* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Winter Newsletter_2016
Patient safety wikipedia , lookup
Reproductive health wikipedia , lookup
Maternal health wikipedia , lookup
Rhetoric of health and medicine wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Theralizumab wikipedia , lookup
Maternal physiological changes in pregnancy wikipedia , lookup
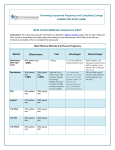
news WINTER 2016 Inside this issue CASE STUDY •Thrombosis in Pregnancy •Idarucizumab and the lab in patients bleeding on Dabigatran CLINICAL TRIAL •The Environmental Determinants of Lupus Flares study (EDOLF) THERAPEUTIC DRUG MONITORING CHANGES Incorporating: • • • • • Northern NSW Hunter New England Mid North Coast Central Coast Northern Sydney FROM THE DIRECTOR A CASE FOR CHANGE? “Would you tell me please, which way I ought to go from here?” said Alice. “That depends a good deal on where you want to get to,” said the Cat. “I don’t much care where…” said Alice. “Then, it doesn’t matter which way you go,” said the Cat. Lewis Carroll: Alice’s Adventures in Wonderland I have often wondered why I was so pleased by Lewis Carroll’s “Alice” stories, but so uninterested in the “heroine”. The uniqueness, vibrancy and range of characters in these stories was astonishing. The peculiarity of the environment in which they existed was fascinating. The laws of physics were irrelevant and an alternate universe; one of the “multiverses” was created. In that world the rules of engagement and conduct were novel and sometimes whimsical. “Sentence first – verdict afterwards.” They often had draconian consequences. “Off with her head!” The Alice novels are, to me, early “fantasy” and “science fiction” genre; a mixture of Game of Thrones and Star Wars. Not that I have ever read any of these novels! Alice seemed so insipid. Things happened to Alice by happenstance. The outcomes were fortuitous but Alice, to me, never apeared in control. Her destiny never appeared to be a consequence of active decision making. “Take care of the sense, and the sounds will take care of themselves.” When she returns to the real world it is bland and staid and static compared to the world “through the looking glass”. To some of you, the realm we now inhabit in pathology may seem like Alice’s fantasy world, populated by Madhatters, Doormice, Cheshire Cats and White Rabbits or, unfortunately, Knaves and Queen of Hearts! The pressure for change in our world is powerful. It is a conflation of social, political, technical and financial imperatives. There are many pressures upon people in our communities and we are committed to assisting them. Pathology North is very aware of the environment in which we work and, unlike Alice, we do know “where we want to go”. We are, therefore, continually intiating new rules of engagement to move in the right direction, to shape our destiny and to deliver a service that is mandated by our clients, customers and patients. Being a part of NSW Health Pathology assists us in improving the services required by the Local Health Districts, the community and the private sector. It is important to note however, one thing that will not change is our commitment to our community. We will continue to bulk bill for pathology services wherever Commonwealth MBS funding arrangements allow. We will continue our service to the entire NSW Health community and we appreciate your support in delivering this goal. Dr. Stephen Braye Director, Pathology North Front Cover:Benicar Tindall, Technical Officer (Grafton Laboratory) working in the haematology department. She is about to spread a blood film so it can be stained for her to examine under the microscope. 2 DR BRYONY ROSS, MBBS, FRACP, FRCPA Staff Specialist Haematologist Dr Ross specialises in Paediatric Haematology and Oncology and Laboratory Haematology therapy are switched to LMWH prior to 6 weeks gestation. The direct oral anticoagulants (DOACs) are not recommended for use in pregnancy or lactation (e.g. rivaroxaban, apixaban, dabigatran). Which patients require anticoagulation in pregnancy? Antepartum Postpartum anticoagulation anticoagulation [email protected] Thrombosis in Pregnancy Rose is a 32 year old G2P1 who presents to her regular GP after a positive urine pregnancy test at home. She unfortunately developed a DVT at 36 weeks gestation in her last pregnancy. She was initially treated with low molecular weight heparin (LMWH) and converted to warfarin at approximately 6 weeks post-partum. She completed a total of 6 months of anticoagulation and has presented today as she recalls being told that she would likely require treatment with anticoagulants in subsequent pregnancies. What are the changes in haemostasis seen in a normal pregnancy? Normal pregnancy is a prothrombotic state. This protects the mother from excessive bleeding at the time of delivery and placental separation BUT it also