV blocks (PR ≥ 0.21 sec., except in athletes), second-degree and third-degree AV blocks Thus, the ECG in apical HCM carriers may vary substantially in some cases, when the ventricular hypertrophy progresses to other regions of the ventricular myocardium, or when all or most of the left ventricular apical region necroses. (71) B. Echocardiography HCM is morphologically defined as a hypertrophied non-dilated left ventricle, in the absence of any other systemic or cardiac disease capable of producing such magnitude of parietal thickening; (8) that is why the echocardiography is so relevant as diagnostic tool. Transthoracic Doppler echocardiography is the most common method for HCM diagnosis, either by confirming the clinical and/or electrocardiographic diagnostic presumption, or by incidental finding. It Fig. 2. ECG of a patient with apical HCM. also provides information about type and morphology of HCM, diastolic and systolic ventricular function, occurrence and severity of dynamic left ventricular outflow tract obstruction, degree and cause of mitral regurgitation, prognosis, some physiopathological aspects, and acute and chronic response to therapies. The best diagnostic criterion is the presence of left ventricular hypertrophy that should be ≥ 15 mm in some ventricular region (20), and that it is often over 20 mm thick. In some patients, it may be below 15 mm; in these patients, HCM diagnosis must be considered when the parietal thickening cannot be explained by other cardiac or extracardiac reasons, such as hypertension, stenosis or aortic regurgitation, amyloidosis, athletes, glycosphingolipid intracellular deposits, or Fabry disease). Asymmetric septal hypertrophy is another echocardiographic finding, defined as the septum/posterior wall relation ≥ 13 mm. It is also closely associated with HCM diagnosis. (72) To differentiate HCM from athletes heart, it is necessary to include information related to parietal thickening, distribution patterns of hypertrophy, cavity size, assessment of diastolic function, Doppler tissue imaging, family history, and response to rest from sports activities in certain cases. (73, 74) As regards prognosis, echocardiographic detection of parietal thickening e 30 mm is considered a major risk factor of sudden death, mainly among adolescents and young adults. (7577) Moreover, the echocardiogram determines the type and extent of the ventricular hypertrophic compromise, which often varies from one patient to another; it may be concentric and symmetric, septal with or without left ventricular outflow tract obstruction, apical, a compromise of the left ventricular free wall, or of the right ventricle. (78, 78 bis) In some cases, the nuclear magnetic resonance allows to detect hypertrophic regions that are subdiagnosed by the echocardiography (3 out of 48 cases). (79) Papillary muscle hypertrophy has recently been defined as a diastolic thickness of more than 11 mm. (16) In fact, little is known about the clinical meaning of solitary papillary muscle hypertrophy. Some cases in which solitary papillary muscle hypertrophy preceded the development of HCM phenotype have been CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY detected when screening HCM patients first degree relatives. Solitary papillary muscle hypertrophy is presumed to represent either a subtype of localized HCM or the early-stage of HCM. (79 bis) In addition, the echocardiography allows for the detection of left ventricular outflow tract obstruction, expressed by systolic anterior motion of the mitral valve, and a dynamic subaortic gradient at that level, which can be screened by color Doppler, and measured by continuous Doppler. Left ventricular outflow tract obstruction is detected in 25% of the HCM cases, and may be basal or be caused by a physical effort, by the Valsava manouver, or by pharmacologic tests (amyl nitrite) that reduce the precharge or post-charge, or else that increase contractility. In every echocardiographic study of HCM, Valsalva maneuver is indicated to trigger an increase in the subaortic gradient. The presence of a gradient > 30 mm Hg is considered a low risk factor of sudden death given its low positive predictive value (14), and it is an independent evolutive predictor of heart failure; the assessment by stress echocardiography identifies the dynamic obstructive component in 70% of HCM patients, making it one of the most adequate forms of detection. (13) (4) It is important to remember, particularly if a therapy is being defined, that more than one dynamic obstructive component may coexist, or that it may only occur at midventricular level. (83) Mitral valve regurgitation generally occurs together with the obstructive form, since it is the result of the systolic anterior motion of the mitral valve, and its severity is variable; in this situation, the jet is usually directed to the left atrial posterior wall. (84) But it is also observed in the 20-30% of non-obstructive forms. In this case, regurgitation is generally mild, and is the result of an abnormality in the valve, such as prolapse, cordal rupture, valve ring calcification, chordae tendineae rupture, (85) or altered papillary muscle position. A jet not directed toward the posterior wall suggests some kind of structural abnormality associated with these mechanisms. The presence of diastolic dysfunction screened by pulsed Doppler may precede the symptomatic stage, and be detected even in the absence of dynamic obstruction. It is a risk factor for sustained ventricular tachycardia and death in children. (86) Myocyte hypertrophy, myofibrillar disorganization, alteration of ventricular geometry, ischemia, and fibrosis are the mechanisms responsible for disturbed ventricular relaxation that may reach a restrictive filling pattern of E/A higher than 2, and shortened mitral deceleration time (140 msec). Tissue Doppler imaging measures the myocardial velocity gradient, and allows not only to assess diastolic dysfunction, but also to differentiate the diagnosis from athletes hypertrophy or hypertrophy in hypertensive patients. A tissue e wave 7 cm/sec differentiates HCM patients from healthy athletes, whereas a tissue a wave 2 cm/sec identifies HCM 7 patients from hypertensive patients. (87) Some studies suggest that alterations in the tissue Doppler precede the development of hypertrophy in HCM, so it would be an early marker of the disease. (88, 89) Systolic function assessment by tissue Doppler imaging also shows significant alterations with clear reduction of tissue s wave at septal level; this alteration is present even in the absence of hypertrophy, and it is the field of study for preclinical and differential diagnosis of this condition. (90, 91) Serial assessment of the systolic ventricular function is also a follow-up parameter, since a small number of patients progress at a later stage to the systolic dysfunction with parietal thinning and ventricular dilation, developing congestive heart failure in its final stage. (81, 92, 93) On the other hand, it is important to point out that the rates of ventricular ejection function are not helpful to define the contractile state involvement in this condition. Systolic myocardial deformation (systolic strain) is involved in HCM, and it has been shown in several works that its assessment is helpful for the differential diagnosis between non-obstructive HCM and hypertensive hypertrophy, (94, 95) since all systolic strain components (longitudinal, transverse, circumferential, and radial) are decreased compared to the control. (96) Since HCM spreads in autosomal dominant form, with a high level of penetrancy in most of its forms, echocardiographic assessment should be prescribed to all first-degree relatives. (82, 97) For children under 12, assessment is indicated only when symptoms appear, when the family history of HCM includes high risk factors, or when the child takes part in competitive sports. Most HCMs develop between the ages of 12 and 18, so it is advisable to start echocardiographic assessment during adolescence, and repeat it annually. For people over 21, reassessment is encouraged every 5 years. The transesophageal echocardiography is another useful tool for the transthoracic approach in patients with inadequate echographic window, for completing the assessment of complex mechanisms of mitral regurgitation, or during the therapeutic processes of septal alcoholization and myomectomy. Detection of new alterations in 17% of the cases and modifications in intraoperative behavior in up to 9% of the cases has been reported for this condition. (98) Contrast echocardiography is a fundamental support for the treatment of HCM with dynamic subaortic gradient, through septal ablation with ethanol infusion. It allows accurate delimitation of the extent of the vascular territory corresponding to the septal artery, prior to infusion; this way, the extent of the infarction is determined, since it should be remembered that this is a case of planned but uncontrolled infarction. In addition, since the anatomy of the septal perforating branches is extremely variable from one patient to another, (99) the use of contrast has reduced the procedure time, the amount of injected al- 8 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 cohol, and the infarction size; it has also improved the procedure success (100) by reducing complications such as the atrioventricular block that requires permanent pacemaker implantation. (101103) The echocardiography and the Doppler have proved very useful in late follow-up after therapeutic procedures such as septal alcoholization, by documenting progressive gradient reduction with the passing of time, with parietal thickening reduction. (104) Conclusions and recommendations Transthoracic Doppler echocardiography Class I (level of evidence B) Confirm HCM diagnosis by determining parietal thickening, extent of the compromise, and presence, magnitude and location of the dynamic gradient. Research for HCM in first-degree relatives. -Reassessment after changes in clinical evolution, or after therapeutic maneuvers. -Myocardial contrast echo to assess the infarction size resulting from septal ablation. Class II (level of evidence C) IIa Reassessment of relatives for HCM, annually between the ages of 12 and 18, and every five years over 21. IIa Tissue Doppler imaging to differentiate HCM from hypertrophy due to hypertension, or in athletes. IIb Exercise echo stress test in symptomatic patients with exertion in their daily lives who do not show significant gradient at rest or with Valsalva manouver. Transesophageal Doppler echocardiography Class I (level of evidence B) -Patients with inadequate transthoracic ultrasound window. Determine the valve involvement, mechanism, and magnitude of mitral valve regurgitation when they are not clearly set by transthoracic echocardiography (TTE). Intraoperative study in myomectomy and in septal ablation with ethanol procedures. Class IIa (level of evidence B) Study to clarify the mechanism of atypical mitral regurgitation. In order to provide surgeons with more orientation, the following should be assessed in the operating room: marked septal protrusion - more mitral-septal contact 6. NATURAL HISTORY AND TREATMENT Life expectancy for HCM patients is variable, but in general it does not differ from that of the general population (1% mortality/year). However, there are fami- lies with multiple early sudden deaths (SD); their identification continues to be one of the biggest challenges in this disease. (4, 5) Likewise, progress of symptoms is very heterogeneous; some patients are asymptomatic for a long time, and others experience a complicated progress that includes congestive heart failure (CHF) symptoms, atrial fibrillation (with systemic embolism and/or CHF), SD, or, rarely, progress to endstage phase with CHF or SD. (7, 105) Infective endocarditis may develop in 4% to 5% of the patients, especially in the presence of LVOTO; it is usually located in the mitral valve, and sometimes in the aortic valve. (106, 107) Typical symptoms of this disease include dyspnea on exertion, precordial pain (angor pectoris), and presyncope and syncope which, as mentioned above, generally occur with a non-dilated ventricle and preserved systolic function, and due mainly to diastolic dysfunction with myocardial ischemia and/or outflow tract obstruction either with mitral regurgitation or not. (7) A. Medical treatment HCM treatment focuses mainly on relieving the symptoms of heart failure and preventing sudden death. Medical treatment is the first step, and it is generally based on works carried out in 1960s. It is important to point out that none of the drugs have proved useful to prevent sudden death or improve survival. 1) Beta blockers Beta blockers are the cornerstone of the medical therapy; most of the few works were carried out with propranolol. (108116) It was observed that beta blockers, together with exercise and decrease in basal obstructive gradient, improved angor, dyspnea, dizziness, and syncope. The negative inotrope and chronotrope effects reduce myocardial oxygen consumption, and also improve diastole by prolonging it rather than by a direct lusitropic action. (92) In patients with LVOTO, this improvement in diastolic filling entails an increase in volume and a decrease of the obstruction. (117) The first double blind study with propranolol versus practolol and placebo was carried out in England in 1973: 16 patients (15 with LVOTO) were treated for 4 weeks. With respect to placebo, propranolol reduced the frequency of angina and dyspnea, and practolol was less effective (which has a certain degree of inherent sympathomimetic action). (118) 2) Calcium blockers Since a significant number of patients do not respond adequately to beta blockers, other negative inotrope drugs have been tested, such as calcium blockers. The most studied one is verapamil; (119131) it reduces LVOTO and improves symptoms due to its negative inotropic effect. (123126, 129, 131) Moreover, it has a pronounced effect on diastole, since it causes ventricu- CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY lar relaxation, diastolic filling improvement, and decrease of LV filling pressure; (123131) coronary perfusion improves by direct effect. (128, 129) Verapamil should be used very carefully, since due to its pharmacological effects (peripheral vasodilation, contractility decrease, and electrical conduction decrease), it may cause a reduction in postcharge with increase of LVOTO, reflex tachycardia, and acute pulmonary edema or sudden death in LVOTO patients with pulmonary hypertension or congestive lung. It may also cause sinus dysfunction, and different degrees of AV block. (121) Verapamil was compared to a beta blocker in two studies. In the first double-blind study, Rosing et al. administered placebo, verapamil, or propranolol to 19 patients with HCM (17 with LVOTO). Both drugs had similar beneficial effects regarding exercise duration improvement. The subjective assessment favored verapamil, largely because of the asthenia on propranolol. (130) Gilligan et al. compared placebo to 80 mg of nadolol and 240 mg of verapamil in a double blind design on 18 HCM patients (8 with LVOTO); despite neither of the drugs showed objective improvement on exercise, symptoms were reduced with verapamil. (131) In spite of its beneficial effects on the diastolic function, diltiazem (132, 133) and dihydropyridines (nifedipine) (134, 135) should be avoided in HCM because of their significant vasodilation effect. 