* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Kawasaki disease
Transmission (medicine) wikipedia , lookup
Compartmental models in epidemiology wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Epidemiology of metabolic syndrome wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Public health genomics wikipedia , lookup
Seven Countries Study wikipedia , lookup
Epidemiology wikipedia , lookup
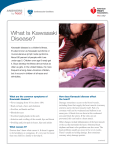
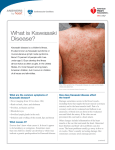
Kawasaki disease Author: Doctor Alfred Mahr1 Creation date: June 2001 Update: June 2004 Scientific Editor: Professor Loïc Guillevin 1 Service de médecine interne, CHU Hôpital Cochin, 27 Rue du Faubourg Saint-Jacques, 75014 Paris France. [email protected]. Abstract Key words Disease name and synonyms Excluded diseases Diagnostic criteria/definition Differential diagnosis Epidemiology Clinical description Management including treatment Etiology Diagnostic methods Unresolved questions References Abstract Mucocutaneous lymph node syndrome, or Kawasaki disease (KD), is an infantile vasculitis of mediumand small-sized arteries. It is a disorder affecting predominantly children younger than 4 years and more frequently subjects of Asian origin. KD is an acute illness characterized by high fever, conjunctivitis, oral mucosal changes, cheilitis, cervical lymph nodes, rash, and reddening of the palms and soles with subsequent desquamation. The disease generally resolves spontaneously within several days. However, approximately 20% of all untreated patients develop coronary aneurysms that are potentially lifethreatening. As there is no specific laboratory test, KD is diagnosed based on clinical criteria and, possibly, on the detection of coronary aneurysms by echocardiography or by coronary angiography. Treatment of KD combines a single course of intravenous immunoglobulins and aspirin. Instituted during the acute phase of the illness, this treatment reduces the frequency of coronary artery-lesions to less than 5%. Some patients unresponsive to this treatment may receive Infliximab as alternative therapy. The etiology of KD remains unknown but might be infectious. Nevertheless, no specific agent has yet been recognized. Key words Kawasaki disease, Kawasaki syndrome, mucocutaneous lymph node syndrome, vasculitis, coronary aneurysms, acquired ischemic heart disease of childhood Disease name and synonyms Kawasaki disease (KD), Kawasaki syndrome, mucocutaneous lymph node syndrome Excluded diseases Infantile polyarteritis nodosa Diagnostic criteria/definition Diagnostic guidelines for KD were established by the Japanese Kawasaki Disease Research Committee [1]. Accordingly, the diagnosis of KD Mahr A. Kawasaki disease. Orphanet Encyclopedia, June 2004: http://www.orpha.net/data/patho/GB/uk-kawasaki.pdf can be retained in the presence of fever lasting for more than 5 days in association with 4 of the following 5 criteria: 1) polymorphous rash; 2) bilateral nonexudative conjunctivitis; 3) changes of the oral mucosa (cheilitis and/or "strawberry" tongue and/or pharyngitis); 4) non-purulent cervical lymphadenopathy (at least 1.5 cm); 5) changes of the hands and feet (erythema on the palms or soles and/or 1 indurative edema and/or desquamation from the fingertips). When coronary abnormalities are present, KD can be diagnosed in patients with fever and only 3 of the other diagnostic criteria. However, because these diagnostic guidelines are not entirely specific to KD, other diseases with similar symptoms have previously to be excluded. A small subset of children present with "atypical" or "incomplete" KD, as defined by the presence of fever and fewer than 4 of the other features. Atypical cases are more difficult to recognize and, unfortunately, at greatest risk for coronary disease [2-4]. Differential diagnosis The differential diagnosis of KD encompasses a multitude of diseases including bacterial infections (toxic shock syndrome, staphylococcal scalded skin syndrome, scarlet fever, rickettsiosis, leptospirosis, yersinia), viral exanthema (measles, adenovirus, Epstein-Barr virus, cytomegalovirus, parvovirus, retrovirus), drug-induced skin reaction (Stevens - Johnson syndrome), connective tissue disease (systemic onset juvenile rheumatoid arthritis, Reiter's syndrome) or other vasculitides (infantile polyarteritis