* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CEA - Nuclear astrophysics
Planetary nebula wikipedia , lookup
Cosmic distance ladder wikipedia , lookup
Big Bang nucleosynthesis wikipedia , lookup
Chronology of the universe wikipedia , lookup
Nuclear drip line wikipedia , lookup
Main sequence wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Star formation wikipedia , lookup
Standard solar model wikipedia , lookup
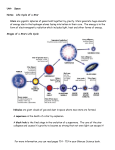
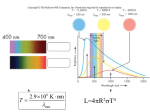
> The science of the stars and the cosmos THE COLLECTION 1 > The atom 2 > Radioactivity 3 > Radiation and man 4 > Energy 5 > Nuclear energy: fusion and fission 6 > How a nuclear reactor works 7 > The nuclear fuel cycle 8 > Microelectronics 9 > The laser: a concentrate of light 10 > Medical imaging 11 > Nuclear astrophysics 12 > Hydrogen FROM RESEARCH TO INDUSTRY 11 > Nuclear astrophysics THE PRINCIPLE OF NUCLEOSYNTHESIS THE STARS THE SUN SUPERNOVAE COSMIC RADIOACTIVITY SEEN BY THE INTEGRAL SATELLITE © Commissariat à l’Énergie Atomique aux Energies Alternatives, 2005 Atomique,et2003 Communication Division Direction de la communication Bâtiment Siège Gif-sur-Yvette cedex 31-33, rue de la- 91191 Fédération www.cea.fr 75752 Paris Cedex 15 – www.cea.fr ISSN 1637-5408. > CONTENTS > INTRODUCTION The aim of nuclear astrophysics is to explain the origin, evolution and abundance of elements in the Universe. © ESA/Soho - Hubble/AURA/STScI/Nasa - PhotoDisc THE PRINCIPLE OF NUCLEOSYNTHESIS 4 What is a nuclear fusion reaction? 5 Periodic table and Abundance table 6 and 7 Where does energy come from? 8 The different types of nucleosynthesis 8 A © PhotoDisc Globular cluster. THE STARS What is a star? Why do the stars shine? The birth of stars The life of stars The death of stars 9 10 10 12 13 13 THE SUN What is the Sun made of? A model of the Sun Life expectancy of the Sun and stars 15 16 16 19 SUPERNOVAE 20 What is a supernova? 21 The different types of supernovae 21 Thermonuclear and gravitational supernovae 21 © D. Malin/Anglo-Australian Observatory Nuclear astrophysics 2 Cloud lit from the inside. Supernovae, the source of heavy elements Supernovae, the source of cosmic radiation COSMIC RADIOACTIVITY SEEN BY THE INTEGRAL SATELLITE 23 24 25 introduction stronomy deals with the position and observation of the objects in our Universe, from planets to galaxies. It is the oldest of the sciences. Astrophysics is the study of the physical properties of these objects. It dates from the start of the 20th century. Nuclear astrophysics is the marriage of nuclear physics, a laboratory science concerned with the infinitely small, and astrophysics, the science of what is far away and infinitely large. Its aim is to explain the origin, evolution and abundance of the elements in the Universe. It was born in 1938 with the work of Hans Bethe, an American physicist who won the Nobel Prize for physics in 1967, on the nuclear reactions that can occur at the center of stars. It explains where the incredible energy of the stars and the Sun comes from and enables us to understand how they are born, live and die. The matter all around us and from which we are made, is made up of ninety-two chemical elements that can be found in every corner of the Universe. Nuclear astrophysics explains the origin of these chemical elements by nucleosynthesis, which is the synthesis of atomic nuclei in different astrophysical environments such as stars. Nuclear astrophysics provides answers to fundamental questions: • Our Sun and the stars in general shine because nuclear reactions are taking place within them. • The stars follow a sequence of nuclear reaction cycles. Nucleosynthesis in the stars enables us to explain the origin and abundance of elements essential to life, such as carbon, oxygen, nitrogen and iron. • Star explosions, in the form of supernovae, disperse the nuclei formed by nucleosynthesis into space and explain the formation of the heaviest chemical elements such as gold, platinum and lead. Nuclear astrophysics is still a growing area of