3) Disopiramide This drug is not available in our country. It is a class I antiarrhythmic medication that could particularly benefit those patients with atrial fibrillation and HCM; this drug has a negative inotropic effect mediated through sodium-calcium exchange. (7, 117, 136, 140) It decreases the gradient for LVOTO by means of this mechanism, and also by causing systemic vasoconstriction. It has anticholinergic effects (with uncomfortable side effects, such as urinary retention and dry mucoses) that may cause accelerated atrioventricular nodal conduction; for this reason, it should be administered together with beta blockers. A recent retrospective non-randomized multicentric study poses a proarrhythmic effect of this drug; therefore, QT should be periodically checked. (141) In general, it is used when symptoms cannot be relieved with betablockers or verapamil. B. Therapy options for patients who are refractory to drug therapy 1) Surgical treatment There is a small group of patients (5% from non-specialized centers; may reach 30% from referral centers) whose symptoms persist (dyspnea and/or angor NYHA class III or IV) in spite of maximum doses of medication; they also have resting LVOTO or during exercise, equal to or higher than 50 mm Hg. At present, 9 transaortic septal myectomy (also known as Morrows surgery) is considered the election therapy and the gold standard in the treatment of these patients. (92, 142, 147) The classical septal myectomy is performed with extracorporeal circulation and cardiac arrest with cardioplegia; by the resection of the proximal septal muscle (2 to 5 grams), it enlarges the LVOT, and eliminates the drag forces (Venturi effect) that cause the contact between the anterior mitral valve leaflet and the hypertrophied septum, resulting in reduction or abolition of LVOTO. Very recently, a group of surgeons created a modification consisting in an enlarged resection with greater distal expansion that allows for more adequate LVOT reconstruction, necessary for certain patients. (148, 150) Mitral valve repair or replacement surgery may be performed in patients with severe mitral regurgitation due to intrinsic mitral valve disease (myxomatous valve). (148150) The learning curve to perform this procedure is important; the initial surgical experience was associated with complete atrioventricular block, VSD, damage to the mitral and/or aortic valves, incomplete decrease of the LVOT obstruction, and operative mortality equal to or lower than 5%. However, over the past years, the outcomes of this surgery in the few specialized centers of United States and Canada have been positive, with a mortality rate of almost 0%. (151) Surgical risk is usually higher for elderly patients with comorbidities, highly symptomatic and with pulmonary hypertension, and for those undergoing other heart surgery procedures (coronary surgery, valve replacements). At present, complete atrioventricular block (which requires permanent pacemaker implantation) and iatrogenic ventricular septal defect are very rare (1-2%), whereas incomplete or complete left bundle branch block is an almost inevitable outcome of a correct myomectomy, and does not imply future risks. (146) As it has already been mentioned, intraoperative TEE is very useful for the surgeon in guiding the septal myectomy extension, and in assessing the mitral valve disease and the outcome on regurgitation. (98) Both the obstruction relapse and the need for reoperation are very rare. Myectomy outcomes are immediate and permanent: there is abolition of the LVOT mechanical obstruction (and of the mitral regurgitation), with normalization of ventricular pressures and decrease or disappearance of dyspnea and syncope, and with improvement of the functional capacity and quality of life. (142, 147, 149, 152, 154) In a recent analysis, 85% of the patients were asymptomatic or slightly symptomatic (NYHA class I or II dyspnea) in mean follow-up of 8 years (and up to 25 years) after septal myectomy. (155) In addition to symptomatic improvement, septal myectomy has improved survival for these patients compared to those who did not undergo surgery, and has achieved a life expectancy similar to that of patients without that condition. Survival free from allcause mortality is 98%, 96%, and 83% in 1, 5, and 10 10 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 years, and survival free from HCM mortality (sudden death and heart failure) is 99%, 98%, and 95% respectively. (155, 156) In short, the advantages of septal myomectomy are the immediate and permanent reduction of the symptoms, and survival improvement; as the surgeon has a direct view of LVOT, he can identify mitral valve pathology correctly; it does not form scar, it rarely requires reoperation or pacemaker implantation, and it allows to repair associated cardiac defects (subaortic stenosis, mitral and aortic valve diseases, coronary surgery). The disadvantages include the need for an experienced surgeon, and the median sternotomy that requires an in-hospital recovery period of 4 to 7 days. (157) Indication for myomectomy Class I (level of evidence B) Patients showing NYHA class III-IV symptoms that are refractory to medical treatment, and a gradient at rest or during exercise ≥ 50 mm Hg with septal thickness ≥ 18 mm, at surgery centers with experience in this condition and low morbimortality. 2) Percutaneous septal ablation In 1995, Sigwart (158) was the first one to describe the occlusion effect followed by absolute ethanol embolization of septal perforating branch in LVOT pressure gradient in HCM patients with LVOTO (PSA). At the same time, two centers from Germany shared their experience on several hundreds of patients, which they called transcoronary ablation of septal hypertrophy (TASH). (159) The major experience in the United States corresponds to the group from the Baylor College of Medicine, in Houston, (160) whereas in Europe, the German PSA registry has reported a significant number of cases. (161) At present, this technique has been spread all over the world, and about 3,000 patients have been reported in a recently published revision. (162) As we have mentioned, percutaneous septal ablation (PSA) with alcohol is a less invasive alternative to surgery. This is a percutaneous technique that consists of injecting absolute ethanol in order to cause a controlled infarction of the basal interventricular septum to decrease its thickness. The final outcome is the reduction in LVOT pressure gradient. (162, 163) At first, gradient reduction is due to deterioration in basal septum contraction, but then there is further reduction due to thinning and fibrosis at this level. This process leads to an increase of left ventricle diameters, a decrease of the systolic thickness and of mitral regurgitation and, therefore, the subsequent ventricular remodeling. (164) However, correct selection of patients as to who and when undergo surgery is controversial; moreo- ver, interventional physicians are not properly trained. This issue has induced some groups with vast experience in this cardiomyopathy to suggest that this technique should be indicated only for those patients with comorbidities that increased their surgical risk. (157, 165) The procedure is performed under the general characteristics of a percutaneous coronary intervention, but taking into account that: 1. Coronary arteriography provides anatomic information regarding septal perforating branches (their degree of development, magnitude of the irrigated area, the presence of collaterals, etc.). But the presence of possible obstructive coronary lesions can also be identified; this would probably lead to rule out the procedure for these patients. 2. A pacemaker lead should be placed inside the right ventricle, since nearly 20-30% of the patients have transient AVB during ablation. As opposed to surgery, PSA usually causes right bundle branch block. 3. Next, what follow are the steps in a conventional coronary angioplasty, complete heparinization, guide catheter, wire 0.014" used to canalize selectively the first septal branch or, otherwise, the major adjacent septal branch (the first septal branch should be avoided if it is of small caliber and extension, since it often provides abundant collaterals to the venous system). An over the wire (OTW) balloon, with 1.5 or 2.0 mm diameter, is inserted. Once in place, the balloon is then inflated until the desired branch is occluded, in order to eliminate the flow and avoid the eventual retrograde effusion. The wire is then removed; dye is injected through the balloon lumen to opacify the distal territory of the involved branch. This is a very important step of the procedure, because the amount of myocardium at risk, the dynamic characteristics (magnitude of gradient reduction), the presence of collaterals to other coronary territories, etc. are assessed. It is at this step when the contrast echocardiography and the experience of the operator play a key role, since the procedure should not be continued if there are no significant changes in the gradient (this piece of information can also be obtained through the invasive measurement of simultaneous left ventricular and aortic pressures), but even more if motility disorders are observed in a wide territory that expresses the potential infarction that alcoholization can cause. The use of contrast echocardiography has probably helped to better identify the involved territory, resulting in less complications. (99102) 4. Before deciding to administer alcohol, gradient reduction of at least 30% should be checked for. In general, for both basal and provoked gradients, the reduction is 50%, or they disappear completely; this usually occurs a minute after the balloon has been inflated, and once the contrast dye has been injected. Then the absolute alcohol infusion is started 11 CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY (96% ethanol), between 1-2 ml, slowly, because it has been demonstrated that the incidence of total AV block is related to the rate of administration, among other reasons. (166) 5. Once the procedure is over, the patient will stay in the intensive care unit for two days. PSA is considered successful when there is a gradient reduction in the cardiac catheterization laboratory. Creatine kinase increases as an expression of the infarction size (about 1,000, which would be equivalent to a necrosis area of 20% of the septum size, or 310% of the left ventricular mass). A progressive gradient reduction in the following 6 to 12 months is sometimes observed, moment in which it reaches the same outcome as the surgery. In other cases, gradient reduction can be obtained with a bimodal response, that is, an initial reduction followed by an increase of almost 50% the following day, and then a definitive reduction in the following months. (160162) This may occur because once the infarction occurs, it is followed by an inflammatory process with interstitial edema, resulting in a new reduction of the LVOT. Finally, once the scar is consolidated with the septal and left ventricular reconstruction, definitive gradient reduction is achieved, with no significant fall in left ventricular ejection fraction. Improvement of symptoms, and therefore of quality of life, is immediate in most patients, who show a decrease in functional class (mostly in FC I), and an increase in exercise tolerance and oxygen consumption. (160, 162) Since its introduction, PSA has undergone modifications that have lead to significant reduction in morbimortality. The rate of complications is relatively low; intraprocedural mortality is 0-5%, and it is generally due to anterior descending artery dissection, cardiac tamponade, or cardiogenic shock. (167) The most common complication is the atrioventricular block, which was close to 20-30% according to initial series. At present, it is about 10%, probably due to decreasing the total volume of injected alcohol and the rate of infusion, among other reasons. However, the early success of PSA over time is discussed, especially if it is compared to septal myomectomy outcomes. Even though there are few studies that compare surgery with PSA directly, and none of them are randomized, trials show similar outcomes, although it is possible that myectomy could get higher early reduction in LVOT gradient. (103) Observational studies prove that gradient reduction holds a value of ≥ 20 mm Hg in a two-year followup (166), and there are data that these values are held for more than 5 years. Exercise tolerance, functional class, and diastolic function undergo a similar evolution to that of the gradient. To date, the true impact on sudden death incidence is still unknown, since some argue that, due to the scar it forms, PSA is adding an arrhythmogenic substrate to a disease that already has one. (157) Conversely, studies have been carried out in which induction of re-entry ventricular arrhythmias was attempted, with no success. These data are important when considering this treatment for a young person. As selection criteria, we could confirm that eligible patients are those who have symptoms uncontrolled with complete medical treatment, those with symptoms of heart failure (functional class III), with asymmetric septal hypertrophy of at least 18 mm thick confirmed by ultrasound, with the classical anterior movement (SAM) of the mitral valve, and those patients who have a gradient at rest or during exercise ≥ 50 mm Hg showed by the Doppler register, with accessible septal branch, absence of atherosclerotic disease in the proximal anterior descending artery, or severe involvement of three vessels. (117) As a result of what has been said, we could undoubtedly confirm that percutaneous septal ablation is effective in reducing the LVOT gradient in hypertrophic cardiomyopathy, and in achieving significant improvement of the symptoms of this disease. However, these outcomes depend on a correct selection of the patients to be treated, together with an adequate learning curve on the part of the interventionist group. While data from late follow-up do not exceed 10 years, initial outcomes would seem to be the same over time, and are compared to those obtained through surgery. (168) Further randomized trials compared with septal myomectomy would be necessary to give an answer to this concern. Chances to carry them out are low, or in some experts opinions even impossible. (169) Indication for percutaneous septal ablation Class IIa (level of evidence B) Patients showing NYHA class III-IV symptoms who are refractory to medical treatment, with a gradient at rest or during exercise e 50 mm Hg and septal thickness e 18 mm, with accessible septal branch, no lesion in the anterior descending artery and no three-coronary vessels lesion, and with comorbidities that contraindicate surgical myomectomy, in centers with experience in this procedure and low morbimortality. 