increases the risk of venous thromboembolism during pregnancy and post-partum. Changes to haemostasis during pregnancy include: • The anticoagulant protein S decreases •Procoagulant factors (fibrinogen, Factors II, VII, VIII, X, XII, and XIII) increase by 20 – 200% • Von Willebrand Factor increases • Activity of fibrinolytic inhibitors increase The net effect is an increase in the tendency for thrombus formation and extension, particularly in the post-partum period. Normalisation of coagulation parameters and factor levels varies, but normally these return to baseline by 6-8 weeks post-partum. However, there are also other multiple acquired risk factors for venous thrombosis in pregnancy that need to be considered, including increasing age, obesity, smoking, dehydration due to hyperemesis as well as many chronic medical conditions. Which anticoagulants can be used in pregnancy? Low molecular weight heparins (for example enoxaparin) are the most commonly used anticoagulants in pregnancy (LMWH). Vitamin K antagonists (VKA - e.g. Warfarin) are contraindicated in pregnancy. It is suggested that women on long term VKA Previous VTE • single episode of VTE with transient risk factor, • not associated with pregnancy or oestrogen ✗ Previous VTE ✓ • unprovoked VTE (prophylactic • pregnancy or or intermediate oestrogendose) related VTE ✓ (prophylactic or intermediate dose) ✓ (prophylactic or intermediate dose) Long term VKA prior to pregnancy ✓ (treatment dose LMWH) ✓ (treatment dose LMWH) Increased risk of VTE after caesarean section ✗ ✓ (prophylactic dose) ✗ ✓ (prophylactic or intermediate dose) Homozygous Factor V Leiden or Prothrombin 20210A mutation • no personal hx of VTE • no family hx of VTE Homozygous Factor V Leiden or Prothrombin ✓ 20210A mutation (prophylactic or • no personal hx of intermediate dose) VTE • with family hx of VTE All other thrombophilias • no personal hx of VTE • no family hx of VTE ✓ (prophylactic or intermediate dose) ✗ ✗ All other thrombophilias • no personal hx of VTE • with family hx of VTE ✗ ✓ (prophylactic or intermediate dose) Antiphospholipid antibody syndrome • with hx of 3 or more pregnancy losses ✓ (prophylactic LMWH and aspirin) ✓ (prophylactic LMWH and aspirin) 3 Prophylactic or intermediate dose depends on the LMWH heparin used (for example with enoxaparin prophylactic dose = 40mg daily; intermediate dose = 40mg BD). For women with mechanical heart valves, treatment dose anticoagulation is required – please seek specialist advice. It is best that these patients be cared for during their pregnancy by a multidisciplinary team experienced in the care of patients with thrombotic disorders. A comprehensive anticoagulation plan for pregnancy, labour and delivery should be formulated. Consider referral to a centre with a maternal-foetal medicine and haematology services. Should I be testing pregnant women for inherited thrombophilias? The association between a diagnosis of an inherited thrombophilia and pregnancy morbidity is weak: PROFESSOR CHRISTOPHER WARD BMedSc, MBCHB, PhD, FRACP, FRCPA Haematologist Prof Ward is the current Head of the Department of Haematology and Transfusion Medicine at Royal North Shore Hospital and the Director of Research. His primary clinical and laboratory expertise is in coagulation disorders, and he oversees the diagnostic investigation of platelet function disorders at Pathology North. His research interests include hypercoagulable states and novel anticoagulants, platelet disorders and heparin-induced thrombocytopenia. He is Past President of the Australasian Society on Thrombosis and Haemostasis and represents Australia on the Executive Council of the Asia-Pacific Society on Thrombosis and Haemostasis. Prof Ward has conducted several clinical trials at Royal North Shore Hospital using novel anticoagulants and thrombopoietin receptor agonists and contributes to local and national guidelines on these therapies. [email protected] DR CATHERINE TANG Dr Tang is an Advanced Trainee in Haematology at Royal North Shore Hospital in Sydney. [email protected]. gov.au 4 •The laboratory tests are imprecise (particularly in the setting of pregnancy when normal ranges are incomplete, and the physiologic changes of pregnancy can interfere with interpretation) •The tests that are available do not comprehensively assess the genetic framework of thrombophilia •Both laboratory abnormalities and pregnancy morbidity are common, so inevitably with increased investigation, abnormalities are frequently found Based on the current evidence, we would not recommend thrombophilia testing in pregnancy. What happened to our patient? Rose was treated with prophylactic LMWH (enoxaparin 40mg daily) throughout her pregnancy and for 6 weeks post-partum. She delivered a beautiful baby boy with no complications. Idarucizumab and the lab in patients bleeding on Dabigatran A 93 year-old woman was admitted