science. Designed and produced by Spécifique - Cover photo by © PhotoDisc - Illustrations by YUVANOE - Printed by Imprimerie de Montligeon - 04/2005 The science of the stars and the cosmos 11 > Nuclear astrophysics The science of the stars and the cosmos 11 > Nuclear astrophysics 3 4 > THE PRINCIPLE OF NUCLEOSYNTHESIS THE NUCLEAR MICROCOSM AND ASTRONOMICAL MACROCOSM ARE CLOSELY LINKED. The principle of nucleosynthesis Nucleosynthesis is the formation of atomic nuclei in different astrophysical environments. It is intimately linked with nuclear physics. WHAT IS A NUCLEAR FUSION REACTION? The nuclear fusion reaction produces one heavy nucleus from two lighter atomic nuclei. It is accompanied by a huge release of energy. Fusion is difficult to achieve because it involves two different, opposing forces: – the strong nuclear interaction that binds the neutrons and protons into the nucleus. This very intense force only works over very small distances no bigger than the radius of the nucleus, – the electromagnetic interaction to which all charged particles are subject. This works over a long distance. It prevents positively charged atomic nuclei from coming very close to one another, by creating a kind of barrier of repulsion. To succeed in crossing this barrier and getting close enough to fuse, the nuclei must be in a THE ATOM Matter is made up of atoms, which consist of a nucleus with electrons orbiting around it. The atomic nucleus is an assembly of protons and neutrons concentrated in a very small space. The neutrons are electrically neutral and the protons have a positive electrical charge (e+). Representation of the electron cloud in an oxygen atom Atomic nucleus made up of 8 protons+ 8 neutrons The electrons have a negative electrical charge (e-). For the atom to be electrically neutral, the number of protons must be exactly equal to the number of electrons in orbit. 8 electrons The protons and neutrons have almost the same mass. Their mass is a thousand times greater than the mass of an electron. A chemical element is defined by the number of protons it has (e.g. oxygen has eight protons). Atoms of a chemical element with different numbers of neutrons are isotopes of that element. © Digital Vision For example, in the hydrogen family, hydrogen itself has only one proton, while deuterium has one proton and one neutron, and tritium has one proton and two neutrons. Deuterium and tritium are both isotopes of hydrogen (see also The atom booklet). The science of the stars and the cosmos 11 > Nuclear astrophysics The science of the stars and the cosmos Electron cloud 11 > Nuclear astrophysics 5 > THE PRINCIPLE OF NUCLEOSYNTHESIS > THE PRINCIPLE OF NUCLEOSYNTHESIS Mendeleev’s periodic table of elements Period 1 1 2 H 1.00794 3 4 2 Li Be Na Mg 5 Al 22.9898 24.3050 21 19 20 Ca K 4 B C 7 8 N 9 O F 4.00206 10 Si P S Cl 26.9815 28.0855 30.9736 32.066 22 Sc 23 Ti V 24 Cr 25 Mn 26 Fe 27 Co 28 Ni 29 Cu 30 Zn 31 32 Ga Ge 33 34 As Se Ar 35.4527 39.948 35 Br 36 Kr 39.0983 40.078 44.956 47.88 50.942 51.996 54.9309 55.847 58.9332 58.69 63.546 65.39 69.723 72.61 74.9216 78.96 79.904 83.80 38 39 41 45 53 54 44 46 47 48 49 50 52 37 42 43 51 40 Rb 5 85.468 Sr Zr Y 87.62 88.906 Nb 58 57 La Ce Mo 92.906 91.224 Tc Ru Rh Pd Ag In Cd Sn Sb Te Xe I 127.60 126.905 131.29 101.07 102.906 106.42 107.868 112.411 114.82 118.710 121.75 (98) 95.94 59 71 69 63 61 65 67 68 60 66 70 64 62 Ho Lu Tb Pr Nd Pm Sm Eu Er Tm Yb Dy Gd 15 140.908 44.24 (145) 50.36 151.965 57.25 158.925 62.50 164.930 67.26 168.934 73.04 174.967 1 1 1 1 1 1 138.906 140.1 6 55 Cs 56 57 Ba 72 to 132.905 137.327 71 88 89 87 73 Hf Ta 74 W 75 Re 76 Os 77 Ir 78 Pt 79 Au 80 Hg 81 82 Tl Pb 83 Bi 84 Po 85 At 86 Rn (210) (222) 178.49 180.948 183.85 186.207 190.2 192.22 195.08 196.967 200.59 204.383 207.2 208.980 (209) 109 111 105 106 107 112 110 104 108 KEY Rf Fr to Db Sg Bh Hs Mt Uun* Uuu* Uub* Ra Atomic number = number of protons = number of electrons 1 272 277 268 (223) 226.025 103 (261.11) 262.11 263.12 264.12 265.13 269 H Symbol 1 1 91 93 99 95 97 01 03 1.00794 2 0 Atomic