3) Pacemaker Electrical stimulation in hypertrophic cardiomyopathy DDD Stimulation with short AV When the stimulus sent by a DDD pacemaker with programmed short AV interval reaches the tip of the RV before the stimulus coming from the normal conducting system, preexcitation in the tip of the RV and the LV occurs. The contraction sequence is inverted and it causes dyssynchrony across the septal basal segments, which produces paradoxical movement of the septum. The 12 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 septum is separated from the mitral annulus; this increases LTOV diameter, reduces flow speed, and reduces the systolic anterior movement (SAM) of the mitral valve. As a result, the degree of mitral regurgitation, LTOV gradient, intraventricular pressure, and parietal stress decrease, and the percentage of atrial contribution to ventricular filling increases. (170173) Initial uncontrolled studies showed that DDD stimulation with short AV reduces the LVOT gradient and improves the functional class in patients who are refractory to medical treatment. (174176) Later on, a randomized crossover study on patients with LVOT gradient at rest > 30 mm Hg showed that DDD PM with short AV reduced the LTOV gradient and the functional class, and improved symptoms with a 3 year-long-term effect. (177, 178) However, these outcomes did not coincide with the outcomes of two minor randomized crossover studies. In one of them, the benefit was only evident in patients over 65 years old with alteration of LV diastolic function. The gradient was reduced in 3 months, but clinical improvement was only observed in 12 months, which suggests a placebo effect. (179) Despite DDD pacing with short AV produces improvement in many patients, there is no clear evidence of who will benefit from this pacing. Gradient reduction is not correlated to clinical improvement in patients, so it is difficult to predict which ones will be responders. (177179) For better therapeutic results: 1. The lead should be placed in the tip of the RV. 2. The short AV interval (shorter than the PR interval) should be the longest interval that, by causing maximum pre-excitation (wider QRS), provides the longest diastole duration with the lowest LVOT gradient and the optimal systolic volume. 3. The maximum programmed frequency limit should be above the maximum heart rate during exercise, so that the RV pre-excitation is not lost with the stress. There is no evidence that the DDD PM stops the progress of the disease or reduces survival. Clinical improvement and LVOT gradient reduction are lower than alcohol ablation or myomectomy. Its main advantage is the programming and simplicity of the surgical procedure. In view of the above said, it is recommended in the following cases: Class I Patients with obstructive HCM and sinus node disease or AV block, as a result of the natural evolution of the disease, secondary to septal myomectomy or septal alcoholization, or as a consequence of the use of drugs required for the treatment, when other alternatives are not valid. Level of evidence C. Class IIa Patients with obstructive HCM, refractory to drugs, and contraindication for septal myomectomy or alcoholization. Particularly in the case of elderly patients. Level of evidence B. Patients with high risk of sudden death that have indication for ICD with significant LVOT gradient at rest or with provocation maneuvers may benefit from DDD-ICD and short AV interval. 7. SUDDEN DEATH HCM is a disease characterized by having a benign natural history for most patients. (7) However, one of the biggest challenges in the assessment of this condition is identifying those patients with increased risk for sudden death (SD). (75, 180) At present, HCM is recognized as the most common cause of SD among young people and those who practice sports competitively. (181185) It should be pointed out that SD is usually the first manifestation of this disease and may occur in patients who have no premonitory symptoms. (75) The incidence of SD in HCM is almost 6% in tertiary care centers, and below 1% in non-selected populations. While SD is more frequent among young patients, it may occur in middle-aged persons, and in 20% of the people over 65. (186) Several physiopathologic mechanisms leading to SD have been proposed; these include atrial and ventricular tachyarrhythmias, diastolic dysfunction, systemic arterial hypotension, and myocardial ischemia. (7) According to the International Consensus on HCM in 2003, cardiac arrest due to documented ventricular fibrillation, spontaneous sustained ventricular tachycardia, family history of early SD, syncope of unexplained origin, extreme ventricular hypertrophy, nonsustained ventricular tachycardia on Holter ECG, and abnormal blood pressure response during exercise are considered major predictors of SD (Figure 3). (7) Possible risk factors for certain patients included atrial fibrillation, myocardial ischemia, resting left ventricular outflow tract obstruction, high-risk genetic mutations, and vigorous exercise. (7) Based on different publications from 2003 to date, this group should also include young patients, patients in the end-stage phase of HCM, patients who have associated coronary artery disease, patients with muscular bridges, those treated with alcohol ablation, and those who evidence myocardial fibrosis. (27, 75, 187193) Sudden death due to documented ventricular fibrillation and spontaneous sustained ventricular tachycardia Patients who have survived a ventricular fibrillation event or spontaneous ventricular tachycardia are in high risk of suffering a recurrent event, and should 13 CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY receive an ICD as secondary prevention of SD. (194, 195) Family history of early sudden death Family history of early SD refers to one or more SD in first-degree relatives before the age of 40. (92) Different studies have found out families whose members have identical genotype, but significant phenotypical heterogeneity and completely different prognosis. (196, 197) However, in clinical practice, the presence of early sudden death in a family particularly if it is multiple is still a strong predictor of SD. (198, 199) Unexplained syncope In HCM, syncope has low sensitivity and specificity as SD predictor, since it may be caused by multiple mechanisms, such as neurocardiogenic causes, supraventricular and ventricular arrhythmias, LV out- flow tract obstruction, etc. (7, 200) Unexplained syncope has a value in children and young patients, or when it is recurrent or occurs during exercise. (7, 201) Extreme ventricular hypertrophy Extreme ventricular hypertrophy is defined as the ventricular wall thickness ≥ 30 mm, or its equivalent in children. (76) There is a direct relationship between the magnitude of cardiac hypertrophy and the risk of SD. (76, 204) It is unusual to find such phenotypical expression in patients over 50 years of age; this phenomenon could indicate that most of them died early or presented ventricular remodeling with wall thinning. (77, 188) It is important to point out that while this is a significant and easy to recognize risk factor, the fact that the patient does not have extreme hypertrophy is not a synonym of absence of SD risk. (7) Most patients with SD have a wall thickness < 30 * En los pacientes menores de 40 años se puede considerar la indicación de un CDI con un solo criterio mayor, salvo en el caso de la respuesta anormal de la presión arterial durante el ejercicio, que tiene bajo valor predictivo positivo en forma aislada, pero su valor aumenta cuando se asocia con mutaciones de la troponina T. Fig. 3. Risk assessment for sudden cardiac death in HCM. 14 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 mm, and some even have normal wall thickness, as in the case of those with troponin I and T gene mutations. (7, 196, 202) On the other hand, there are other sites of hypertrophy, as in the case of apical HCM, that in the absence of other risk factors usually have a good prognosis. (7, 203) Non-sustained ventricular tachycardia Non-sustained ventricular tachycardia (NSVT) is considered a risk predictor of SD in HCM when the episodes are multiple and/or prolonged (with a HR3 120 bpm), and are evident on serial Holter ECG. (238) The risk of SD due to NSVT in HCM is higher, especially for young patients (under 30 years of age), and for those who have a history of syncope or deterioration of systolic function. (187, 205, 210) Abnormal blood pressure response during exercise Abnormal blood pressure response during exercise occurs independently from whether the patient has some form of obstruction or not. An exaggerated fall in systemic vascular resistance or a diffuse subendocardial ischemia that leads to systolic dysfunction have been the possible mechanisms proposed. (32, 211) The point to be made is that this finding is clinically significant if the stress test is performed with no drug effect. Blood pressure response may be plain: high systolic blood pressure < 20-25 mm Hg, or hypotensive: low blood pressure > 15 mm Hg). It occurs in 22-37% of the patients; in individual cases, it has a low positive predictive value, and its value increases when it occurs in patients under 50 years of age (especially in children or adolescents), and in cases associated with troponin T mutations. (7, 212) Left ventricular outflow tract obstruction It has recently been demonstrated that HCM is mainly an obstructive disease. (13) It was evidenced in two different series that the left ventricular outflow tract obstruction (LVOTO) has a low predictive value for SD, 7% and 9% respectively. (14, 214) Therefore, LVOTO is not enough to indicate an ICD as primary prevention, and its predictive value increases when it is associated with other risk factors. (215) Atrial fibrillation As commented above, atrial fibrillation (AF) in HCM is an independent predictor of morbidity and mortality due to heart failure and stroke. (31, 105) At the same time, atrial fibrillation can trigger malign ventricular arrhythmias, especially in the case of high ventricular response. (216) However, evidence is not yet enough to determine if the control of AF frequency or rhythm may have an impact on SD prevention in HCM patients. (199) Myocardial fibrosis and ischemia The main mechanisms that can cause ischemia in HCM include: intramural small vessel disease, left ventricular hypertrophy, muscular bridges, microvascular dysfunction with inadequate vasodilation, and epicardial coronary artery disease, which occurs with the same frequency as it does in the general population. (23, 217, 220) A relationship between SD in HCM and the presence of ischemia assessed with different non-invasive methods has been found out. (23, 217, 219) At the same time, the areas with myocardial fibrosis in HCM patients act as substrate for the appearance of potentially malign ventricular tachyarrhythmias. The areas with myocardial fibrosis may be detected by nuclear magnetic resonance imaging with gadolinium. (221) There is not enough evidence to indicate an ICD for primary prevention of SD, just by presenting ischemia and/or fibrosis in the absence of other risk factors. (199) Vigorous exercise HCM is the most frequent cause of SD in athletes. (183, 184) It can occur with no previous symptoms, and it has a significant social and emotional impact. Patients diagnosed with HCM should be discouraged from taking part in sports; they can only practice lowintensity sports like golf or bowling (184, 185) (see Exercise and HCM). Young age The age at which it occurs is considered a significant variable for risk stratification. (189, 199, 222) Sorajja et al. have demonstrated, for example, that the annual incidence of SD in patients with extreme LVH is 2.2% for individuals under 30, and 0.73% for the age group of 30-59. (189, 199) Monserrat et al. have demonstrated that the odds ratio for SD in patients with documented NSVT is 4.35 (p = 0.0006) for those under 30, and 2.16 (not significant) for patients over 30. (205) End-stage phase of hypertrophic cardiomyopathy During the end-stage phase of HCM, defined as an ejection fraction lower than 50% at rest, patients usually progress to severe clinical deterioration; it is also an important risk factor of SD. (187) For that reason, this subgroup of patients should be indicated for an ICD as a bridge to cardiac transplant. (213) Muscular bridges Muscular bridges in the anterior descending artery increase the risk of sudden death in children, probably mediated by myocardial ischemia. (218, 223) However, this relationship is not very clear in adults, and the need to perform a routine invasive coronary angiography to discard this condition reduces the incidence of muscular bridges as a risk factor of SD. (7) Alcohol ablation Alcohol ablation favors the development of a myocardial scar that acts as a substrate for ventricular arrhythmias in a condition that is inherently 15 CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY arrhythmogenic by nature. (224) Both early ventricular arrhythmias that may occur during or immediately after alcohol ablation and late ventricular arrhythmias (i.e. that occur 72 hours following postablation) have been described. (191, 193) HCM patients usually have polymorphic VT; however, patients who underwent alcohol ablation evidenced monomorphic VT with left bundle branch block image, which is a patent consistent with arrhythmias due to re-entry in the infarcted area and whose outflow tract is at the interventricular septum level. (191) It is precisely that risk for ventricular arrhythmias that made experts suggest that this procedure be carried out only in patients contraindicated for surgery, such as the elderly or those with concomitant diseases that increase surgical risk. (7) Genetic testing The diagnostic performance of genetic testing is 40% on average (38, 225); so far, no prognostic usefulness has been shown, since the phenotype depends not only on the primary causal mutation, but also on the interaction with other genes, and on environmental factors. (226, 227) The tests to assess the genotype-phenotype correlation were performed on a reduced number of patients or selected families, and in fact a large number of families with positive genotype would be