* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cordeiro et al. Am J Physiol Heart Circ Physiol
Survey
Document related concepts
Transcript
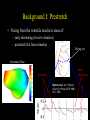
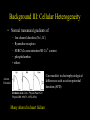
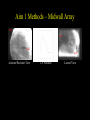
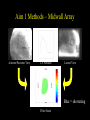
Ventricular Mechanics of Asynchronous Activation Ben Coppola Senate Proposal 01/09/07 Motivation • Heart failure leads to: – Reduced pump function – Structural remodeling • Ventricular pacing in normal hearts leads to: – Reduced pump function – Structural remodeling • Common link: Asynchronous Activation • 170,000/yr receive pacemakers in the U.S. alone. – Some resynchronize while others dyssynchronize Background I: Prestretch • Pacing from the ventricle results in areas of: – early shortening (close to stimulus) – prestretch (far from stimulus) Pacing site * Activation Time early shortening prestretch * Strain Wyman et al. Am J Physiol Heart Circ Physiol 276: H881891, 1999. Background II: Fiber Anatomy • Myofiber angle varies across the wall in a nearly linear fashion Sample Data (Anterior Wall) 80 R2 = 0.9773 60 Fiber Angle (º) 40 20 0 -20 0 5 10 -40 -60 -80 Depth from Epicardium (mm) Streeter et al. Circ Res 24: 339-347, 1969. 15 20 Background II: Sheet Anatomy sheet fibers • Fibers are arranged in sheets – Approximately 4 cells thick – Loosely coupled by collagen – Shearing allows for large changes in wall thickness – Complex distribution base 5 mm slice perpendicular to fibers apex LeGrice et al. Circ Res 1995;77:182-193 Background III: Cellular Heterogeneity • Normal transmural gradients of: – Ion channel densities (Na+, K+) – Ryanodine receptors – SERCA2a concentration/SR Ca2+ content – phospholamban + others Can manifest in electrophysiological differences such as action potential duration (APD) Action Potential Cordeiro et al. Am J Physiol Heart Circ Physiol 286: H1471-1479, 2004. Many altered in heart failure Specific Aims 1. To test the hypothesis that prestretch is a diastolic phenomenon and that its functional consequences are the result of length-dependent activation. 2. To test the hypothesis that the deformation of the laminar sheets is dependent on the pattern of activation, not just their anatomical orientation. 3. To test the hypothesis that observed patterns of transmural strains during normal and altered activation are influenced by the presence of cellular heterogeneity. Aim 1 • To test the hypothesis that prestretch is a diastolic phenomenon and that its functional consequences are the result of length-dependent activation. • Experimental measurements in this aim: – – – – – Midwall bead arrays Transmural bead arrays Bipolar electrode recordings LV Pressure Aortic Flow Midwall bead & electrode array Aim 1 Methods – Midwall Array Base Base Apex Apex Anterior/Posterior View LV Pressure Lateral View Aim 1 Methods – Midwall Array Anterior/Posterior View LV Pressure Lateral View Blue = shortening Fiber Strain Aim 1 Methods – Transmural Array • Same general approach but now: – 3 columns of 4-6 beads are implanted across the wall. – Can get 3D strain tensor in one small area instead of 2D strains over a broader area. – Non-homogeneous Fit Omens et al. Circulation 84: 1235-1245 McCulloch et al. J Biomech 24: 539-548, 1991. Aim 1a: Timing • To test the hypothesis that prestretch occurs prior to ventricular end diastole, due to early-activated regions generating intracavitary pressure and passively inflating late-activated regions before they become electrically active. • Rationale: – ‘Diastole’ continuing in areas not yet active • Experimental Methods: – – – – – 5 open-chest dogs Bead array in anterior midwall Pacing from posterior wall Bipolar electrode array LV Pressure & Aortic Flow Example, Expt. #1 Timing *: Ventricular Stimulus Local Activation Time (ms) 44 Fiber Strain .016 End Diastole Aortic Valve Opening N=5 Local Activation Example, Expt. #1 Timing *: Ventricular Stimulus Local Activation End Diastole Time (ms) 44 55 Fiber Strain .016 .019 Aortic Valve Opening N=5 Local Activation End Diastole Example, Expt. #1 Timing *: Ventricular Stimulus Local Activation End Diastole Aortic Valve Opening Time (ms) 44 55 134 Fiber Strain .016 .019 .060 N=5 Local Activation End