mass = number of protons 0 0 98 96 90 92 94 89 1 1 A Np Pu Pa Es Fm Md No Lr + neutrons = number of nucleons in the nucleus m Cm Bk Cf Ac Th U 38 231.036 38.029 237.048 (244) (243) (247) (247) (251) (252) (257) (258) (259) (260) The figures between brackets indicate 227.028 232.0 2 the mass number of the most stable isotope. * Names and symbols of these elements are temporary. According to Handbook of Chemistry and Physics, 7 th 74 Ed. 1993, CRC Press and Pure and Applied Chemistry, 1997, 69, 2471 • Abundance table of the elements H 10 10 A He C Ne N 10 6 C Mg Si Fe F 10 2 Ti P Li Ge B Se B Kr Kr Sn Be 0 Te Ba Pb Pt Dy 40 The science of the stars and the cosmos – C the most abundant nuclei after that are carbon C (with 12 neutrons and protons), oxygen O (16), neon Ne (20), magnesium Mg (24), silicon Si (26) and iron Fe (56). These are also the most stable nuclei in the Universe; D Zn 1 10 –2 Ni Na 10 4 The abundance table of the elements in the solar system • indicates, for every element in the periodic table ¶ , the quantity of this element to be found in the solar system. The reference basis for this is the abundance of one particular element, silicon. Its abundance was arbitrarily set at 106. The abundance of the other elements is given relative to the abundance of silicon in powers of ten: 10-2 = one hundredth, 1, 102 = 100, 104 = 10,000, 106 = 1,000,000 (a million), 108 = one hundred million, 1010 = ten billion. The table was developed from measurements and observations, and is of great value to astrophysicists. It shows that: – B there is a significant gap between helium He and carbon C, explained by the fragility of the nuclei of lithium Li, beryllium Be and boron B; Ar Ca Mendeleev’s periodic table of elements ¶ enables us to classify and name the different chemical elements discovered to date by the number of protons in the nucleus, from 1 for hydrogen to 92 for uranium and even higher for laboratoryproduced nuclei that do not exist in the natural state. It specifies the chemical properties of the elements, which depend on the number of electrons in the atom. – A the most abundant elements are hydrogen H and helium He (one gram of matter on average contains 98% of these). This is true for the whole of the observable Universe; 10 8 O PERIODIC TABLE AND ABUNDANCE TABLE Ne 10.811 12.011 14.0067 15.9994 18.9984 20.1797 13 14 15 16 17 18 6.941 9.0122 12 11 3 6 He 80 Yb Hf Hg Th U – D nuclei heavier than iron (Fe) are much more rare. Iron is the most stable nucleus in the Universe. © PhotoDisc ¶ Relative abundance (Si = 106) 6 A globular cluster is a concentration of thousands of stars bound by gravitation. very agitated state. They reach this state when they are brought to a very high temperature. So fusion naturally occurs in the extremely hot environment of stars, like the Sun. At the Sun’s core the temperature reaches 15 million degrees, making the fusion of the lightest nuclei such as hydrogen (one proton) and helium (two protons and two neutrons) possible. In more massive stars than the Sun, core temperatures are even higher, enabling the fusion of heavier nuclei than hydrogen. These reactions produce carbon, oxygen and iron nuclei. 120 160 200 240 Number of nucleons (protons and neutrons) in the nucleus 11 > Nuclear astrophysics The science of the stars and the cosmos 11 > Nuclear astrophysics 7 9 > THE PRINCIPLE OF NUCLEOSYNTHESIS STARS OPERATE ON THE PRINCIPLE OF THERMONUCLEAR FUSION. WHERE DOES ENERGY COME FROM? The result of the fusion of hydrogen in the Sun is that four hydrogen nuclei form a helium nucleus (see diagram on p.18). This releases energy. In this reaction the sum of the masses of the four original nuclei is greater than the mass of the final nucleus. According to the equation of equivalence between mass and energy, E = mc2, known as Einstein’s law, the missing mass ‘m’ has changed into energy, E. Where does this energy go? It is mainly emitted in the form of heat and light. The energy radiated in the form of light is enough to make the Sun shine, and the energy radiated in the