necessary in order to determine if this relationship is present with other genes. (228) Genetic testing should not be indicated in a routine way to stratify the risk of SD, and its usefulness is currently limited to research studies. (227, 228) Other complementary tests The analysis of variability in the autonomic tone, the high-resolution electrocardiogram, and the QT interval dispersion may be altered, but they are not useful to identify high risk patients. (229, 230) Patients can also have sinus node dysfunction, bradyarrhythmias, AV block, and supraventricular arrhythmias. (230) As for the electrophysiological study (EPS), it does not appear to be efficient in the risk stratification of these patients. (231, 232) It is important to perform the following tests to identify high-risk patients, particularly in those under 60 years of age: research into personal and family history; cardiac echo-Doppler to assess the magnitude of the hypertrophy and the ejection fraction, and to determine if there is outflow tract obstruction; 48hour Holter monitoring to detect NSVT; graduated stress test or, preferably, exercise stress echocardiogram to detect ischemia; assess blood pressure during effort to determine if the patient has a latent or provocable gradient with exercise. (7, 229) These tests should be performed periodically, and when clinical changes are perceived. (7, 233) Recently, it has been observed that T wave alternans voltage is present in high-risk HCM patients, compared to controls; also, a significant association between this finding and the presence of NSVT in Holter monitor has been found. (234, 235) Anyway, the evidence is currently poor to consider this factor a SD predictor for HCM patients. (199) Therapeutic alternatives for HCM patients with high risk of SD Implantable cardioverter-defibrillator for primary and secondary prevention of sudden death Patients resuscitated from SD, and symptomatic patients due to ventricular tachycardia and/or syncope related to ventricular arrhythmia have indication for implantable cardioverter-defibrillator (ICD) as secondary prevention. (7, 180) A consensus has also been currently reached on the indication for ICD as primary prevention for patients with significant risk factors of SD. (7, 180) About 45% of HCM patients have evidence of increased risk, and only 3% of those with no risk factors experience SD. (75) It is also important to point out that prognosis is more ominous when two or more risk factors are associated. (75) At present, whether it is necessary only one risk factor for primary prevention or whether patients must combine two or more criteria is still controversial. It should be pointed out that indication for ICD as primary prevention is considerably different depending on the country of origin, healthcare system, accessibility to devices, and opinions from different experts. (182, 204, 233) Maron et al. studied the ICD efficacy in preventing SD on 128 high-risk HCM patients over a three-year period. For those who received ICD for secondary prevention, the discharge rate was 11% per year in 40% of patients, and for those who received ICD for primary prevention the appropriate discharge rate was 5% for 11.8% of the patients. (236) Maron et al. (6) have recently published a work that included 506 patients from 42 different centers, with a follow-up period of 3.7 years. Appropriate ICD discharge rates for primary and secondary prevention were 11% and 4.4% per year, respectively. In this work, it was evidenced that the time from ICD implantation to the first appropriate shock may reach up to 10 years, and also that one third of the patients under primary prevention who received appropriate shocks presented only one risk factor. (6) This way, authors consider that just one risk factor is enough to indicate ICD implantation for primary prevention of SD in certain patients. (6) However, the authors clarify that indication for ICD when there is just one risk factor should not apply for all patients. (6) For instance, SD is relatively less frequent in elderly patients, and ICD should not be indicated for these patients if the only risk factor is, for example, syncope of unknown cause. (6, 237) In Table 4, there is a summary of recommendations and levels of evidence available, to indicate for ICD for secondary and primary prevention of sudden cardiac death in HCM. (237, 238) On the basis of this, we 16 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 have developed an algorithm to be used as a guide to select patients who can benefit from this therapy (see Figure 3). The rate of inappropriate shocks and complications related to ICD implantation is high, and it should be taken into account at the time of deciding whether or not to implant these devices. (6, 239) left ventricular remodeling with cavity dilation, and this process is not complete in 48% of these patients, including those with pronounced hypertrophy and non-dilated cavity. (187) Clinical evolution of the endstage phase is variable, but it is generally unfavorable, and usually adopts an aggressive course with significant clinical deterioration. (187, 242247) Mortality rate for end-stage phase is 11% per year, and it increases to 50% per year for advanced cases. (187, 242) The mechanisms responsible for the transformation of typical HCM into end-stage phase are not fully clarified. Fighali et al. (243) showed that the incidence for heart disease, muscular bridges, dynamic outflow tract obstruction, and previous septal myomectomy is similar among patients who develop left ventricular hypokinesia, and among those who do not develop it. It is postulated that this process is the result of recurrent myocardial ischemia due to microvascular dysfunction. (218, 248, 251) Several arterioles show an increase in their wall thickness with the resulting luminal narrowing, which provides the mechanism that determines ischemia and fibrosis replacement. (218, 248, 251) HCM occurs with a decrease in the coronary blood flow reserve, together with structural changes in microcirculation (coronary remodeling), including a reduction of the arteriolar density that is inversely correlated to myocyte hypertrophy. (22, 252, 253) Microvascular dysfunction may precede clinical deterioration for several years. (218) By means of the positron emission tomography (PET), Cecchi et al. (218) evaluated the response to dipiridamol, and showed that the degree of microvascular dysfunction is an independent predictor of clinical deterioration and death. Later, Olivotto et al. (248) Pharmacological therapy Various drugs have been proposed to prevent SD in HCM, including β-blockers, calcium blockers, and various antiarrhythmics, but none of them has proved effective in reducing this risk. (240, 241) Recently, Maron et al. evidenced that 25% of the patients with appropriate ICD shocks were taking amiodarone; this shows the relative inefficacy of antiarrhythmic drugs to prevent SD in these patients. (6, 15) In spite of this, amiodarone therapy is used both for secondary and primary prevention when ICD is not available or is rejected by the patient (238) (see Table 4). 8. END-STAGE PHASE OF HCM While HCM is usually associated to diastolic dysfunction, a few number of patients show a progressive deterioration of systolic function, called end-stage (ES) phase, defined by an ejection fraction at rest < 50%. (187, 242) The reported prevalence of the end-stage phase varies from 2.4% to 15% in different series. (187) Harris et al. (187) studied 1,259 HCM patients and found out 44 (3.5%) in end-stage phase with systolic dysfunction (previous bibliography was limited to smallgroups, and have overestimated end-stage phase occurrence). Most patients in end-stage phase show Recommendation Level of evidence I B Amiodarone C HCM patient resuscitated from SD or syncope with SVT and/or documented VF (when the ICD is not available or is rejected by the patient) IIa C Primary prevention ICD HCM with a major factor and two minor factors, or with two or more major risk factors for SD IIa C Amiodarone HCM with a major factor and two minor factors, or with two or MORE major risk factors for SD (when the ICD is not available or is rejected by the patient) IIb C Secondary prevention ICD Patient with HCD resuscitated from SD or syncope with SVT and/or documented VF HCM: Hypertrophic cardiomyopathy SVT: Sustained ventricular tachycardia. VF: Ventricular fibrillation. ICD: Implantable cardioverter-defibrillator. SD: Sudden death. Table 4. Prevention of sudden cardiac death in patients with hypertrophic cardiomyopathy CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY confirmed that microvascular dysfunction is a longterm strong predictor of adverse left ventricular remodeling and systolic dysfunction in HCM. On the other hand, some authors suggest that progression to dilation may be genetically determined. (254, 256) Some patients improve with an optimal pharmacological therapy, or with other therapeutic alternatives such as biventricular pacing, resynchronization therapy, but a large number of them evolve with rapid clinical deterioration and death due to heart failure or sudden death. (187, 242, 257, 259) These patients should be assessed for cardiac transplant, and the indication for an ICD as a bridge to such procedure should also be considered for them. (242, 244, 245, 260) 9. EXERCISE AND HCM The relationship between exercise and HCM can be approached from two different perspectives. On the one hand, by assessing the disease through stress tests, and on the other hand, by recommending HCM patients regarding sports and different physical activities. A. Exercise tests and HCM For a long time, ergometric test has been contraindicated in HCM patients. At present, its use has been spread to stratify the risk, and to get prognostic information. The stress test provides independent value to identify patients with an increased risk for sudden death, and is included in the prognostic algorithm for decision-making of preventive treatments. Together with the measurement of peak O2 consumption (VO2), this test has a key role in the differential diagnosis from other conditions related to left ventricular hypertrophy (LVH), such as the athletes heart. Assessment of functional capacity Many HCM patients have their exercise capacity reduced. Cardiopulmonary exercise test is particularly useful to evaluate the degree of intolerance to exercise and the causes of this intolerance. The peak VO2 is a useful marker of functional capacity in this population. When compared to healthy controls, HCM patients have lower peak VO2 values. Recent studies have shown a positive correlation between the peak values of ventilation equivalents for CO2 with pulmonary pressure measures at rest, whereas there has been a negative correlation between peak VO 2 and these hemodynamic measures. (261) From the cardiological point of view, the VO2 is determined by the minute volume (MV) and the arteriovenous oxygen difference (dAVO2). During exercise, VO2 increases as a result of the increase of these components. HCM patients may show an inadequate increase in SV during exercise, which may be due to inadequate 17 diastolic filling caused by ischemia, left ventricular hypertrophy, and/or intramuscular fibrosis. On the same note, some HCM patients present skeletal myopathy with lower mitochondrial density and lower peripheral extraction of O2. In patients with LVOTO and intraventricular gradient at rest, there is an inverse linear correlation between the gradient magnitude and the peak VO2. This suggests that severe mechanical obstruction is an important factor in exercise limitation. Patients with abnormal response to systolic blood pressure during exercise have significantly lower peak VO2 anaerobic threshold, O2 pulse, and higher VE/ VCO2 slope, in comparison with those with normal pressor response. (262) In several HCM patients, the anaerobic threshold is recorded early during exertion; this variable is due to the inadequate reduced systolic volume caused by metabolic demands of the exercised muscle. In these patients, the O2 pulse (VO2 per beat) a reflection of the systolic volume is usually reduced. The ventilation/VCO2 slope is usually higher than in healthy individuals. Two mechanisms are involved: 1) hyperventilation, or 2) ventilation/inadequate perfusion due to lung perfusion abnormality during exercise, secondary to the low cardiac output. Cardiopulmonary exercise test is a useful method for the differential diagnosis between HCM and the athletes heart or physiologic hypertrophy. While there are multiple electrocardiographic and ultrasound variables and signs that help differentiate them, there is a small number of individuals who fall in the shaded area, in which the differential diagnosis may be more difficult. Sharma, McKenna et al. compared four groups of patients: 1) HCM patients, 2) recreacional athletes, 3) elite athletes with LVH, and 4) elite athletes without LVH. There were no significant differences in cardiopulmonary parameters between the elite athletes with and without LVH. (263) Peak VO2, aerobic threshold, and O2 pulse values are high in elite athletes. Recreational athletes had higher peak VO2 than HCM patients. There was no overlap in values among the groups. A cut- off peak VO2 value > 50 ml/kg/min or > 20% over the theoretical maximum, an AT > 55% over the theoretical maximun, and an O2 pulse > 20 ml/beat differentiated HCM individuals from trained athletes. Arrhythmia assessment While the stress test is not the method to be chosen for studying arrhythmias in HCM, sometimes it can show them up even if they have not been detected through 24-hour Holter monitoring. ST segment changes observed during stress test ST segment depression is frequent when there is LVH; its specificity to detect associated coronary artery disease is low, and it is one of the confounding factors in diagnostic stress test interpretation. 