to the Emergency Department with severe per-rectal bleeding resulting in haemodynamic instability. Her past medical history included atrial fibrillation resulting in significant embolic events, and prior nephrectomy with a reduced GFR of approximately 43mL/min. She was on Dabigatran 110mg BD, last taken 10 hours prior to her presentation. Her coagulation profile on admission demonstrated a markedly deranged Thrombin Time >120.0s (15-19.0s), Prothrombin Time 29.9s (11-15.0s) and Activated Partial Thromboplastin Time 69.5 (24-36.0s). A dilute thrombin time (dTT) was measured using the Hemoclot Assay which was significantly prolonged at 103s correlating to a Dabigatran level of 534ng/mL. The patient was then given the reversal agent Idarucizumab as part of the REVERSE-AD trial with immediate normalisation of her dTT (below detection limit <35s) and haemodynamic status (See Table 1). Discussion There has been increasing use of direct oral anticoagulants (DOACs) in management of conditions such as atrial fibrillation within the community. Of concern are elderly patients who, across multiple studies, have been shown to have an increased risk of gastrointestinal bleeding on DOACs versus warfarin1. The monitoring of these DOACs is not routinely required, however guidelines have been developed to use standard coagulation assays to determine anticoagulant effect2 Recently, Idarucizumab has been approved as a reversal agent for Dabigatran in emergent settings. Idarucizumab is a monoclonal antibody which binds to both free and thrombin-bound dabigatran at high affinity to neutralize the anticoagulant effect of Dabigatran3. The advent of Idarucizumab necessitates appropriate assays to determine the anticoagulant effect of Dabigatran in emergent settings to direct appropriate use of this reversal agent. Prior pharmacokinetic and pharmacodynamics correlation studies in the initial development of Dabigatran have demonstrated the deficiencies of standard coagulation assays in determining levels of Dabigatran4. The PT is often insensitive with only modest elevation even at high plasma concentrations of Dabigatran; the aPTT demonstrates a curvilinear response that flattens at higher concentrations whilst the TT is too sensitive with results often exceeding the upper limit of the coagulometer. The dTT has been found to demonstrate a linear dose response curve to plasma concentrations of Dabigatran. (See figure) Whilst there is still no defined “therapeutic range” for Dabigatran the preliminary data of the REVERSE-AD trial suggests that there are a significant percentage of patients who present with significant bleeding but actually have low levels of Dabigatran on board compared to the 10-90th percentile of patients enrolled in the RE-LY trial3,5. Therefore early use of dTT to identify patients who have significant plasma levels of Dabigatran could help direct the rationale of Idarucizumab. Additional research to define dabigatran levels associated with haemorrhagic vs thrombotic events would further improve the clinical utility of dTT assays. Table 1: Results of coagulation tests and clinical status of patient pre and post Idarucizumab Timepoint PT(s) APTT (s) TCT (s) dTT (s) References: Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-62. Tran H, Joseph J, Young, L, McRae S, Curnow J, Nandurkar H, Wood P and McLintock C. New oral anticoagulants: a practical guide on prescription, laboratory testing and peri-procedural / bleeding management. Internal Medicine Journal. 2014; 44(6):525-36 Pollack CV, Jr., Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, et al. Idarucizumab for Dabigatran Reversal. The New England journal of medicine. 2015;373(6):511-20. Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. British journal of clinical pharmacology. 2007;64(3):292-303. Lehr T, Haertter S, Liesenfeld KH, Staab A, Clemens A, Reilly PA, et al. Dabigatran etexilate in atrial fibrillation patients with severe renal impairment: dose identification using pharmacokinetic modeling and simulation. Journal of clinical pharmacology. 2012;52(9):1373-8. Dabigatran (ng/mL) Clinical Notes Presentation 29.9 69.5 >120 103 533.99 PR Bleed, on DABI Just prior to Rx 29.7 71.2 >120 98 445.6 Bleed; pre reversal agent After 1 vial 16.1 26.6 16.7 <35 0.0 post reversal agent Dose 1 After 2 vial 16.4 28.1 18.4 <35 0.0 post reversal agent Dose 2 st nd Figure 1: R elative performance of available clotting assays to determine Dabigatran concentration 5 MARLINE SQUANCE Cert Path., Cert Bio Tech., Dip BioMed, CT (ASC), CT (IAC), B. Env Sc (Health) Hons., PhD. Ms Squance is the Chief Executive Officer of the Autoimmune Resource and Research Centre (ARRC) based in the Hunter. The centre offers education, resources and support services as well as access to innovative research projects for people living with autoimmune illnesses in particular Lupus, Scleroderma, Sjogren’s