form of heat is enough to maintain life on Earth (see also the Nuclear Energy: fusion and fission booklet). Paradoxically, the power emitted into space by the Sun is very weak, at only 0.2 microwatts per gram, i.e. 10,000 times less than the energy given off by a human being, which is a few milliwatts per gram. THE DIFFERENT TYPES OF NUCLEOSYNTHESIS The syntheses of atomic nuclei in different astrophysical environments may be defined as follows: • During the first three minutes of the Universe’s existence, the primordial nucleosynthesis took place. This explains the abundance of hydrogen, its isotope (see inset on p. 5) deuterium (1 proton, 1 neutron) and the two stable isotopes of helium (helium-3 [2 protons, 1 neutron] and helium-4 [2 protons, 2 neutrons]). The science of the stars and the cosmos The stars “The fusion of hydrogen in the Sun releases enough energy to make it shine and support life on Earth.” • The formation of certain slightly heavier nuclei such as lithium (Li), beryllium (Be) and boron (B) is explained by spallation reactions. • Within stars, A reaction characterized by the action of a natural flux of high fusion reactions energy particles present in space, occur that transcosmic radiation. This flux makes the heaviest nuclei present in the form atomic interstellar environment (carbon, nuclei in what is nitrogen, etc.) explode, and the nuclei produced (lithium, known as stellar beryllium, boron) are dispersed. nucleosynthesis. • Fusion reactions are not possible for nuclei heavier than iron, so these elements are more rare and their synthesis is due to a different type of nuclear reaction, neutron capture, which occurs in This is where a nucleus captures one or supernovae. more neutrons in succession. It then becomes unstable and disintegrates by Thus all the beta emission during which one neutron turns into a proton. This creates a heavier chemical nucleus (one extra proton). elements in the periodic table (see p. 6) are present in the Universe. © Nasa/A. Schaller 8 11 > Nuclear astrophysics The science of the stars and the cosmos 11 > Nuclear astrophysics 10 > THE STARS > THE STARS One aim of nuclear astrophysics is to understand how stars are born, live and die. WHAT IS A STAR? Stars are balls of very high temperature gas. This gas is ionized, which means that the negatively electrically charged electrons are totally or partially separated from the positively electrically charged nuclei. This gas is also known as plasma. With the naked eye or a telescope, we can only see the bright surface of the stars. In astrophysics, many discoveries have been made over recent years using ground-based telescopes or telescopes mounted on satellites. The entire electromagnetic spectrum is used, from radio waves to X- and gamma rays (see Radioactivity booklet), because each spectral range provides particular information: – infrared rays tell us where and how the stars and planets were formed; – visible light tells us about the different nuclear reactions produced within the stars throughout their life; – radio waves, X-rays and gamma rays show us the sometimes very violent phenomena that occur at the end of a star’s life: supernovae, pulsars, neutron stars and black holes. By interpreting the data from all these types of radiation, we can work out how much energy is produced by the star, what the temperature is on its surface, and its chemical composition. In stellar gas, hydrogen and helium are of all the chemical elements by far the most common, followed by oxygen, carbon and nitrogen. For every 1,000 billion hydrogen atoms, there are 100 billion helium atoms and approximately 1 billion oxygen atoms. © Hubble/AURA/STScI/Nasa WHY DO THE STARS SHINE? Globular cluster. The science of the stars and the cosmos The stars are generally stable objects within the Universe. A star is an enormous sphere of hot gas kept in balance by two opposing effects: – on the one hand, gravitation, which prevents the gas from dispersing and tends to attract particles to the center; – on the other, internal pressure due to thermal agitation of the gas, which counteracts this inward force. 