18 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 The probable mechanisms are related to systolic compression of intraparietal small vessels, poor capillary development compared to the increase of myocardial mass, structural disease in small caliber arteries with disturbance in the vasodilatory capacity, imbalance in O2 supply and demand due to increased myocardial mass. Repeated episodes of ischemia during heavy exercise could contribute to the onset of intramuscular fibrosis. The presence of ST segment depression during stress test may have prognostic significance for SD in young patients, mainly children and adolescents. SD, syncope, or cardiac arrest would be primarily ischemic episodes for children under 14 years old. Most patients at this age show ischemia in perfusion tests, and ST segment depression during exercise, even in the absence of ventricular arrhythmias in Holter or EPS. (264) An association between ST segment depression during exercise and systolic dysfunction has been discovered. Shimzu et al. studied 53 HCM patients with ergometry and ambulatory ventricular function monitoring, and they found out that patients with ST segment depression showed a fall in the left ventricular ejection fraction during stress. Conversely, those patients with no alteration of the ST segment had a normal response of ejection fraction. (265) To sum up, the presence of ST segment shifts in stress tests on HCM patients reveals ischemia, and not coronary artery disease; therefore, its diagnostic value is poor, but it may be a significant prognostic marker. Abnormal blood pressure response during stress The most studied ergometric variable related to prognosis in HCM patients is the systolic blood pressure (SBP) response to stress. The widespread and accepted definitions of abnormal SBP response include: 1) the fall ≥ 20 mm Hg compared to the previous record or the continuous decrease during stress that reaches 20 mm Hg, and/or 2) the little increase or flat curve in SBP, defined as an increase lower than 20 mm Hg compared to basal, and over the course of the test. Abnormal SPB response in the stress test is associated to poor late prognosis in patients under 50 years of age. It does not identify in isolation a high risk population, but it should be considered together with the rest of the variables. It has a screening value for low risk patients, due to its high negative predictive value, together with the absence of the rest of the risk factors. (140, 162, 266) Current consensuses include the abnormal SPB response among the major risk factors to predict sudden death in HCM patients. (7) Indication for stress test on HCM patients Class I (level of evidence B) Asymptomatic patients with no high-risk variables as associated element in prognostic stratification. -Asymptomatic patients with no high-risk variables who want to do recreational exercises. Ergospirometry as associated element in the differential diagnosis between HCM and athletes heart. Class Ila (level of evidence C) Patients with equivocal symptoms not associated with other high-risk variables. Submaximal stress test for patients with cardioverter-defibrillator who want to do low-intensity sports, in order to assess their tolerance and heart rate response to exercise. Class III Patients with high-risk variables and no cardioverter-defibrillator. Conventional stress test in athletes for differential diagnosis between HCM and physiologic hypertrophy (athletes hypertrophy). B. Sports and HCM HCM diagnosis implies the athletes exclusion from competitive sports practice. (267) This assertion includes all HCM cases, even those which do not present risk clinical variables, or whose genotype is associated with low risk. HCM prevalence is low in high-performance athletes, because there would be a natural selection for this type of practice in patients with functional and structural changes, which are secondary to this condition. (268) Some exceptions are possible for asymptomatic patients with no risk variables, who are involved in lowintensity sports, such as golf, cricket, bowling, or shooting. Whereas it is true that many athletes have been diagnosed with HCM once their competitive and even professional sport activity was over, there are no clinical or genetic studies that ensure good prognosis. Intense exercise may be the cause of severe arrhythmias, increase of left ventricular outflow tract obstruction, and ischemia due to compression of the small vessels (and eventual fibrosis as a result of repetitive ischemia). These alterations may cause the athletes sudden death during competence or training. The extension of genetic studies, even more to relatives of HCM patients, has posed the situation of individuals with positive genotype and negative phenotype for HCM. This situation generates discussion about the recommendations on competitive sports for these patients. At present, if there are no diagnostic clinical data or risk variables, there is no strong evidence to discourage sports practice. However, certain troponin T gene mutations (chromosome 1) may not manifest as ventricular hypertrophy, but have histopathological alterations in myocardial muscle fibers and result in sudden death. (42) The intense exertion needed to practice recreational (non-competitive) sports should be taken into account. It is not always feasible to assess this inten- 19 CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY sity, since the sport in itself will require different psychological and physical loads from the different participants. As a general rule, it could be said that those sports that require sudden changes slowing down and speeding up unexpectedly (acyclic) should be avoided, as in the case of soccer, tennis, basketball, and handball, among others. Preference is given to cyclic activities such as jogging, biking, swimming, or walking in which energy expenditure is largely low and stable. In certain cases in which the physician is well aware of the patients reasoning and habits, it is possible to indicate the practice of acyclic sports as the ones first above mentioned (soccer, tennis, basketball, or handball), but with a detailed explanation as to adapting to low intensity and no competitiveness. Some consensuses categorize sports with regard to the level of physical intensity required, and suggest whether patients with hypertrophic cardiomyopathy may or may not practice them. (269) The American Heart Association Group suggests levels of recommendation numbered from 0 to 5, with 0 to 1 designating activities strongly discouraged, and 4 to 5 indicating activities probably permitted. The intermediate recommendation is subject to the physicians consideration on an individual basis, and its level goes from 2 to 3. As an example, 0 to 1 values correspond to recreational sports, such as basketball, single tennis, windsurfing, soccer and football, weight lifting, and diving. (264) The following recommendations arise from experiences and consensus: Attending physicians should assess the patients on an individual basis, including physical, psychological, and educational aspects. Recommendations for patients with HCM who want to practice sports Class I (level of evidence C) HCM patients should be excluded from competitive sport activities with the possible exception of low-intensity ones (golf, shooting, billiard, bowling), for individuals with no high-risk variables of sudden death, and for low-risk patients. Recreational sports that require high intensity or sudden intensity changes are not recommended. Individuals with positive genotype and negative phenotype (with no clinical evidence of the disease) can practice sports, even competitive ones, provided they undergo regular screenings. Patients with no high-risk variables and with normal stress test can perform recreational, cyclic, and low-intensity exercise. Patients with implanted cardioverter-defibrillator should be discouraged from practicing contact sports. Class Ila Patients with no high-risk variables and with normal stress test can practice recreational contact sports of low intensity and volume, with regular breaks, provided they can control the exertion intensity and the psychological burden of their participation. Class III Asymptomatic HCM patients, with low clinical and genetic risk, high functional capacity and no family history of sudden death, can practice high-intensity competitive sports. BIBLIOGRAPHY 1. Brock R. Functional obstruction of the left ventricle; acquired aortic subvalvar stenosis. Guys Hosp Rep 1957; 106:221-38. 2. Teare D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J 1958; 20:1-8. 3. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDlA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995; 92:785-9. 4. Maron BJ, Roberts WC, Epstein SE. Sudden death in hypertrophic cardiomyopathy: a profile of 78 patients. Circulation 1982; 65:1388-94. 5. Fay WP, Taliercio CP, llstrup DM, Tajik AJ, Gersh BJ. Natural history of hypertrophic cardiomyopathy in the elderly. J Am Coll Cardiol 1990; 16:821-6. 6. Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS et al. lmplantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 2007; 298:405-12. 7. Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, et al; Task Force on Clinical Expert Consensus Documents. American College of Cardiology; Committee for Practice Guidelines. European Society of Cardiology. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003; 42:1687-713. 8. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology lnterdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology lnterdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006; 113:1807-16. 9. Ommen SR, Nishimura RA. Hypertrophic cardiomyopathy. Curr Probl Cardiol 2004; 29:233-91. 10. Spirito P, Maron BJ. Patterns of systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy: assessment by twodimensional echocardiography. Am J Cardiol 1984; 54:1039-46. 11. Wigle ED. Cardiomyopathy: The diagnosis of hypertrophic cardiomyopathy. Heart 2001; 86:709-14. 12. Klues HG, Roberts WC, Maron BJ. Anomalous insertion of papillary muscle directly into anterior mitral leaflet in hypertrophic car- 20 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 diomyopathy. Significance in producing left ventricular outflow obstruction. Circulation 1991; 84:1188-97. 13. Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006; 114:22329. 14. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003; 348:295303. 15. Autore C, Bernabé P, Stella Barilli C, Bruzzi P, Spirito P. The prognostic importance of left ventricular outflow obstruction in hypertrophic cardiomyopathy varies in relation to the severity of symptoms. J Am Coll Cardiol 2005; 45:1076-80. 16. Wynne J, Braunwald E. The Cardiomyopathies and Myocarditides. In: Braunwald E. Heart Disease. 5th ed. Philadelphia: WB Saunders Company; 1997. 17. Nihoyannopoulos P, Karatasakis G, Frenneaux M, McKenna WJ, Oakley CM. Diastolic function in hypertrophic cardiomyopathy: relation to exercise capacity. J Am Coll Cardiol 1992; 19:536-40. 18. Briguori C, Betocchi S, Romano M, Manganelli F, Angela Losi M, Ciampi Q, et al. Exercise capacity in hypertrophic cardiomyopathy depends on left ventricular diastolic function. Am J Cardiol 1999; 84:309-15. 19. Spirito P, Maron BJ. Relation between extent of left ventricular hypertrophy and diastolic filling abnormalities in hypertrophic cardiomyopathy. J Am Coll Cardiol 1990; 15:808-13. 20. Maron BJ. Hypertrophic cardiomyopathy: A systematic review. JAMA 2002; 287:1308-20. 21. Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 40:2156-64. 22. OGara PT, Bonow RO, Maron BJ, Damske BA, Van Lingen A, Bacharach SL, et al. Myocardial perfusion abnormalities in patients with hypertrophic cardiomyopathy: assessment with thallium-201 emission computed tomography. Circulation 1987; 76:1214-23. 23. Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, et al. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation 2007; 115:2418-25. 24. Schwartzkopff B, Mundhenke M, Strauer BE. Alterations of the architecture of subendocardial arterioles in patients with hypertrophic cardiomyopathy and impaired coronary vasodilator reserve: a possible cause for myocardial ischemia. J Am Coll Cardiol 1998; 31:1089-96. 25. Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Hum Pathol 2000; 31:988-98. 26. Maron BJ, Wolfson JK, Epstein SE, Roberts WC. lntramural (small vessel) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol 1986; 8:545-57. 27. Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Berger PB, Tajik AJ. Adverse prognosis of patients with hypertrophic cardiomyopathy who have epicardial coronary artery disease. Circulation 2003; 108:2342-8. 28. Sherrid MV, Chu CK, Delia E, Mogtader A, Dwyer EM Jr. An echocardiographic study of the fluid mechanics of obstruction in hypertrophic cardiomyopathy. J Am Coll Cardiol 1993; 22:816-25. 29. Petrone RK, Klues HG, Panza JA, Peterson EE, Maron BJ. Coexistence of mitral valve prolapse in a consecutive group of 528 patients with hypertrophic cardiomyopathy assessed with echocardiography. J Am Coll Cardiol 1992; 20:55:00-61. 30. Robinson K, Frenneaux MP, Stockins B, Karatasakis G, Poloniecki JD, McKenna WJ. Atrial fibrillation in hypertrophic cardiomyopathy: longitudinal study. J Am Coll Cardiol 1990; 15:1279-85. 31. Olivotto l, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. lmpact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001; 104:2517-24. 32. Frenneaux MP, Counihan PJ, Caforio AL, Chikamori T, McKenna WJ. Abnormal blood pressure response during exercise in hypertrophic cardiomyopathy. Circulation 1990; 82:1995-2002. 33. Olivotto l, Maron BJ, Montereggi A, Mazzuoli F, Dolara A, Cecchi F. Prognostic value of systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy. J Am Coll Cardiol 1999; 33:2044-51. 34. Ciampi Q, Betocchi S, Lombardi R, Manganelli F, Storto G, Losi MA, et al. Hemodynamic determinants of exercise-induced abnormal blood pressure response in hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 40:278-84. 35. Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, et al. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell 1994; 77:701-12. 36. Arad M, Maron BJ, Gorham JM, Johnson WH Jr, Saul JP, PerezAtayde AR, et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med 2005; 352:362-72. 37. Pare JA, Fraser RG, Pirozynski WJ, Shanks JA, Stubington D. Hereditary cardiovascular dysplasia. A form of familial cardiomyopathy. Am J Med 1961; 31:37-62. 38. Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, et al; EUROGENE Heart Failure Project. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003; 107:2227-32. 39. Morita H, Larson MG, Barr SC, Vasan RS, ODonnell CJ, Hirschhorn JN, et al. Single-gene mutations and increased left ventricular wall thickness in the community: the Framingham Heart Study. Circulation 2006; 113:2697-705. 40. Olsson MC, Palmer BM, Stauffer BL, Leinwand LA, Moore RL. Morphological and functional alterations in ventricular myocytes from male transgenic mice with hypertrophic cardiomyopathy. Circ Res 2004; 94:201-7. 41. Rottbauer W, Gautel M, Zehelein J, Labeit S, Franz WM, Fischer C, et al. Novel splice donor site mutation in the cardiac myosin-binding protein-C gene in familial hypertrophic cardiomyopathy. Characterization of cardiac transcript and protein. J Clin lnvest 1997; 100:475-82. 42. Sweeney HL, Feng HS, Yang Z, Watkins H. Functional analyses of troponin T mutations that cause hypertrophic cardiomyopathy: insights into disease pathogenesis and troponin function. Proc Natl Acad Sci USA 1998; 95:14406-10. 43. Wang Q, Moncman CL, Winkelmann DA. Mutations in the motor domain modulate myosin activity and myofibril organization. J Cell Sci 2003; 116:4227-38. 44. Fatkin D, McConnell BK, Mudd JO, Semsarian C, Moskowitz lG, Schoen FJ, et al. An abnormal Ca(2+) response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy. J Clin lnvest 2000; 106:1351-9. 