nodosa) [2-5]. Epidemiology Since the initial description in 1967 by the Japanese pediatrician T. Kawasaki [6], more than 170,000 children have been diagnosed with KD in Japan. Recently, series from European countries such as the UK and Italy have been published [23]. In 80%, the affected individuals are 4 years old with a peak incidence between 6 months and 2 years [2-4]. KD is uncommon before 3 months and in children over 4 years, even if it has also been observed occasionally in children older than 10 years [7] or in young adults [8]. Although described in subjects of all ethnic origins, the incidence of KD is highest in Asians or individuals of Asian ancestry. In Japan, the estimated incidence rate exceeds 1,000/1,000,000 under 5 years old [9]. In the United States, an annual incidence of up to 150/1,000,000 children under 5 years old has been reported, with the highest incidence among Asian Americans (333/1,000,000) followed by black (234/1,000,000) and white Americans (127/1,000,000) [10]. The incidence in Europe is compatible to that in USA. Occurrence in siblings is rare, at approximately 2% [11]. KD epidemics have been recorded in many countries with, in particular, 3 large-scale outbreaks in Japan in 1979, 1982 and 1986. KD recurrences are uncommon, occurring in only about 1-3% of the patients [3]. KD is more common in males, with a M:F ratio of 1.5:1. Seasonal variation in the Mahr A. Kawasaki disease. Orphanet Encyclopedia, June 2004: http://www.orpha.net/data/patho/GB/uk-kawasaki.pdf disease incidence has been reported, with peak occurrence in the spring and winter months. Clinical description Clinical presentation. KD is an acute-onset illness characterized by high fever with mucocutaneous changes and adenitis. Fever is a constant feature of KD and generally the first symptom of the disease; it ranges between 38° and 40°C and frequently has irregular spikes; it is often abrupt in onset and unresponsive to antibiotic therapy. Mucocutaneous changes are seen in approximately 90% of the patients and they coincide with the onset of fever or within the hours or days thereafter. The mucosal signs variably include intense bilateral non-exudative conjunctivitis predominantly involving the bulbar conjunctivae; cheilitis with crusting, bleeding lips; "strawberry" tongue; or diffuse erythema of the oropharynx. The skin involvement includes a rash, and changes of the hands and feet associating a clearly delimited erythema of the palms and soles, whereas the dorsa of the hands and feet and the fingers and toes become swollen and indurated. The rash has a remarkably polymorphous presentation and can be morbilliform, scarlatiniform or multiform. It is widespread over the body, affecting the face, the trunk and limbs. However, in some cases, and especially in infants and young children, the rash may preferentially involve the perineal area. Lymphadenitis is less frequent, occurring in approximately 50 - 60% of the patients. Most frequently, the adenitis presents as a large, firm, unilateral lymph node of the cervical or submandibular area; in some cases, it may become the most prominent feature of the disease [2-4, 12]. Associated manifestations. In addition to the previously cited major signs, many other features have been observed in association with KD: aseptic meningitis, vomiting, diarrhea, gall bladder hydrops, sterile pyuria, hepatitis, arthralgia or arthritis, and/or uveitis [2-4, 12]. Irritability (out of proportion to the degree of fever or other signs) is present in the majority of children with KD that can be related to aseptic meningitis. Disease course KD has a typically triphasic course. The acute phase is self-limited with the fever and the other acute signs of inflammation subsiding spontaneously after 1 - 2 weeks. The following subacute phase lasts 1-2 weeks and is characterized by the desquamation of the hand and feet with peeling starting at the tips of the fingers and toes and subsequently involving the entire hands and feet. The conjunctivitis may persist during this phase. 