11 > Nuclear astrophysics © D. Malin (AAO)/ROE/UKS Telescope “Stars are heavenly nuclear reactors.” Stars are born from the material left by other stars. Gravitation depends on the mass of the star and pressure depends on its temperature. The core of the star is extremely hot (several million degrees) but its surface is cooler (several thousand degrees). The temperature difference causes the flow of heat, and there- fore of energy, from the center towards the surface. It is this heat that is radiated by the star and makes it look to us as though it’s shining. Where does the star’s energy come from? It comes from the nuclear fusion reactions that take place in its center, which are also known as thermonuclear reactions. Because these reactions generate energy from matter – the nuclei that make up the star (essentially hydrogen) – the nuclei are known as “fuels”, by analogy with other forms of energy. Stars evolve by slowly burning up their fuel (hydrogen). The heat given off by the nuclear reactions prevents the star from collapsing, and gravity prevents it from dispersing. Stars shine for a long time because the thermonuclear reactions in their core release their enormous quantities of energy slowly. NON-EXPLOSIVE STAGES OF THERMONUCLEAR FUSION IN A STAR WITH 25 TIMES THE MASS OF THE SUN (25 MJ*) FUEL (raw material) TEMPERATURE (degrees) MOST ABUNDANT PRODUCTS OF FUSION LENGTH OF STAGE Hydrogen 20 million helium, nitrogen 7 million years Helium 200 million carbon, oxygen 500,000 years Carbon 800 million oxygen, neon, magnesium 600 years Neon 1.5 billion oxygen, magnesium, silicon 1 year Oxygen 2 billion silicon, sulphur 6 months Silicon 3.5 billion iron, nickel 1 day * Mass of the Sun = M = 1.991 x 1030 kg, compared with the mass of the Earth = 6 x 1024 kg; the mass of the Sun is 300,000 times greater than the mass of the Earth. J The science of the stars and the cosmos 11 > Nuclear astrophysics 11 > THE STARS > THE STARS Cross-section of a star Cross-section of the central part of a star, with a mass 25 times greater than the mass of the Sun (25 MJ), about to explode (according to the American physicist S. Woosley). J l Helium 3.7 M J Carbon 0.2 M THE BIRTH OF STARS J Oxygen 1.6 M J Neon 0.06 M J lMagnesium 0.02 M l Nebulae within galaxies are gigantic clouds of gas and dust. Gravitation or some external event can cause part of these clouds to contract. The mass of gas concentrates and the molecules collide; the temperature rises until hydrogen fusion occurs. A star is born. Astrophysicists can see the birth of stars because of the infrared radiation emitted by the stars through these clouds. l J l Silicon-Calcium 0.6 M J l Iron core 1.4 M EVOLUTION OF THE STARS (HERZSPRUNG-RUSSELL BRIGHTNESS AND COLOR DIAGRAM) 10+5 10+3 0.008 6 0.08 Red giants 10 +2 10 1 3.2 1.8 1.5 1.3 Main sequence Sun 1 0.7 10 -1 30 0.5 White dwarfs 10 0.4 2 4 6 13 70 0.3 -2 3, 00 0 5, 00 0 4, 00 0 6, 00 0 20 , 15 000 ,0 00 10 ,0 9, 00 0 8, 00 0 7, 00 00 0 10 -3 100 Time spent in main sequence (billions of years) 10+4 Red supergiants 17 This diagram represents the brightness of a star according to the temperature on the surface of the star in our galaxy (the reference basis for brightness is the brightness of the Sun = 1). We can see that the stars are grouped into several regions: • The central band known as the main sequence contains the stars in the longest phase of their life, when they are transforming hydrogen into helium in their core. • At the top right are the stars in the most advanced phases of nuclear combustion: fusion of helium into carbon and oxygen, and fusion of carbon into heavier elements, neon, magnesium and silicon. These are red giants and red supergiants. • At the bottom left are the white dwarfs, the final phase of stars with a small mass, like our Sun. Surface temperature (K) The science of the stars and the cosmos 11 > Nuclear astrophysics “The supernova: a very bright explosion marking the death of a star.” THE LIFE OF STARS Stars