and associated illnesses of Pulmonary Arterial Hypertension and Raynauds Phenomenon. ARRC works closely with Specialists and staff of Pathology North and has a patient resource office within our Newcastle building. Marline has degrees in Biomedical Science, Diagnostic Cytology and Environmental Science/ Environmental Health. Marline has worked both internationally and nationally in Health for over 30 years. Marline began her career in 1983 as a Cytotechnologist in the Anatomical Pathology Department of the Royal Newcastle Hospital. She worked for 10 years in this department before leaving to work and live in Canada and also in Switzerland. Upon her return to Newcastle, Marline returned to Cytology with work in local pathology services before moving to research and project management positions with local General Practice network groups and also the NSW Cancer Council. After a brief meeting with A/Professor Glenn Reeves (Immunology) in 2005, Marline returned to Hunter Area Pathology as a research coordinator of studies related to coeliac disease, scleroderma and lupus. Under the supervision of A/Prof Reeves, she took on the management role of ARRC and also completed her doctorate which investigated environmental determinants of lupus flares. The Environmental Determinants of Lupus Flares study (EDOLF) The Environmental Determinants of Lupus Flares study (EDOLF) investigated the relationships between common environmental agents found indoors and self-reported symptom flare days (SRF) in 101 Australian female lupus patients as compared to 41 age matched healthy controls. The study was retrospective and employed mixed methods examining differences in lifestyle behaviours and agent exposure with personal product use. The study showed that the Australian population was similar to other Caucasian populations, with the average number of flares reported to be 29.9 SRF days, with 6.8 discrete flares for the study year. Flare symptoms were consistent with other population profiles published, however the EDOLF Australian population also reported gastrointestinal issues (13.9%) and shortness of breath (9.9%) as common symptoms. Commonly published flare triggers of UV radiation, infection and stress were confirmed, with the addition of new potential triggers: temperature & weather changes, work, and cleaning chemicals. Use of personal care products for home cleaning, personal hygiene and lifestyle activities, resulted in significant increased risk associations for bath oil (IRR 1.008, CI 1.00-1.02) and significant reduced SRF risk for cleansing beauty products (IRR 0.999, CI 0.998-0.999) and a combined makeup group (foundation and sunscreen) (OR 0.998: CI 0.997–1.0). A flare day reduction of 0.15% was calculated for each day of combined makeup group product use. In comparison to control participants, the SLE group showed significant difference in 25(OH)D deficiency (p=0.02), and 25(OH)D levels (means-control 74nmol/L (29.5ng/ml); SLE 58nmol/L (23.1ng/ml), p=0.04). Reduced levels of 25(OH)D were associated with expression of serological autoimmunity (ANA titres of 1:80) with odds ratios (OR) for ANA-positivity declining by 36% of the baseline OR for every two-fold rise in 25(OH)D level. A significant association could not be found between levels of 25(OH)D and SRF. Significant associations were found for Finnish Job Exposure Matrix (FINJEM) occupational exposure classes; manual handling burden (p=0.02, IRR 1.01); iron (p=0.00, IRR 1.37); wood dust (p=0.00, IRR 3.34); and asbestos (p=0.03, IRR 2.48), indicating that participating in occupations such as nursing, teaching and specialist labouring could pose an increased risk to SLE patients. 6 Analysis of lifestyle factors indicates that the EDOLF SLE participants, as compared to the control participants, had reduced levels of QOL on VAS scales, lower levels of physical activity but similar dietary variables. SLE participants also used significantly more whole medical system CAM (p=0.0301). SLE patients commonly used therapies suchas acupuncture, hydrotherapy, massage and dietary supplements including vitamin D and anti-inflammatory homeopathic medications such as fish oils. The retrospective design of the EDOLF study may have resulted in a number of study limitations including misclassification and recall bias; however a number of data validation steps were incorporated to limit bias influences on reported results. One considerable limitation of the retrospective EDOLF study design was that establishment of firm causal relationships was not possible. Therefore, reported results can only infer potential significant relationships and health effects. In conclusion, the EDOLF study provides insight into the patient SLE experience particularly perceived flare symptoms, triggers and management strategies. Each year, the average SLE patient experiences 30 days of symptom flares which