2 The convalescent phase starts when all clinical signs have resolved and end when the laboratory abnormalities have returned to normal, usually 4 - 6 weeks after disease onset. Beau's lines, corresponding to transverse grooves of the fingernails, may develop 1 - 2 months after KD onset [2-4, 12]. Cardiovascular complications Initially described as a harmless disease [6], it rapidly became evident that KD was fatal for 12% of the affected individuals with death most commonly attributed to ischemic heart disease [12]. KD was subsequently recognized as a systemic vasculitis of small- and medium-sized arteries with a marked predilection for the coronary arteries [13]. During the acute phase of the disease, the spectrum of cardiovascular manifestations comprises myocarditis, valvulitis, pericarditis or arrhythmia. However, cardiac involvement during this stage is most commonly asymptomatic but rarely is manifested by congestive heart failure. The most characteristic feature of cardiovascular involvement in KD is the formation of coronary aneurysms that may occur during the subacute phase of the disease and thus when the acute clinical symptoms have already subsided. Aneurysms develop in approximately 20% of untreated patients [14]. Angiographic studies showed that the aneurysms, which are principally located in the proximal portions of the coronary arteries, are commonly associated with other abnormalities, such as vessel dilatation, stenosis or thrombosis. The most severe coronary lesions are giant aneurysms, which are defined by an internal lumen of more than 8 mm [15]. More rarely, aneurysms involve extracoronary arteries, and approximately 2% of the patients present with aneurysms in the axillary, iliac, renal and/or intermammarian arteries [14]. The coronary artery disease may lead to fatal myocardial infarction or rupture of an aneurysm, with most of the deaths occurring within the first 2 months following disease onset [14]. Peripheral ischemia is a rare but severe complication of KD that leads either to death or gangrene [16]. It is unclear what factors account for the occurrence of severe cardiovascular disease in some patients. Male gender, age of less than 1 year at disease onset, prolonged fever duration and highly elevated laboratory markers of inflammation have been cited as predictors of the occurrence of coronary aneurysms [2-4]. Management including treatment The current standard of treatment of KD combines high-dose intravenous immunoglobulins (IVIG) and aspirin administered Mahr A. Kawasaki disease. Orphanet Encyclopedia, June 2004: http://www.orpha.net/data/patho/GB/uk-kawasaki.pdf within the first 10 days of disease onset [2-4, 17]. It has been demonstrated in several therapeutic studies that, compared to aspirin alone, this treatment shortens the duration of the acute signs of the disease and reduces the occurrence of coronary aneurysms and the frequency of long-term coronary abnormalities [18, 19]. Among the various protocols that have been tried, a single, high dose of IVIG (2 g/kg) appeared to be the most efficacious regimen [20]. Aspirin was the first medication widely used to treat KD. Although it has never been proven that this drug has a preventive effect on the coronary abnormalities, it continues to be given for its anti-inflammatory and anti-thrombotic properties. Most commonly, aspirin is administered at an anti-inflammatory dose (80100 mg/kg/d in four divided doses) until the fever subsides and is subsequently reduced to an antiplatelet aggregation dose (3 - 10 mg/kg/d) until being discontinued by 6 weeks after disease onset unless coronary abnormalities persist [24]. Thus, the efficacy of the standard treatment with IVIG and aspirin has only been studied for administration within the first 10 days of illness and, although the precise mechanism of this therapy remains unknown, it is unlikely that it may still be beneficial when the inflammatory response has already subsided [3, 17]. With regard to the atypical presentation in some patients and the importance of early initiation of therapy, this combined treatment should be given to all patients for whom a high index of suspicion exists and other diagnoses have been reasonably excluded [2-4]. In about 10% of the patients, the fever persists or recurs after the completion of the initial therapy with IVIG and aspirin. The management of these patients is not well defined, but most authors have recommended treating again with 1 g/kg of IVIG [17]. Steroids have been abandoned for the treatment of KD since it was advanced that they would favor the formation of coronary aneurysms [21]. More recently, however, the use of steroids has been reconsidered in selected patients, e.g. for patients with IVIG-refractory disease [22]. Other medications include anticoagulants that should be given to patients with giant coronary aneurysms. Inotropic agents may be required for congestive heart failure. The long-term follow-up of children with KD mainly depends on the presence or not of persistent coronary aneurysms beyond the convalescent phase. Small- to medium-sized aneurysms may completely regress within 1-2 years, whereas giant aneurysms generally do not resolve or progress to stenotic lesions and may ultimately necessitate percutaneous or surgical angioplasty. In developed countries, KD has 3 become the major cause of acquired heart disease of childhood [2-4]. Recently, a patient unresponsive to treatment with IVIG and high dose aspirin was treated with infliximab. After the first dose, reduction of fever was observed and his laboratory measures improved [24]. Etiology Despite intense investigation, the etiology of KD remains unknown. The young age at onset, the clinical presentation and the occurrence of outbreaks would point to an infectious, and, in particular, a viral origin. However, none of the various viruses (parvovirus B19, Epstein - Barr virus, human herpesvirus 6 or other Herpesviruses, retrovirus, measles virus) that have been incriminated as potential causative agents could be clearly correlated to KD. Toxinproducing bacteria were implicated because of clinical similarities with the toxic shock syndrome and because of immunological evidence that would suggest an underlying superantigenmediated mechanism. However, further investigation provided conflicting results, and could not confirm the immunological findings or the relationship between staphylococcal or streptococcal infection and KD. An interesting hypothesis refers to the role of IgA plasma cells: these have been found in the vascular walls of KD patients, and an oligoclonal pattern has been demonstrated, consistent with an antigen-driven immune response [25]. Pro-inflammatory cytokines have been shown to play a role in the disease pathogenesis, and a recent study has provided preliminary evidence for an increased frequency of alleles associated with elevated TNF- levels [26]. Autoantibodies in KD have not been found consistently except antiendothelial cell antibodies (AECA) [27]. A genetic factor conferring susceptibility to KD would be supported by the higher incidence in children of Asian ancestry but no particular human leukocyte antigen phenotype (HLA) could be identified. Furthermore, different environmental risk factors have been identified in affected children such as a significantly higher exposure to carpet cleaning and proximity to septic water, but the significance of these results remains unknown [2-4]. Diagnostic methods No specific test is available to diagnose KD. Laboratory findings mainly reflect an intense immune response with leukocytosis, highly elevated erythrocyte sedimentation rate and Creactive protein level, and thrombocytosis. A mild elevation of liver enzymes and sterile pyuria are also commonly observed [2-4]. An interesting finding, possibly related to a cross Mahr A. Kawasaki disease. Orphanet Encyclopedia, June 2004: http://www.orpha.net/data/patho/GB/uk-kawasaki.pdf reactivity of T cells between epitopes of mycobacterial and human heat shock proteins, is the development of erythema and induration at sites of BCG immunization [23] Two-dimensional echocardiography may show coronary artery aneurysms which are a highly suggestive but delayed finding as they generally become visible only during the subacute phase of the illness. During the acute phase, echocardiography may detect coronary dilatation, pericardial effusion, mitral insufficiency and/or left ventricular insufficiency. Because of the high sensitivity of two-dimensional echocardiography in detecting proximal coronary aneurysms [2], coronary angiography is generally reserved for patients with large or multiple aneurysms on echocardiograms, in order to fully investigate the extent of the coronary involvement [4]. Consequently, the diagnosis of KD relies, for the most part, on clinical criteria and, in particular, on the major features that have been outlined by the Japanese Kawasaki Disease Research