are self-regulating nuclear reactors. When a nuclear reaction flares up inside a star, the flexible gas core dilates slightly, the temperature falls and the reaction calms down. Conversely, when a reaction dies down, the core contracts, the temperature rises and nuclear reactions begin again. The star lasts as long as its core remains flexible and gassy. The life of a star is a succession of gravitational contractions and cycles of nuclear combustion. A star that is working well shines, and if it shines, it is slowly burning. The fuel cycle within a star is particularly efficient: the products of one combustion cycle are used as fuel for the next cycle. So helium, the product of hydrogen fusion, burns to give carbon and oxygen through thermonuclear fusion, and these are subsequently transformed into silicon. Gradually, the length of each cycle decreases considerably as the fuel provides less and less energy. THE DEATH OF STARS Stars with only a small mass, like the Sun, can only burn hydrogen and helium. Then, part of their outer layer is expelled, and they become The science of the stars and the cosmos © FORS Team/VLT/ESO l Brightness (Sun = 1) 12 Remains of the supernova 1054 (Crab nebula). white dwarfs – stars that have exhausted their nuclear resources. The most massive stars, between ten and hundreds of times bigger than the Sun, have higher temperatures at their center. Nuclear combustion is faster and goes further than helium combustion. Once the carbon fusion is over, a huge loss of energy resulting from a large emission of neutrinos triggered by the heat, literally Electrically neutral particles of low mass, which interact little with matter. exhausts the star. Thermonuclear combustion stops at iron, the most stable nucleus in the Universe. Iron is not combustible, so when it accumulates at the core of massive stars, it amounts to a death sen11 > Nuclear astrophysics 13 14 15 > THE STARS THE MAIN ELEMENTS OF SOLAR PHYSICS ARE NOW UNDERSTOOD. The Sun tence for them. Their core, having reached a great density, rebounds, producing a shock wave that sweeps through the surrounding matter. The implosion of the core is coupled with the explosion of the star. This star, now a supernova, emits an intense light that can be seen by astrophysicists when they observe the galaxy to which the star belongs. A neutron star or black hole remains at the center of the supernova. The stars in our galaxy are divided into two different populations distinguished by their chemical composition and their distribution in space: The science of the stars and the cosmos Supernova. • stars that contain heavy elements in the same proportions as the Sun, • stars that have a low abundance of elements heavier than helium (they may contain a thousand times less iron, for example). These constitute the globular clusters traveling around the galaxy. © PhotoDisc Globular cluster. © DigitalVision © PhotoDisc “There are two star populations in our galaxy.” 11 > Nuclear astrophysics The science of the stars and the cosmos 11 > Nuclear astrophysics > THE SUN > THE SUN “The Sun is the closest and best studied of the stars.” Internal structure of the Sun WHAT IS THE SUN MADE OF? The science of the stars and the cosmos Core Neutrino Photon trajectory (not to scale) Radiation flux Force of pressure (outwards) Gravitational force (inwards) Temperatures and densities within the Sun SOLAR NEUTRINOS The Sun’s oscillations are superficial movements of its surface. The areas moving closer to the observer are shown in blue; the areas moving away are shown in red. 11 > Nuclear astrophysics The science of the stars and the cosmos Density (g/cm3) 0 0.07 1.3 2.0 344,000 135 0 The neutrino is a particle in the same family as the electron. It carries energy and has a very low mass. Neutrinos are produced when protons change into neutrons. The neutrino flux detected on Earth, which is much smaller than the calculated neutrino flux, has concerned astrophysicists for a long time. But from precise measurements made by underground detectors (Gallex experiment