45. Theis JL, Bos JM, Bartleson VB, Will ML, Binder J, Vatta M, et al. Echocardiographic-determined septal morphology in Z-disc hypertrophic cardiomyopathy. Biochem Biophys Res Commun 2006; 351:896-902. 46. Watkins H, Rosenzweig A, Hwang DS, Levi T, McKenna W, Seidman CE, et al. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med 1992; 326:1108-14. 47. Watkins H, Conner D, Thierfelder L, Jarcho JA, MacRae C, McKenna WJ, et al. Mutations in the cardiac myosin binding protein-C gene on chromosome 11 cause familial hypertrophic cardiomyopathy. Nat Genet 1995; 11:434-7. 48. Kimura A, Harada H, Park JE, Nishi H, Satoh M, Takahashi M, et al. Mutations in the cardiac troponin l gene associated with hypertrophic cardiomyopathy. Nat Genet 1997; 16:379-82. 49. Van Driest SL, Ackerman MJ, Ommen SR, Shakur R, Will ML, Nishimura RA, et al. Prevalence and severity of benign mutations in the beta-myosin heavy chain, cardiac troponin T, and alpha- CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY tropomyosin genes in hypertrophic cardiomyopathy. Circulation 2002; 106:3085-90. 50. Brito D, Richard P, Isnard R, Pipa J, Komajda M, Madeira H. Familial hypertrophic cardiomyopathy: the same mutation, different prognosis. Comparison of two families with a long follow-up. Rev Port Cardiol 2003; 22:1445-61. 51. Hoedemaekers YM, Caliskan K, Majoor-Krakauer D, van de Laar l, Michels M, Witsenburg M, et al. Cardiac beta-myosin heavy chain defects in two families with non-compaction cardiomyopathy: linking non-compaction to hypertrophic, restrictive, and dilated cardiomyopathies. Eur Heart J 2007; 28:2732-7. 52. Alpert NR, Mohiddin SA, Tripodi D, Jacobson-Hatzell J, VaughnWhitley K, Brosseau C, et al. Molecular and phenotypic effects of heterozygous, homozygous, and compound heterozygote myosin heavychain mutations. Am J Physiol Heart Circ Physiol 2005; 288:H1097102. 53. Marian AJ. Modifier genes for hypertrophic cardiomyopathy. Curr Opin Cardiol 2002; 17:242-52. 54. Li HL, Liu C, de Couto G, Ouzounian M, Sun M, Wang AB, et al. Curcumin prevents and reverses murine cardiac hypertrophy. J Clin lnvest 2008; 118:879-93. 55. Miller TE, You L, Myerburg RJ, Benke PJ, Bishopric NH. Whole blood RNA offers a rapid, comprehensive approach to genetic diagnosis of cardiovascular diseases. Genet Med 2007; 9:23-33. 56. Charron P, Dubourg O, Desnos M, lsnard R, Hagege A, Millaire A, et al. Diagnostic value of electrocardiography and echocardiography for familial hypertrophic cardiomyopathy in a genotyped adult population. Circulation 1997; 96:214-9. 57. McKenna WJ, Spirito P, Desnos M, Dubourg O, Komajda M, et al. Experience from clinical genetics in hypertrophic cardiomyopathy: proposal for new diagnostic criteria in adult members of affected families. Heart 1997; 77:130-2. 58. McPherson E. Genetic diagnosis and testing in clinical practice. Clin Med Res 2006; 4:123-9. 59. Acunzo RS. Manifestaciones clínicas y electrocardiográficas, la estratificación del riesgo y el tratamiento de la miocardiopatía hipertrófica. In: Elizari MV, Chiale P, editors. Arritmias cardíacas. Bases celulares y moleculares, diagnóstico y tratamiento. 2nd ed. Buenos Aires, Argentina: Editorial Médica Panamericana SA; 2003. pp. 753-74. 60. Braudo M, Wigle ED, Keith JD. A distinctive electrocardiogram in muscular subaortic stenosis due to ventricular septal hypertrophy. Am J Cardiol 1964; 14:599-607. 61. Marriot HJL. Anomalías electrocardiográficas, trastornos de la conducción y arritmias en la miocardiopatía hipertrófica. In: Friedberg CK, director. Progresos en las enfermedades cardiovasculares. Tomo V. Ed Científico Médica; 1966. cap Vll, p. 10115. 62. Acunzo RS, Konopka lV, Aldariz AE, Cianciulli T, Saccheri MC, Elizari MV et al. Hallazgos electrocardiográficos en pacientes con miocardiopatía hipertrófica. Rev Argent Cardiol 1998; 66:150. Abstract N° 381. 63. Konno T, Shimizu M, Ino H, Yamaguchi M, Terai H, Uchiyama K, et al. Diagnostic value of abnormal Q waves for identification of preclinical carriers of hypertrophic cardiomyopathy based on a molecular genetic diagnosis. Eur Heart J 2004; 25:246-51. 64. Maron BJ, Spirito P, Wesley Y, Arce J. Development and progression of left ventricular hypertrophy in children with hypertrophic cardiomyopathy. N Engl J Med 1986; 315:610-4. 65. Prescott R, Quinn JS, Littmann D. Electrocardiographic changes in hypertrophic subaortic stenosis which simulate myocardial infarction. Am Heart J 1963; 66:42-8. 66. Dumont CA, Monserrat L, Soler R, Rodríguez E, Fernandez X, Peteiro J, et al. lnterpretation of electrocardiographic abnormalities in hypertrophic cardiomyopathy with cardiac magnetic resonance. Eur Heart J 2006; 27:1725-31. 67. McKenna WJ, Spirito P, Desnos M, Dubourg O, Komajda M. Experience from clinical genetics in hypertrophic cardiomyopathy: pro- 21 posal for new diagnostic criteria in adult members of affected families. Heart 1997; 77:130-2. 68. Acunzo RS, Konopka lV, Halpern SM. La semiología del ECG normal y patológico. In: Elizari MV, Chiale P, editors. Arritmias cardíacas. Bases celulares y moleculares, diagnóstico y tratamiento. 2nd ed. Buenos Aires, Argentina: Editorial Médica Panamericana; 2003. Cap. 8, p. 163-204. 69. Corrado D, Pelliccia A, Bjørnstad HH, Vanhees L, Biffi A, Borjesson M, et al; Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2005; 26:516-24. 70. Maron BJ, Thompson PD, Ackerman MJ, Balady G, Berger S, Cohen D, et al; American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology. Foundation. Circulation 2007;115:1643-55. 71. Konopka lV, Acunzo RS, Saccheri MC et al. Historia natural de la miocardiopatía hipertrófica apical. Premio al mejor tema libre 9nas Jornadas de Cardiología del Gobierno de la Ciudad de Buenos Aires. 2000; p. 58. 72. Henry WL, Clark CE, Epstein SE. Asymmetric septal hypertrophy. Echocardiographic identification of the pathognomonic anatomic abnormality of lHSS. Circulation 1973; 47:225-33. 73. Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med 1991; 324:295-301. 74. Abergel E, Chatellier G, Hagege AA, Oblak A, Linhart A, Ducardonnet A, et al. Serial left ventricular adaptations in worldclass professional cyclists: implications for disease screening and followup. J Am Coll Cardiol 2004; 44:144-9. 75. Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 2000; 36:22128. 76. Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 2000; 342:1778-85. 77. Spirito P, Maron BJ. Relation between extent of left ventricular hypertrophy and diastolic filling abnormalities in hypertrophic cardiomyopathy. J Am Coll Cardiol 1990; 15:1521-6. 78. Klues H, Schiffers A, Maron B. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by twodimensional echocardiography in 600 patients. J Am Coll Cardiol 1995; 26:1699. 78 bis. Saccheri MC, Cianciulli TF, Konopka lV, Acunzo RS, Méndez RJ, Gagliardi JA, et al. Right ventricular hypertrophy alone as a new form of presentation of hypertrophic cardiomyopathy. Circulation 2008; 117:143. 79. Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005; 112:855-61. 79 bis. Cianciulli TF, Saccheri MC, Bermann AM, Lax JA, Konopka IV, Acunzo RS, et al. Solitary papillary muscle hypertrophy can be a precursor of hypertrophic cardiomyopathy. Circulation 2008; 117:121. 80. Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 1995; 92:1680-92. 81. Wigle ED, Sasson Z, Henderson MA, Ruddy TD, Fulop J, Rakowski 22 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 H, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis 1985; 28:1-83. 82. Nishimura RA, Holmes DR Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy: N Engl J Med 2004; 350:1320-7. 83. Schwammenthal E, Block M, Schwartzkopff B, L6sse B, Borggrefe M, Schulte HD, et al. Prediction of the site and severity of obstruction in hypertrophic cardiomyopathy by color flow mapping and continuous wave Doppler echocardiography. J Am Coll Cardiol 1992; 20:96472. 84. Yu EH, Omran AS, Wigle ED, Williams WG, Siu SC, Rakowski H. Mitral regurgitation in hypertrophic obstructive cardiomyopathy: relationship to obstruction and relief with myectomy. J Am Coll Cardiol 2000; 36:2219-25. 85. Klues HG, Maron BJ, Dollar AL, Roberts WC. Diversity of structural mitral valve alterations in hypertrophic cardiomyopathy. Circulation 1992; 85:1651-60. 86. Maron BJ, Spirito P, Green KJ, Wesley YE, Bonow RO, Arce J. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1987; 10:733-42. 87. Palka P, Lange A, Fleming AD, Donnelly JE, Dutka DP, Starkey lR, et al. Differences in myocardial velocity gradient measured throughout the cardiac cycle in patients with hypertrophic cardiomyopathy, athletes and patients with left ventricular hypertrophy due to hypertension. J Am Coll Cardiol 1997; 30:760-8. 88. Nagueh SF, McFalls J, Meyer D, Hill R, Zoghbi WA, Tam JW, et al. Tissue Doppler imaging predicts the development of hypertrophic cardiomyopathy in subjects with subclinical disease. Circulation 2003; 108:395-8. 89. Ho CY, Sweitzer NK, McDonough B, Maron BJ, Casey SA, Seidman JG, et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation 2002; 105:2992-7. 90. Nagueh SF, McFalls J, Meyer D, Hill R, Zoghbi WA, Tam JW, et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation 2001; 104:128-30. 91. Chejtman D, Baratta S, Fernández H, Marani A, Ferroni F, Bilbao J. Valor clínico del análisis de la fase sistólica de la contracción ventricular con Doppler tisular en la discriminación de hipertrofia fisiológica de formas patológicas. Rev Argent Cardiol 2006; 74:12935. 92. Spirito P, Seidman CE, McKenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N Engl J Med 1997; 336:77585. 93. Maron BJ, McKenna WJ, Elliott P, Spirito P, Frenneaux MP, Keren A, et al. Hypertrophic cardiomyopathy. JAMA 1999; 282:2302-3. 94. Kato TS, Noda A, lzawa H, Yamada A, Obata K, Nagata K, et al. Discrimination of nonobstructive hypertrophic cardiomyopathy from hypertensive left ventricular hypertrophy on the basis of strain rate imaging by tissue Doppler ultrasonography. Circulation 2004; 110:3808-14. 95. Baratta S, Chejtman D, Fernández H, Ferroni FE, Bilbao J, Kotliar C, et al. Valor clínico de la utilización del strain rate sistólico en el estudio de distintas formas de hipertrofia ventricular izquierda. Rev Argent Cardiol 2007; 75:367-73. 96. Serri K, Reant P, Lafitte M, Berhouet M, Le Bouffos V, Roudaut R, et al. Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy. J Am Coll Cardiol 2006; 47:1175-81. 97. Maron BJ, Seidman JG, Seidman CE. Proposal for contemporary screening strategies in families with hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 44:2125-32. 98. Grigg LE, Wigle ED, Williams WG, Daniel LB, Rakowski H. Transesophageal Doppler echocardiography in obstructive hypertrophic cardiomyopathy: clarification of pathophysiology and impor- tance in intraoperative decision making. J Am Coll Cardiol 1992; 20:42-52. 99. Singh M, Edwards WD, Holmes DR Jr, Tajil AJ, Nishimura RA. Anatomy of the first septal perforating artery: a study with implications for ablation therapy for hypertrophic cardiomyopathy. Mayo Clin Proc 2001; 76:799-802. 100. Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation 1998; 98:2415-21. 101. Nagueh SF, Lakkis NM, He ZX, Middleton KJ, Killip D, Zoghbi WA, et al. Role of myocardial contrast echocardiography during nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 1998; 32:225-9. 102. Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation 1998; 98:2415-21. 103. Nagueh SF, Ommen SR, Lakkis NM, Killip D, Zoghbi WA, Schaff HV, et al. Comparison of ethanol septal reduction therapy with surgical myectomy for the treatment of hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2001; 38:1701-6. 104. Mazur W, Nagueh SF, Lakkis NM, Middleton KJ, Killip D, Roberts R, et al. Regression of left ventricular hypertrophy after nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy. Circulation 2001; 103:1492-6. 105. Maron BJ, Olivotto l, Bellone P, Conte MR, Cecchi F, Flygenring BP, et al. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 39:301-7. 106. Frank S, Braunwald E. ldiopathic hypertrophic subaortic stenosis. Clinical analysis of 126 patients with emphasis on the natural history. Circulation 1968; 37:759-88. 107. Spirito P, Rapezzi C, Bellone P, Betocchi S, Autore C, Conte MR, et al. lnfective endocarditis in hypertrophic cardiomyopathy: prevalence, incidence, and indications for antibiotic prophylaxis. Circulation 1999; 99:2132-7. 108. Harrison DC, Braunwald E, Glick G, Mason DT, Chidsey CA, Ross J Jr. Effects of beta adrenergic blockade on the circulation with particular reference to observations in patients with hypertrophic subaortic stenosis. Circulation 1964; 29:84-98. 109. Cohen LS, Braunwald E. Amelioration of angina pectoris in idiopathic hypertrophic subaortic stenosis with beta-adrenergic blockade. Circulation 1967; 35:847-51. 110. Cohen LS, Braunwald E. Chronic beta adrenergic receptor blockade in the treatment of idiopathic hypertrophic subaortic stenosis. Prog Cardiovasc Dis 1968; 11:211-21. 111. Flamm MD, Harrison DC, Hancock EW. Muscular subaortic stenosis. Prevention of outflow obstruction with propranolol. Circulation 1968; 38:846-58. 112. Sloman G. Propranolol in management of muscular subaortic stenosis. Br Heart J 1967; 29:783-7. 113. Thompson DS, Naqvi N, Juul SM, Swanton RH, Coltart DJ, Jenkins BS, et al. Effects of propranolol on myocardial oxygen consumption, substrate extraction, and haemodynamics in hypertrophic obstructive cardiomyopathy. Br Heart J 1980; 44:488-98. 114. Adelman AG, Shah PM, Gramiak R, Wigle ED. Long-term propranolol therapy in muscular subaortic stenosis. Br Heart J 1970; 32:804-11. 115. Saenz de la Calzada C, Ziady GM, Hardarson T, Curiel R, Goodwin JF. Effect of acute administration of propranolol on ventricular function in hypertrophic obstructive cardiomyopathy measured by non-invasive techniques. Br Heart J 1976; 38:798-803. 116. Bourmayan C, Razavi A, Fournier C, Dussaule JC, Baragan J, Gerbaux A, et al. Effect of propranolol on left ventricular relaxation in hypertrophic cardiomyopathy: an echographic study. Am Heart J 1985; 109:1311-6. 117. Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation 2008; 117:429-39. CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY 118. Hubner PJ, Ziady GM, Lane GK, Hardarson T, Scales B, Oakley CM, et al. Double-blind trial of propranolol and practolol in hypertrophic cardiomyopathy. Br Heart J 1973; 35:1116-23. 119. Rosing DR, Kent KM, Borer JS, Seides SF, Maron BJ, Epstein SE. Verapamil therapy: a new approach to the pharmacologic treatment of hypertrophic cardiomyopathy. l. Hemodynamic effects. Circulation 1979; 60:1201-7. 120. Rosing DR, Condit JR, Maron BJ, Kent KM, Leon MB, Bonow RO, et al. Verapamil therapy: a new approach to the pharmacologic treatment of hypertrophic cardiomyopathy: lll. Effects of long-term administration. Am J Cardiol 1981; 48:545-53. 