Committee [1]. However, with regard to the existence of atypical presentations, the diagnosis should be considered in all children with an undiagnosed febrile illness presenting with any of the diagnostic criteria of KD [2, 4]. Unresolved questions The long-term consequences of KD are a matter of debate. The results of several studies tend to support that arteries with resolved aneurysms remain functionally impaired, but it is unclear if these abnormalities predispose to accelerated atherosclerosis. Further investigation should also be undertaken to define the optimal management of immunoglobulin-refractory disease. Finally, the development of a diagnostic test and a specific treatment awaits the discovery of the etiological agent of KD. References 1. The Japanese Kawasaki Disease Research Committee: Diagnostic guidelines of Kawasaki disease, 4th edition, 1984. 2. Laupland KB, Dele Davies H: Epidemiology, etiology, and management of Kawasaki disease: state of the art. Pediatr Cardiol 1999; 20: 177-83. 3. Rowley AH, Shulman ST: Kawasaki syndrome. Pediatr Clin North Am 1999; 46: 31329. 4. Mason WH, Takahashi M: Kawasaki syndrome. Clin Infect Dis 1999; 28: 169-87. 5. Bligard CA: Kawasaki disease and its diagnosis. Pediatr Dermatol 1987; 4: 75-84. 6. Kawasaki T: Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children (in Japanese). Arerugi 1967; 16: 178222. 4 7. Stockheim JA, Innocentini N, Shulman ST: Kawasaki disease in older children and adolescents. J Pediatr 2000; 137: 250-2. 8. Jackson JL, Kunkel MR, Libow L, Gates RH: Adult Kawasaki disease. Report of two cases treated with intravenous gamma globulin. Arch Intern Med 1994; 154: 1398-405. 9. Yanagawa H, Nakamura Y, Yashiro M, et al.: Incidence survey of kawasaki disease in 1997 and 1998 in Japan. Pediatrics 2001; 107: E33. 10. Davis RL, Waller PL, Mueller BA, Dykewicz CA, Schonberger LB: Kawasaki syndrome in Washington State. Race-specific incidence rates and residential proximity to water. Arch Pediatr Adolesc Med 1995; 149: 66-9. 11. Fujita Y, Nakamura Y, Sakata K, et al.: Kawasaki disease in families. Pediatrics 1989; 84: 666-9. 12. Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H: A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 1974; 54: 271-6. 13. Tanaka N, Sekimoto K, Naoe S: Kawasaki disease. Relationship with infantile periarteritis nodosa. Arch Pathol Lab Med 1976; 100: 81-6. 14. Kato H, Inoue O, Akagi T: Kawasaki disease: cardiac problems and management. Pediatr Rev 1988; 9: 209-17. 15. Suzuki A, Kamiya T, Kuwahara N, et al.: Coronary arterial lesions of Kawasaki disease: cardiac catheterization findings of 1100 cases. Pediatr Cardiol 1986; 7: 3-9. 16. Tomita S, Chung K, Mas M, Gidding S, Shulman ST: Peripheral gangrene associated with Kawasaki disease. Clin Infect Dis 1992; 14: 121-6. Mahr A. Kawasaki disease. Orphanet Encyclopedia, June 2004: http://www.orpha.net/data/patho/GB/uk-kawasaki.pdf 17. Rowley AH, Shulman ST: Current therapy for acute Kawasaki syndrome. J Pediatr 1991; 118: 987-91. 18. Furusho K, Kamiya T, Nakano H, et al.: High-dose intravenous gammaglobulin for Kawasaki disease. Lancet 1984; 2: 1055-8. 19. Newburger JW, Takahashi M, Burns JC, et al.: The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med 1986; 315: 341-7. 20. Newburger JW, Takahashi M, Beiser AS, et al.: A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med 1991; 324: 1633-9. 21. Kato H, Koike S, Yokoyama T: Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics 1979; 63: 175-9. 22. Newburger JW: Treatment of Kawasaki disease: corticosteroids revisited. J Pediatr 1999; 135: 411-3. 23. Cimaz R, Falcini F. An update on Kawasaki disease. Autoimmun Rev. 2003; 2:258-63. 24. Weiss JE, Eberhard BA, Chowdhury D, Gottlieb BS. Infliximab as a novel therapy for refractory Kawasaki disease. J Rheumatol. 2004 Apr;31(4):808-10. 25. Rowley AH, Shulman ST, Spike BT, Mask CA, Baker SC. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol 2001; 166:1334–43. 26. Quasney MW, Bronstein DE, Cantor RM et al. Increased frequency of alleles associated with elevated Tumor Necrosis Factor alpha levels in children with Kawasaki disease. Pediatr Res 2001; 49:686–90. 27. Grunebaum E, Blank M, Cohen S et al. The role of anti-endothelial cell antibodies in Kawasaki disease–in vitro and in vivo studies. Clin Exp Immunol 2002; 130:233–40. 5