in Europe, Superkamiokande experiment in Japan and SNO in Canada), we now know that the Sun is not responsible for the neutrino deficit. It is explained by the fact that these particles oscillate between three states, and that detectors, which are sensitive to only one of these states, can only capture a third of them. 160,000 The relative proportions of the various chemical elements in the solar system and the Sun are known from two main sources: • Analysis of the radiation emanating from the Sun’s photosphere. We cannot observe the inside of the Sun The bright surface of the star and the only observable part. directly. We analyze visible light, but also radiation that is invisible to the naked eye: radio waves, infrared, ultraviolet, X-rays and gamma rays. All these types of radiation make up the Sun’s spectrum. • Laboratory analysis of meteorites enables us to determine the isotopic composition of matter in the Composition in terms of isotopes, solar system. i.e. chemical elements whose atoms have the same number O v e r a l l , o n e of protons and a different number of gram of matter neutrons, e.g. carbon-12 (6 protons and 6 neutrons) and carbon-14 from the Sun (6 protons and 8 neutrons) are is made up two carbon isotopes. o f 0.70 g o f hydrogen, 0.28 g of helium and 0.02 g of all the other chemical elements in the periodic table (see p. 6). But observation is not enough. It must also be accompanied by theoretical studies of physics. the neutrino flux that transports energy from the center of the Sun to its surface, which physicists are now able to detect and study. This flux is the indicator that the solar nuclear reactor is operating correctly. As the Sun is a sphere of gas in perpetual movement, it oscillates. These oscillations are being studied, particularly with the help of the Golf satellite to which CEA made a major contribution. When the model has been finely adjusted and agrees with the observations, the internal properties of the Sun can be deduced. Stars whose initial mass and composition is different, can also be modeled in this way (by computer simulation). Computer simulation has a special place among tools for studying nucleosynthesis in the Big Bang and the stars. Depth (km) 690,000 Researchers are developing a physical model of the Sun. This enables us Conforms to the laws to determine the physical of physics. parameters (density, temperature, pressure, chemical composition, energy level, etc.) of the Sun at all depths, from the surface through to the core. The physical characteristics of the observable surface of the Sun calculated by the model – radius, brightness, and temperature – are compared and adjusted to match the actual observed characteristics of the Sun. Other quantities are used to check that the model is correct: one of the most relevant is 515,000 A MODEL OF THE SUN` The Sun is just one of the hundred billion stars in our galaxy, but it is the star closest to us, at about one hundred and fifty million kilometers away, and therefore the one we have observed best. © CEA/National Solar Observatory 16 Temperature (°K) 15 million 7 million 3 million 1.5 million 6,000 11 > Nuclear astrophysics 17 > THE SUN > THE SUN It can be used to reconstruct and trace the Sun’s evolution from its birth through to the moment when it will shed its outer layer and a compact, dense object, a white dwarf, will remain. The transformation of hydrogen into helium in the Sun Stage 1 LIFE EXPECTANCY OF THE SUN AND STARS Deuterium Neutrino: ν e+ Hydrogen fusion is enough to fuel the Sun throughout most of its luminous life. To determine its life expectancy, we need to compare its energy reserves with its energy consumption. Calculations using the model of the Sun indicate that the total nuclear energy available will run out after about ten billion years. This is twice the age of the oldest rocks on Earth and Stage 2 Helium-3 Deuterium Gamma radiation Stage 3 Helium-3 Helium-3 “The Sun, aged 4.6 billion years, is half way through its life.” the Moon and of meteorites (4.6 billion years). It