121. Epstein SE, Rosing DR. Verapamil: its potential for causing serious complications in patients with hypertrophic cardiomyopathy. Circulation 1981; 64:437-41. 122. Kaltenbach M, Hopf R, Kober G, Bussmann WD, Keller M, Petersen Y, et al. Treatment of hypertrophic obstructive cardiomyopathy with verapamil. Br Heart J 1979; 42:35-42. 123. Bonow RO, Rosing DR, Bacharach SL, Green MV, Kent KM, Lipson LC, et al. Effects of verapamil on left ventricular systolic function and diastolic filling in patients with hypertrophic cardiomyopathy. Circulation 1981; 64:787-96. 124. Bonow RO, Frederick TM, Bacharach SL, Green MV, Goose PW, Maron BJ, et al. Atrial systole and left ventricular filling in hypertrophic cardiomyopathy: effect of verapamil. Am J Cardiol 1983; 51:1386-91. 125. Bonow RO, Dilsizian V, Rosing DR, Maron BJ, Bacharach SL, Green MV. Verapamil-induced improvement in left ventricular diastolic filling and increased exercise tolerance in patients with hypertrophic cardiomyopathy: short- and long-term effects. Circulation 1985; 72:853-64. 126. Hess OM, Murakami T, Krayenbuehl HP. Does verapamil improve left ventricular relaxation in patients with myocardial hypertrophy? Circulation 1986; 74:530-43. 127. Udelson JE, Bonow RO, OGara PT, Maron BJ, Van Lingen A, Bacharach SL, et al. Verapamil prevents silent myocardial perfusion abnormalities during exercise in asymptomatic patients with hypertrophic cardiomyopathy. Circulation 1989; 79:1052-60. 128. Gistri R, Cecchi F, Choudhury L, Montereggi A, Sorace O, Salvadori PA, et al. Effect of verapamil on absolute myocardial blood flow in hypertrophic cardiomyopathy. Am J Cardiol 1994; 74:363-8. 129. Petkow Dimitrow P, Krzanowski M, Nizankowski R, Szczeklik A, Dubiel JS. Effect of verapamil on systolic and diastolic coronary blood flow velocity in asymptomatic and mildly symptomatic patients with hypertrophic cardiomyopathy. Heart 2000; 83:262-6. 130. Rosing DR, Kent KM, Maron BJ, Epstein SE. Verapamil therapy: a new approach to the pharmacologic treatment of hypertrophic cardiomyopathy. ll. Effects on exercise capacity and symptomatic status. Circulation 1979; 60:1208-13. 131. Gilligan DM, Chan WL, Joshi J, Clarke P, Fletcher A, Krikler S, et al. A double-blind, placebo-controlled crossover trial of nadolol and verapamil in mild and moderately symptomatic hypertrophic cardiomyopathy. J Am Coll Cardiol 1993; 21:1672-9. 132. lwase M, Sotobata l, Takagi S, Miyaguchi K, Jing HX, Yokota M. Effects of diltiazem on left ventricular diastolic behavior in patients with hypertrophic cardiomyopathy: evaluation with exercise pulsed Doppler echocardiography. J Am Coll Cardiol 1987; 9:1099-105. 133. Betocchi S, Piscione F, Losi M A, Pace L, Boccalatte M, PerroneFilardi P, et al. Effects of diltiazem on left ventricular systolic and diastolic function in hypertrophic cardiomyopathy. Am J Cardiol 1996; 78:451-7. 134. Betocchi S, Cannon RO 3rd, Watson RM, Bonow RO, Ostrow HG, Epstein SE, et al. Effects of sublingual nifedipine on hemodynamics and systolic and diastolic function in patients with hypertrophic cardiomyopathy. Circulation 1985; 72:1001-7. 135. Lorell BH, Paulus WJ, Grossman W, Wynne J, Cohn PF. Modification of abnormal left ventricular diastolic properties by nifedipine in patients with hypertrophic cardiomyopathy. Circulation 1982; 65:499-507. 23 136. Kondo N, Mizukami M, Shibata S. Negative inotropic effects of disopyramide on guinea-pig papillary muscles. Br J Pharmacol 1990; 101:789-92. 137. Pollick C, Kimball B, Henderson M, Wigle ED. Disopyramide in hypertrophic cardiomyopathy. l. Hemodynamic assessment after intravenous administration. Am J Cardiol 1988; 62:1248-51. 138. Fifer MA, OGara PT, McGovern BA, Semigran MJ. Effects of diltiazem on left ventricular systolic and diastolic function in hypertrophic cardiomyopathy. Am J Cardiol 1994; 74:405-8. 139. Matsubara H, Nakatani S, Nagata S, lshikura F, Katagiri Y, Ohe T, et al. Salutary effect of disopyramide on left ventricular diastolic function in hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 1995; 26:768-75. 140. Pollick C. Disopyramide in hypertrophic cardiomyopathy. ll. Noninvasive assessment after oral administration. Am J Cardiol 1988; 62:1252-5. 141. Sherrid MV, Barac l, McKenna WJ, Elliott PM, Dickie S, Chojnowska L, et al. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005; 45:1251-8. 142. McCully RB, Nishimura RA, Tajik AJ, Schaff HV, Danielson GK. Extent of clinical improvement after surgical treatment of hypertrophic obstructive cardiomyopathy. Circulation 1996; 94:467-71. 143. Robbins RC, Stinson EB. Long-term results of left ventricular myotomy and myectomy for obstructive hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 1996; 111:586-94. 144. Schulte HD, Borisov K, Gams E, Gramsch-Zabel H, L6sse B, Schwartzkopff B. Management of symptomatic hypertrophic obstructive cardiomyopathy- long-term results after surgical therapy. Thorac Cardiovasc Surg 1999; 47:213-8. 145. ten Berg JM, Suttorp MJ, Knaepen PJ, Ernst SM, Vermeulen FE, Jaarsma W. Hypertrophic obstructive cardiomyopathy. lnitial results and long-term follow-up after Morrow septal myectomy. Circulation 1994; 90:1781-5. 146. Heric B, Lytle BW, Miller DP, Rosenkranz ER, Lever HM, Cosgrove DM. Surgical management of hypertrophic obstructive cardiomyopathy. Early and late results. J Thorac Cardiovasc Surg 1995; 110:195-206. 147. Morrow AG, Reitz BA, Epstein SE, Henry WL, Conkle DM, ltscoitz SB, et al. Operative treatment in hypertrophic subaortic stenosis. Techniques, and the results of pre and postoperative assessments in 83 patients. Circulation 1975; 52:88-102. 148. Minakata K, Dearani JA, Nishimura RA, Maron BJ, Danielson GK. Extended septal myectomy for hypertrophic obstructive cardiomyopathy with anomalous mitral papillary muscles or chordae. J Thorac Cardiovasc Surg 2004; 127:481-9. 149. Schoendube FA, Klues HG, Reith S, Flachskampf FA, Hanrath P, Messmer BJ. Long-term clinical and echocardiographic follow-up after surgical correction of hypertrophic obstructive cardiomyopathy with extended myectomy and reconstruction of the subvalvular mitral apparatus. Circulation 1995; 92 (9 Suppl):ll122-7. 150. D6rge H, Schmitto JD, Liakopoulos OJ, Walther S, Sch6ndube FA. Extended myectomy for hypertrophic obstructive cardiomyopathy after failure or contraindication of septal ablation or with combined surgical procedures. Thorac Cardiovasc Surg 2004; 52:344-8. 151. Smedira NG, Lever HM, Miluk M, Krishnaswamy G, Laing G, Thamilarasan M, et al. Septal myectomy: 324 consecutive procedures without a mortality. J Am Coll Cardiol 2006; 47 (Suppl A):275A. Abstract. 152. Dearani JA, Ommen SR, Gersh BJ, Schaff HV, Danielson GK. Surgery insight: Septal myectomy for obstructive hypertrophic cardiomyopathy the Mayo Clinic experience. Nat Clin Pract Cardiovasc Med 2007; 4:503-12. 153. Morrow AG, Reitz BA, Epstein SE, Henry WL, Conkle DM, ltscoitz SB, et al. Operative treatment in hypertrophic subaortic stenosis. Techniques, and the results of pre and postoperative assessments in 83 patients. Circulation 1975; 52:88-102. 154. Schoendube FA, Klues HG, Reith S, Flachskampf FA, Hanrath 24 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 P, Messmer BJ. Long-term clinical and echocardiographic follow-up after surgical correction of hypertrophic obstructive cardiomyopathy with extended myectomy and reconstruction of the subvalvular mitral apparatus. Circulation 1995; 92 (9 Suppl):ll122-7. 155. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005; 46:470-6. 156. Seiler C, Hess OM, Schoenbeck M, Turina J, Jenni R, Turina M, et al. Long-term follow-up of medical versus surgical therapy for hypertrophic cardiomyopathy: a retrospective study. J Am Coll Cardiol 1991; 17:634-42. 157. Maron BJ. Controversies in cardiovascular medicine. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation 2007; 116:196-206. 158. Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 1995; 346:211-4. 159. Gietzen FH, Leuner CJ, Raute-Kreinsen U, Dellmann A, Hegselmann J, Strunk-Mueller C, et al. Acute and long-term results after transcoronary ablation of septal hypertrophy (TASH). Catheter interventional treatment for hypertrophic obstructive cardiomyopathy. Eur Heart J 1999; 20:1342-54. 160. Lakkis NM, Nagueh SF, Dunn JK, Killip D, Spencer WH 3rd. Nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy: one-year follow-up. J Am Coll Cardiol 2000; 36:852-5. 161. Kuhn H, Seggewiss H, Gietzen FH, Boekstegers P, Neuhaus L, Seipel L. Catheter-based therapy for hypertrophic obstructive cardiomyopathy. First in-hospital outcome analysis of the German TASH Registry. Z Kardiol 2004; 93:23-31. 162. Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J lnterv Cardiol 2006; 19:319-27. 163. Flores-Ramirez R, Lakkis NM, Middleton KJ, Killip D, Spencer WH 3rd, Nagueh SF. Echocardiographic insights into the mechanisms of relief of left ventricular outflow tract obstruction after nonsurgical septal reduction therapy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2001; 37:208-14. 164. van Dockum WG, Beek AM, ten Cate FJ, ten Berg JM, Bondarenko O, G6tte MJ, et al. Early onset and progression of left ventricular remodeling after alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Circulation 2005; 111:2503-8. 165. Maron BJ, Dearani JA, Ommen SR, Maron MS, Schaff HV, Gersh BJ, et al. The case for surgery in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 44:2044-53. 166. Veselka J, Duchonová R, Páleníckova J, Zemánek D, Tiserová M, Linhartová K, et al. lmpact of ethanol dosing on the long-term outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy: a single-center prospective, and randomized study. Circ J 2006; 70:1550-2. 167. Knight CJ. Alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Heart 2006; 92:1339-44. 168. Ralph-Edwards A, Woo A, McCrindle BW, Shapero JL, Schwartz L, Rakowski H, et al. Hypertrophic obstructive cardiomyopathy: comparison of outcomes after myectomy or alcohol ablation adjusted by propensity score. J Thorac Cardiovasc Surg 2005; 129:351-8. 169. Olivotto I, Ommen SR, Maron MS, Cecchi F, Maron BJ. Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Will there ever be a randomized trial? J Am Coll Cardiol 2007; 50:831-4. 170. Jeanrenaud X, Goy JJ, Kappenberger L. Effects of dual-chamber pacing in hypertrophic obstructive cardiomyopathy. Lancet 1992; 339:1318-23. 171. Pavin D, de Place C, Le Breton H, Leclercq C, Gras D, Victor F, et al. Effects of permanent dual-chamber pacing on mitral regurgitation in hypertrophic obstructive cardiomyopathy. Eur Heart J 1999; 20:203-10. 172. Nishimura RA, Hayes DL, llstrup DM, Holmes DR Jr, Tajik AJ. Effects of sublingual nifedipine on hemodynamics and systolic and diastolic function in patients with hypertrophic cardiomyopathy. Acute Doppler echocardiographic and catheterization hemodynamic study. J Am Coll Cardiol 1996; 27:421-30. 173. Betocchi S, Losi MA, Piscione F, Boccalatte M, Pace L, Golino P, et al. Effects of dual-chamber pacing in hypertrophic cardiomyopathy on left ventricular outflow tract obstruction and on diastolic function. Am J Cardiol 1996; 77:498-502. 174. McDonald K, McWilliams E, OKeeffe B, Maurer B. Functional assessment of patients treated with permanent dual chamber pacing as a primary treatment for hypertrophic cardiomyopathy. Eur Heart J 1988; 9:893-8. 175. Fananapazir L, Cannon RO 3rd, Tripodi D, Panza JA. lmpact of dual-chamber permanent pacing in patients with obstructive hypertrophic cardiomyopathy with symptoms refractory to verapamil and beta-adrenergic blocker therapy. Circulation 1992; 85:2149-61. 176. Fananapazir L, Epstein ND, Curiel RV, Panza JA, Tripodi D, McAreavey D. Long-term results of dual-chamber (DDD) pacing in obstructive hypertrophic cardiomyopathy. Evidence for progressive symptomatic and hemodynamic improvement and reduction of left ventricular hypertrophy. Circulation 1994; 90:2731-42. 177. Kappenberger L, Linde C, Daubert C, McKenna W, Meisel E, Sadoul N, et al. Pacing in hypertrophic obstructive cardiomyopathy. A randomized crossover study. PIC Study Group. Eur Heart J 1997; 18:1249-56. 178. Kappenberger L, Linde C, Daubert C, McKenna W, Meisel E, Sadoul N, et al. Clinical progress after randomized onloff pacemaker treatment for hypertrophic obstructive cardiomyopathy. Pacing in Cardiomyopathy (PlC) Study Group. Europace 1999; 1:77-84. 179. Maron BJ, Nishimura RA, McKenna WJ, Rakowski H, Josephson ME, Kieval RS. Assessment of permanent dual-chamber pacing as a treatment for drug-refractory symptomatic patients with obstructive hypertrophic cardiomyopathy. A randomized, double-blind, crossover study (M-PATHY). Circulation 1999; 99:2927-33. 180. Maron BJ, Shen WK, Link MS, Epstein AE, Almquist AK, Daubert JP, et al. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med 2000; 342:365-73. 181. Maron BJ, Chaitman BR, Ackerman MJ, Bayés de Luna A, Corrado D, Crosson JE, et al; Working Groups of the American Heart Association Committee on Exercise, Cardiac Rehabilitation, and Prevention; Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation 2004; 109:2807-16. 182. Maron BJ. Contemporary considerations for risk stratification, sudden death and prevention in hypertrophic cardiomyopathy. Heart 2003; 89:977-8. 183. Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation 2006; 114:1633-44. 184. Maron BJ. Sudden death in young athletes. N Engl J Med 2003; 349:1064-75. 185. Maron BJ, Chaitman BR, Ackerman MJ, Bayés de Luna A, Corrado D, Crosson JE, et al; Working Groups of the American Heart Association Committee on Exercise, Cardiac Rehabilitation, and Prevention; Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation 2004; 109:2807-16. 186. Maron BJ, Olivotto l, Spirito P, Casey SA, Bellone P, Gohman TE, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 2000; 102:858-64. 187. Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 2006; 114:216-25. CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY 188. Maron BJ, Piccininno M, Casey SA, Bernabýÿ P, Spirito P. Relation of extreme left ventricular hypertrophy to age in hypertrophic cardiomyopathy. Am J Cardiol 2003; 91:626-8. 189. Sorajja P, Nishimura RA, Ommen SR, Ackerman MJ, Tajik AJ, Gersh BJ. Use of echocardiography in patients with hypertrophic cardiomyopathy: clinical implications of massive hypertrophy. J Am Soc Echocardiogr 2006; 19:788-95. 190. Olivotto l, Maron BJ, Montereggi A, Mazzuoli F, Dolara A, Cecchi F. Prognostic value of systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy. J Am Coll Cardiol 1999; 33:2044-51. 191. McGregor JB, Rahman A, Rosanio S, Ware D, Birnbaum Y, Saeed M. Monomorphic ventricular tachycardia: a late complication of percutaneous alcohol septal ablation for hypertrophic cardiomyopathy. Am J Med Sci 2004; 328:185-8. 192. Boltwood CM Jr, Chien W, Ports T. Ventricular tachycardia complicating alcohol septal ablation. N Engl J Med 2004; 351:1914-5. 