Diastole Aortic Valve Opening Timing * P < 0.05 vs. Local Activation † P < 0.05 vs. End Diastole. Aim 1b: Initial Fiber Length • To test the hypothesis that myocardial fibers in the late-activated regions are longer at the onset of ejection during ventricular pacing than during atrial pacing, resulting in increasing ejection shortening in these late-activated regions. • Rationale: Compensatory work done by late-activated regions Aortic Valve Opening Strain Ejection Strain Eff 0.10 0.00 0.00 -0.05 -0.05 Eff Ecc Ecc Efc Efc 0.08 Strain 0.04 0.02 -0.10 -0.15 Strain 0.06 Strain Ref: Atrial Pacing, onset of ejection Def: Vent. Pacing, onset of ejection -0.10 -0.15 * 0.00 Eff, Fiber Strain Ecc, Cross-fiber Strain -0.20 Efc,-0.20 Shear Strain Atrial Ventricular Control Atrial Ventricula Aim 1c: AV Delay • To test the hypothesis that the length of AV delay modulates the prestretch response by changing the preload. • Rationale: – 35 ms AV delay resulted in same fiber length as atrial pacing – Hemodynamic function varies with AV delay Aortic Valve Opening Strain 1.5 0.05 1.4 0.04 1.3 0.03 1.2 0.02 1.1 Fiber Strain Normalized Stroke Volume Stroke Volume 1 0.9 0.8 0.01 0 -0.01 0 10 20 30 40 -0.02 0.7 -0.03 0.6 -0.04 0.5 0 10 20 30 40 50 60 70 -0.05 AV Delay • Need to create AV block in order to get long AV delays AV Delay (ms) 50 60 70 Aim 1c: AV Delay Stroke Volume Normalized Stroke Volume 1.5 1.4 1.3 1.2 1.1 1 0.9 0.8 0.7 0.6 0.5 0 50 100 150 200 250 AV Delay (ms) Truncated atrial kick Filling while ventricle not fully relaxed Stroke Volume follows expected pattern; strains not yet analyzed Aim 1d: Conduction Velocity • To test the hypothesis that decreasing the conduction velocity (without changing activation sequence) will increase prestretch in a finite element model of ventricular pacing. Activation Time Approach: Use same activation pattern but scale activation times Aim 1d: Conduction Velocity • Suppose that all areas activated X ms after stimulus are prestretched – Increase in volume of late-activated tissue – Decrease in stroke volume • Alternative outcome: – Reduced rate of pressure development – No change in pattern of prestretch relative to cardiac cycle – No change in stroke volume Aim 1 Summary • Most of aims 1a, 1b, 1c complete • 1d may already have been looked at by Roy – If so, could look at other factors such as contractility Aim 2 • To test the hypothesis that the deformation of the laminar sheets is dependent on the pattern of activation, not fixed by their anatomical orientation. Fibers in plane of screen Fibers go into screen Yellow lines demarcate a sheet in each electron micrograph (LeGrice, I. J. et al. Circ Res 1995;77:182-193) Aim 2 • • • • Sheet angle (b) varies through the wall Multiple sheet families can exist at one location Strains can be expressed in a fiber-sheet coordinate system Changes in sheet angle can be derived from strains Ashikaga et al. Am J Physiol Heart Circ Physiol 286: H640-647, 2004. Large S.D.’s probably due to averaging of 2 sheet populations Aim 2a: Sheet Angle Change • To test the hypothesis that pacing from the LV epicardium alters the systolic sheet angle change compared to normal activation. • Rationale: – Magnitude and gradient of sheet extension (ESS) reduced by epicardial pacing Atrial pacing LV epicardial pacing Ashikaga et al. Am J Physiol Heart Circ Physiol 286: H2401-2407, 2004. Aim 2a: Sheet Angle Change • Multiple sheet families can exist at one location • Direction of approach of activation wavefront may preferential activate one of these families Methods: • • • • Open-chest dogs Transmural bead array 4 Pacing Sites Histology X X X X X: Epicardial Pacing Site Expected Results: Sheet angle change dependent on pacing site (only in areas of multiple sheet families?) Aim 2b: Transmural Activation • To determine the relative contribution of sheet deformation to wall thickening with normal and reversed transmural activation. • Rationale: – Preliminary data suggests epicardial pacing is functionally inferior to endocardial pacing (though not significantly) – The order of activation of the sheets may be important – For example, earlier activation of endocardial sheets may cause them to deform first, and through tissue tethering epicardial sheets may