are commonly self-managed with no extra physician assistance. The study also identified that everyday behaviours and exposures in day-to-day life activities, including both home and work environments, could potentially trigger exacerbation of SLE symptoms. In addition, the use of UV protective products, whilst potentially reducing symptom exacerbation and flare days, may paradoxically influence serum 25(OH)D in a group of patients with a higher incidence of deficiency and insufficiency as compared to the general population. Importantly, the EDOLF study provides insight into future research directions that will better inform appropriate protective measures that people living with SLE can adopt to reduce adverse health impacts and improve life potential and quality. List of publications to date 1. The lived experience of lupus flares: features, triggers and management in an Australian female cohort. Journal: International Journal of Chronic Diseases Authors: Marline Squance, Glenn EM Reeves, Howard Bridgman. www.hindawi.com/journals/ijcd/2014/816729/ 2. Exploring lifetime occupational exposure and SLE flare: a patient focussed pilot study. Journal: Lupus, Science & Medicine Authors: Marline Squance, Maya Guest, Glenn Reeves, John Attia, Howard Bridgman www.lupus.bmj.com/content/1/1/e000023 3. Vitamin D levels are associated with expression of SLE, but not flare. Journal: International Journal of Rheumatology Authors: Marline Squance, Glenn Reeves, Huy Tran www.hindawi.com/journals/ijr/2014/362834/ 4. Self-reported Lupus flare: associations with everyday home and personal product exposure. Journal: Toxicology Reports Authors: Marline Squance, Glenn Reeves, John Attia, Howard Bridgman, Maya Guest www.sciencedirect.com/science/article/pii/S2214750015300044 5. Patient reported lupus flare: exploring associations with foundation makeup and sunscreen use. Journal: Journal of Cosmetics, Dermatological Sciences and Applications Authors: Marline Squance, Glenn Reeves, and John Attia www.scirp.org/journal/PaperDownload.aspx?paperID=52242 For more information about EDOLF email: [email protected] For more information about ARRC and its services www.autoimmune.org.au or [email protected] 7 CHANGE OF UNITS FOR THERAPEUTIC DRUG MONITORING As part of a national harmonization initiative, Pathology North will be changing the reporting of therapeutic drugs to mass units as of 4th July 2016. The use of mass units follows recommendations from the Royal Australasian College of Physicians, The Royal College of Pathologists of Australasia, the Australasian Association of Clinical Biochemistry and the Australasian Society for Clinical and Experimental Toxicology as published in Med J Aust, 2013; 198 (7): 368-369 (www.mja.com.au). This aligns drug concentration measurements with the units used for drug dosing and with the major English language sources of information on drug efficacy, metabolism and toxicity. The aim is for therapeutic drug measurements from all laboratories in Australia to be in the standardised units to avoid clinical errors. In general the use of mass units (e.g. mg/L or ug/L) will apply, with the following exceptions: • • • • • Methotrexate in umol/L Lithium in mmol/L (as these are currently fully harmonised in these units) Iron in umol/L Free thyroxine (fT4) in pmol/L Vitamin D (25-Hydroxy Vitamin D) in nmol/L These are examples of drugs which are also endogenous substances where the current units are retained. Results in the new mass units will be reported in parallel with the old units for a transitional period of at least 12 months. During this dual reporting period, on the printed and eMR laboratory report, two sets of results will be shown for each unit of measurement of the drug in question; e.g.: • • Paracetamol (mg/L) 10 Paracetamol (umol/L) 66 PLEASE ENSURE THAT THE CORRECT UNIT IS USED IN THE PARACETAMOL NOMOGRAM (Available at: Med J Aust 2015; 203: 215-218. doi:10.5694/mja15.00614.) Drug results will be marked with a footnote for a period of time to alert users to the change. On the 4th July 2016, this change over may delay Therapeutic Drug reports for up to four hours. Pathology North staff apologises for the inconvenience. For further information, or for clinical enquiries, please contact the following Pathology North staff: Northern Sydney and Central Coast Peter Ward, Royal North Shore Hospital Tel: 9926 4142 Email: [email protected] Newcastle/Hunter and Taree region Dr A Caswell, John Hunter Hospital Tel: 4921 4401 Email: [email protected] New England Don Clausen, Tamworth Base Hospital Tel: 6767 7813 Email: [email protected] Mid North Coast Dileep Kumar, Coffs Harbour Hospital Tel: 6656 7509 Email: [email protected] Northern NSW Lyndall Palmer, Lismore Base Hospital Tel: 6620 2915 Email: [email protected] Are you interested in receiving the newsletter? Please email: [email protected] or [email protected]