193. Simon RD, Crawford FA 3rd, Spencer WH 3rd, Gold MR. Sustained ventricular tachycardia following alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Pacing Clin Electrophysiol 2005;28:1354-6. 194. Cecchi F, Maron BJ, Epstein SE. Long-term outcome of patients with hypertrophic cardiomyopathy successfully resuscitated after cardiac arrest. J Am Coll Cardiol 1989; 13:1283-8. 195. Elliott PM, Sharma S, Varnava A, Poloniecki J, Rowland E, McKenna WJ. Survival after cardiac arrest or sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1999; 33:1596-601. 196. Mogensen J, Murphy RT, Kubo T, Bahl A, Moon JC, Klausen lC, et al. Frequency and clinical expression of cardiac troponin l mutations in 748 consecutive families with hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 44:2315-25. 197. Ko YL, Tang TK, Chen JJ, Hshieh YY, Wu CW, Lien WP. ldiopathic hypertrophic cardiomyopathy in identical twins. Morphological heterogeneity of the left ventricle. Chest 1992; 102:783-5. 198. Moolman JC, Corfield VA, Posen B, Ngumbela K, Seidman C, Brink PA, et al. Sudden death due to troponin T mutations. J Am Coll Cardiol 1997; 29:549-55. 199. Miller MA, Gomes JA, Fuster V. Risk stratification of sudden cardiac death in hypertrophic cardiomyopathy. Nat Clin Pract Cardiovasc Med 2007; 4:667-76. 200. Fernández A, Casabé HJ, Coronel R, Galizio N, Torino A, Valero de Pesce E. Alternativas terapéuticas en la miocardiopatía hipertrófica. Rev Argent Cardiol 2003; 71:294-301. 201. Kofflard MJ, Ten Cate FJ, van der Lee C, van Domburg RT. Hypertrophic cardiomyopathy in a large community-based population: clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J Am Coll Cardiol 2003; 41:987-93. 202. Mogensen J, Kubo T, Duque M, Uribe W, Shaw A, Murphy R, et al. ldiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin l mutations. J Clin lnvest 2003; 111:209-16. 203. Eriksson MJ, Sonnenberg B, Woo A, Rakowski P, Parker TG, Wigle ED, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 39:638-45. 204. Elliot PM, Gimeno Blanes JR, Mahon NG; Poloniecki JD, McKenna WJ. Relation between severity of left ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet 2001; 357:420-4. 205. Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol 2003; 42:873-9. 206. Spirito P, Rapezzi C, Autore C, Bruzzi P, Bellone P, Ortolani P, et al. Prognosis of asymptomatic patients with hypertrophic cardiomyopathy and nonsustained ventricular tachycardia. Circulation 1994; 90:2743-7. 207. Adabag AS, Casey SA, Kuskowski MA, Zenovich AG, Maron BJ. Spectrum and prognostic significance of arrhythmias on ambulatory 25 Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol 2005; 45:697-704. 208. McKenna WJ, England D, Doi YL, Deanfield JE, Oakley C, Goodwin JF. Arrhythmia in hypertrophic cardiomyopathy. l: lnfluence on prognosis. Br Heart J 1981; 46:168-72. 209. Maron BJ, Savage DD, Wolfson JK, Epstein SE. Prognostic significance of 24 hour ambulatory electrocardiographic monitoring in patients with hypertrophic cardiomyopathy: a prospective study. Am J Cardiol 1981; 48:252-7. 210. Cecchi F, Olivotto l, Montereggi A, Squillatini G, Dolara A, Maron BJ. Prognostic value of non-sustained ventricular tachycardia and the potential role of amiodarone treatment in hypertrophic cardiomyopathy: assessment in na unselected non-referral based patient population. Heart 1998; 79:331-6. 211. Counihan PJ, Frenneaux MP, Webb DJ, McKenna WJ. Abnormal vascular responses to supine exercise in hypertrophic cardiomyopathy. Circulation 1991; 84:686-96. 212. Sadoul N, Prasad K, Elliott PM, Bannerjee S, Frenneaux MP, McKenna WJ. Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy. Circulation 1997; 96:2987-91. 213. Sadoul Boriani G, Maron BJ, Shen W-K, Spirito P. Prevention of sudden death in hypetrophic cardiomyopathy. But which defibrillator for which patient? Circulation 2004; 110:438-42. 214. Elliott PM, Gimeno JR, Tomé MT, Shah J, Ward D, Thaman R, et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J 2006; 27:1933-41. 215. Maron BJ, Olivotto l, Maron MS. The dilemma of left ventricular outflow tract obstruction and sudden death in hypertrophic cardiomyopathy: do patients with gradients really deserve prophylactic defibrillators? Eur Heart J 2006; 27:1895-7. 216. Cha YM, Gersh BJ, Maron BJ, et al. Electrophysiologic manifestations of ventricular tachyarrythmias provoking appropriate defibrillator interventions in high-risk patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2007; 18:483-7. 217. Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol 2000; 35:1493-501. 218. Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 2003; 349:1027-35. 219. Yamada M, Elliott PM, Kaski JC, Prasad K, Gane JN, Lowe CM, et al. Dipyridamole stress thallium-201 perfusion abnormalities in patients with hypertrophic cardiomyopathy. Relationship to clinical presentation and outcome. Eur Heart J 1998; 19:500-7. 220. Fernández A, Vigliano C, Casabé JH, et al. Myocardial fibrosis and progression to left ventricular dilatation in patients with hypertrophic cardiomyopathy. Eur Heart J 2005; 26:392 (abstract). 221. Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003; 41:1561-7. 222. Maron BJ, Casey SA, Poliac LC, Gohman TE, Almquist AK, Aeppli DM. Clinical course of hypertrophic cardiomyopathy in a regional United States cohort. JAMA 1999; 281:650-5. 223. Yetman AT, McCrindle BW, MacDonald C, Freedom RM, Gow R. Myocardial bridging in children with hypertrophic cardiomyopathya risk factor for sudden death. N Engl J Med 1998; 339:1201-9. 224. Valeti US, Nishimura RA, Holmes DR, Araoz PA, Glockner JF, Breen JF, et al. Comparison of surgical septal myectomy and alcohol septal ablation with cardiac magnetic resonance imaging in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2007; 49:350-7. 225. Van Driest SL, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Yield of genetic testing in hypertrophic cardiomyopathy. Mayo Clin Proc 2005; 80:739-44. 26 REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 77 Nº 2 / MARCH-APRIL 2009 226. Ho TC, Lai WW. Combination therapy for choroidal neovascularization. Ophthalmology 2006; 113:1470-1. 227. Bos JM, Ommen SR, Ackerman MJ. Genetic basis of hypertrophic cardiomyopathy: one, two, or more diseases? Curr Opin Cardiol 2007; 22:193-9. 228. Maron BJ, Seidman JG, Seidman CE. Proposal for contemporary screening strategies in families with hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 44:2125-32. 229. Brugada J. Sudden death in hypertrophic myocardiopathy. Rev Esp Cardiol 1998; 51: 991-6. 230. McKenna W, Deanfield J, Faruqui A, England D, Oakley C, Goodwin J. Prognosis in hypertrophic cardiomyopathy: role of age and clinical, electrocardiographic and hemodynamic features. Am J Cardiol 1981; 47:532-8. 231. Brugada P, Abdollah H, Heddle B, Wellens HJ. Results of a ventricular stimulation protocol using a maximum of 4 premature stimuli in patients without documented or suspected ventricular arrhythmias. Am J Cardiol 1983; 52:1214-8. 232. Fananapazir L, Chang AC, Epstein SE, McAreavey D. Prognostic determinants in hypertrophic cardiomyopathy. Prospective evaluation of a therapeutic strategy based on clinical, Holter, hemodynamic, and electrophysiological findings. Circulation 1992; 86:730-40. 233. Maron BJ, Estes NA 3rd, Maron MS, Almquist AK, Link MS, Udelson JE. Primary prevention of sudden death as a novel treatment strategy in hypertrophic cardiomyopathy. Circulation 2003; 107:2872-5. 234. Momiyama Y, Hartikainen J, Nagayoshi H, Albrecht P, Kautzner J, Saumarez RC, et al. Exercise-induced T-wave alternans as a marker of high risk in patients with hypertrophic cardiomyopathy. Jpn Circ J 1997; 61:650-6. 235. Kuroda N, Ohnishi Y, Yoshida A, Kimura A, Yokoyama M. Clinical significance of T-wave alternans in hypertrophic cardiomyopathy. Circ J 2002; 66:457-62. 236. Maron BJ, Shen WK, Link MS, Epstein AE, Almquist AK, Daubert JP, et al. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med 2000; 342:365-73. 237. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for lmplantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008; 117:e350-408. 238. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, et al; American College of Cardiology/American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association and the Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur Heart J 2006; 27:2099-140. 239. Woo A, Monakier D, Harris L, Hill A, Shah P, Wigle ED, et al. Determinants of implantable defibrillator discharges in high-risk patients with hypertrophic cardiomyopathy. Heart 2007; 93:1044-5. 240. Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 1995; 92:1680-92. 241. Fananapazir L, Leon MB, Bonow RO, Tracy CM, Cannon RO 3rd, Epstein SE. Sudden death during empiric amiodarone therapy in symptomatic hypertrophic cardiomyopathy. Am J Cardiol 1991; 67:169-74. 242. Yacoub MH, Olivotto l, Cecchi F. End-stage hypertrophic cardiomyopathy: from mystery to model. Nat Clin Pract Cardiovasc Med 2007; 4:232-3. 243. Fighali S, Krajcer Z, Edelman S, Leachman RD. Progression of hypertrophic cardiomyopathy into a hypokinetic left ventricle: higher incidence in patients with midventricular obstruction. J Am Coll Cardiol 1987; 9:288-94. 244. Shirani J, Maron BJ, Cannon RO 3rd, Shahin S, Roberts WC. Clinicopathologic features of hypertrophic cardiomyopathy managed by cardiac transplantation. Am J Cardiol 1993; 72:434-40. 245. Coutu M, Perrault LP, White M, Pelletier GB, Racine N, Poirier NC, et al. Cardiac transplantation for hypertrophic cardiomyopathy: a valid therapeutic option. J Heart Lung Transplant 2004; 23:413-7. 246. Thaman R, Gimeno JR, Reith S, Esteban MT, Limongelli G, Murphy RT, et al. Progressive left ventricular remodeling in patients with hypertrophic cardiomyopathy and severe left ventricular hypertrophy. J Am Coll Cardiol 2004; 44:398-405. 247. Hina K, Kusachi S, lwasaki K, Nogami K, Moritani H, Kita T, et al. Progression of left ventricular enlargement in patients with hypertrophic cardiomyopathy: incidence and prognostic value. Clin Cardiol 1993; 16:403-7. 248. Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, et al. Relevance of coronary microvascular flow impairment to longterm remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol 2006; 47:1043-8. 249. Takemura G, Takatsu Y, Fujiwara H. Luminal narrowing of coronary capillaries in human hypertrophic hearts: an ultrastructural morphometrical study using endomyocardial biopsy specimens. Heart 1998; 79:78-85. 250. Waller TA, Hiser WL, Capehart JE, Roberts WC. Comparison of clinical and morphologic cardiac findings in patients having cardiac transplantation for ischemic cardiomyopathy, idiopathic dilated cardiomyopathy, and dilated hypertrophic cardiomyopathy. Am J Cardiol 1998; 81:884-94. 251. Fernández A, Casabé JH, Favaloro RR, et al. Prevalence and clinical course of the end stage phase of hypertrophic cardiomyopathy in patients referred to a tertiary care center. Circulation 2008; 117:50 (abstract). 252. Krams R, Ten Cate FJ, Carlier SG, Van Der Steen AF, Serruys PW. Diastolic coronary vascular reserve: a new index to detect changes in the coronary microcirculation in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 43:670-7. 253. Krams R, Kofflard MJ, Duncker DJ, Von Birgelen C, Carlier S, Kliffen M, et al. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation 1998; 97:230-3. 254. Fujino N, Shimizu M, lno H, Yamaguchi M, Yasuda T, Nagata M, et al. A novel mutation Lys273Glu in the cardiac troponin T gene shows high degree of penetrance and transition from hypertrophic to dilated cardiomyopathy. Am J Cardiol 2002; 89:29-33. 255. Maron BJ, Niimura H, Casey SA, Soper MK, Wright GB, Seidman JG, et al. Development of left ventricular hypertrophy in adults in hypertrophic cardiomyopathy caused by cardiac myosin-binding protein C gene mutations. J Am Coll Cardiol 2001; 38:315-21. 256. Marian AJ. Pathogenesis of diverse clinical and pathological phenotypes in hypertrophic cardiomyopathy. Lancet 2000; 355:58-60. 257. Rogers DP, Marazia S, Chow AW, Lambiase PD, Lowe MD, Frenneaux M, et al. Effect of biventricular pacing on symptoms and cardiac remodelling in patients with end-stage hypertrophic cardiomyopathy. Eur J Cardiothorac Surg 2008; 10:507-13. 258. Ashrafian H, Mason MJ, Mitchell AG. Regression of CONSENSUS ON HYPERTROPHIC CARDIOMYOPATHY dilatedhypokinetic hypertrophic cardiomyopathy by biventricular cardiac pacing. Europace 2007; 9:50-4. 259. Pezzulich B, Montagna L, Lucchina PG. Successful treatment of end-stage hypertrophic cardiomyopathy with biventricular cardiac pacing. Europace 2005; 7:388-91. 260. Biagini E, Spirito P, Leone O, Picchio FM, Coccolo F, Ragni L, et al. Heart transplantation in hypertrophic cardiomyopathy. Am J Cardiol 2008; 101:387-92. 261. Arena R, Owens DS, Arevalo J, Smith K, Mohiddin SA, McAreavey D, et al. Ventilatory efficiency and resting hemodynamics in hypertrophic cardiomyopathy. Med Sci Sports Exerc 2008; 40:799805. 262. Sharma S, Elliott P, Whyte G, Jones S, Mahon N, Whipp B, et al. Utility of cardiopulmonary exercise in the assessment of clinical determinants of functional capacity in hypertrophic cardiomyopathy. Am J Cardiol 2000; 86:162-8. 263. Sharma S, Elliott PM, Whyte G, Mahon N, Virdee MS, Mist B, et al. Utility of metabolic exercise testing in distinguishing hypertrophic cardiomyopathy from physiologic left ventricular hypertrophy in athletes. J Am Coll Cardiol 2000; 36:864-70. 264. Dilsizian V, Bonow RO, Epstein SE, Fananapazir L. Myocardial ischemia detected by thallium scintigraphy is frequently related to cardiac arrest and syncope in young patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1993; 22:796-804. 27 265. Shimizu M, lno H, Okeie K, Emoto Y, Yamaguchi M, Yasuda T, et al. Exercise-induced ST-segment depression and systolic dysfunction in patients with nonobstructive hypertrophic cardiomyopathy. Am Heart J 2000; 140:52-60. 266. Brión G, Peidro R, Angelino A, Zalazar T, Kriskovich J, Casabé H, et al. Prognostic value of exercise testing in patients with hypertrophic cardiomyopathy. Eur Heart J 2005; 26:1696 (abstract). 267. Maron B, Ackerman M, Nishimura R, Pyeritz R, Towbin J, Udelson J. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. In: Maron B, Zipes D. 36th Bethesda Conference: Eligibility Recommendations for Competitive Athletes with Cardiovascular Abnormalities. J Am Coll Cardiol 2005; 45:29-33. 268. Basavarajaiah S, Wilson M, Whyte G, Shah A, McKenna W, Sharma S. Prevalence of hypertrophic cardiomyopathy in highly trained athletes: relevance to pre-participation screening. J Am Coll Cardiol 2008; 51:1033-9. 269. Maron BJ, Chaitman BR, Ackerman MJ, Bayés de Luna A, Corrado D, Crosson JE, et al; Working Groups of the American Heart Association Committee on Exercise, Cardiac Rehabilitation, and Prevention; Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation 2004; 109:2807-16.