deform even before being activated. Aim 2b: Transmural Activation • Can calculate contributions of fiber-sheet strains to wall thickening (ERR = ESScos2b + ENNsin2b + ESNsinbcosb) • During systole, primary mechanism is sheet extension, ESS. Contribution to wall thickening ESS ENN ESN 66% -5% 41% (Anterior wall, ¼ of distance from apex to base) Costa et al. Am J Physiol 276: H595-607, 1999. Expected Results (Epi pacing compared to Endo): • Decrease in overall wall thickening • Change in contributions to wall thickening, sheet angle change Aim 2c: Maximum Shear Predicted Measured • To test the hypothesis that sheets are oriented along the plane of maximum shear (constrained to contain the myofibers) only when normally activated. • Rationale: – The sheet angles can be predicted based on measured deformations, using the assumption that they are aligned along the direction of maximum shear that contains the fibers. Arts et al. Am J Physiol Heart Circ Physiol 280: H2222-2229, 2001. Aim 2c: Maximum Shear Rationale(cont.) • Altered activation changes the overall pattern of deformation • Direction of maximum shear is likely changed as well such that it no longer coincides with the sheet direction • Could be a stimulus for the remodeling of sheets that is seen with chronic ventricular pacing (Helms, 2006) Expected Results (based on experiments of 2a & 2b): • Pacing at the bead set will change the direction of maximum shear, making it closer to the radial direction • This is the direction in which the sheet angle remodels chronically Summary of Aim 2 • Use existing techniques to measure deformation • Make histological measurements of fibers and sheets • Determine role of sheets in abnormal activation Aim 3 • To test the hypothesis that observed patterns of transmural strains during normal and altered activation are influenced by the presence of cellular heterogeneity. Action Potential Unloaded Cell Shortening Cordeiro et al. Am J Physiol Heart Circ Physiol 286: H1471-1479, 2004. • Methods – Computational modeling, no new experiments Aim 3a: Normal Gradients • To test the hypothesis that cellular heterogeneity influences observed strain patterns with normal activation. • Rationale: Transmural conduction time – Preliminary data shows that a finite element model with homogeneous cellular properties incorrectly predicts epicardial prestretch (due to endocardial contraction) Endo AP – Transmural conduction time is thought to be balanced byEpi differences in electrical-mechanical delays, resulting in a synchronous contraction (Cordeiro, 2004) Cell Shortening Schematic representation based on data from Cordeiro et al. Am J Physiol Heart Circ Physiol 286: H1471-1479, 2004. Aim 3a: Normal Gradients • Methods: – Incorporate cellular model which is transmurally heterogeneous (Flaim, 2006) into current finite element model – Compare experimentally measured strains to: • Simulations with homogeneous cellular properties • Simulations with heterogeneous cellular properties • Expected Results: – Eliminate erroneous prestretch – Improve end-systolic strains? (radial or transverse shear) Aim 3b: Abnormal Activation • To test the hypothesis that cellular heterogeneity creates transmural dyssynchrony during epicardial pacing. • Rationale: Retrograde conduction time – If the transmurally heterogeneous properties are “designed” to make the wall contract more synchronously, then reversing Endo the direction of activation should have a dramatic effect. AP Cell Shortening Epi Aim 3b: Abnormal Activation • Methods – Compare Epi & Endo pacing experimentally to: • Simulation with homogeneous cellular properties • Simulation with heterogeneous cellular properties • Expected Results – Endocardial pacing will be improved w/heterogeneity – Epicardial pacing will generate endocardial prestretch (may not be reflected experimentally – Likely to have effects on relaxation as well but this may be difficult to address with model Summary of Aims To look at the significance of the following during asynchronous activation: 1. Late Activated Tissue (3 months) 2. Fiber-Sheet Architecture (6 months) 3. Cellular Heterogeneity (3-6 months) Acknowledgments: Hiroshi Ashikaga Roy Kerckhoffs Rachel Alexander Aundrea Graves Katrina Go Jay Yee Leonard Lee