is believed that all the bodies in the solar system were born at around the same time. So the Sun, aged 4.6 billion years, is half way through its life. CALCULATION OF THE SUN’S LIFE EXPECTANCY Helium-4 More than 10% of the Sun’s mass has been converted into helium. As a first approximation, we can assume that it originally consisted of pure hydrogen. As 0.7% of the mass of the hydrogen will be converted into energy by the formation of helium nuclei, the total quantity of energy the Sun has is J Enuc = 0.1 x 0.007 x M c2 = 2.10 x 1033 Mev. Like any other star, the Sun is a huge nuclear reactor. Nuclear fusion reactions take place in its core, during which hydrogen is changed into helium, releasing energy. The temperature in the center of the Sun is fifteen million degrees and the density is one hundred and fifty times that of water (150 g/cm3). The transformation of hydrogen into helium is complex, and takes place in three stages: • Stage one: two protons interact to form deuterium. During this process, one proton is changed into a neutron by emitting a positron (or positively charged electron) and a neutrino, a particle from the same family as the electron, which carries energy and has a very low mass. • Stage two: one deuterium nucleus combines with a proton to form helium-3, releasing energy in the form of a gamma ray (or photon). • Stage three: two helium-3 nuclei combine to form helium-4 by ejecting two protons. When all the hydrogen in the Sun’s core has transformed into helium, the Sun will contract under the force of gravitation. The temperature at its center will increase until it triggers the nuclear fusion of helium. The lifespan of the Sun is obtained by dividing the energy remaining by its consumption. We obtain the figure of approximately 10 billion years. An electron volt is a unit of measurement = 1.602 x 10-19 joules (1 MeV = 1 million electron volts). © Eso/ANTU/UT1 18 J M = solar mass = 1.991 x 1030 kg (see p. 11). Death of a star like the Sun. The science of the stars and the cosmos 11 > Nuclear astrophysics The science of the stars and the cosmos 11 > Nuclear astrophysics 19 20 > SUPERNOVAE SPECTACULAR BUT RARE, SUPERNOVAE ARE CATACLYSMIC EXPLOSIONS OF CERTAIN TYPES OF STARS. Supernovae From generation to generation of stars, the galaxy is becoming richer in heavy elements. The most powerful driving forces behind the chemical evolution of galaxies are supernovae. and of their spectrum. Supernovae can be classified by comThe spectrum is the analysis of emitted light; it can be used to pa ring these determine which chemical elements are present and their abundance. different data. WHAT IS A SUPERNOVA? THE DIFFERENT TYPES OF SUPERNOVAE A supernova is a star that explodes after the implosion, or collapse, of its core. It becomes as bright as billions of stars. Supernovae are the result of spectacular but rare events; there are about three per century in galaxies like ours. Supernovae are important for understanding our galaxy. They heat the interstellar environment, scatter heavy elements within it and accelerate cosmic rays (see pp. 23 and 24). The observation of supernovae is based on their light curve, i.e. the evolution of their brightness over time, of their maximum brightness Previously, the presence or absence of hydrogen in the spectrum was used to classify a supernova as one of two different types: I (absence of hydrogen) or II (presence of hydrogen). However, this traditional spectroscopic classification has recently been replaced by a physical distinction characterizing the way they exploded: thermonuclear or gravitational. THERMONUCLEAR SUPERNOVAE When two stars are in close proximity, they orbit around one another in a binary system. © Hubble/Aura/STScI/Nasa Explosion of a supernova Pre-explosive star The science of the stars and the cosmos 11 > Nuclear astrophysics Collapse of the core The science of the stars and the cosmos Interaction of the shockwave with the outer layer falling towards the core Explosive ejection of the envelope Expanding envelope 11 > Nuclear astrophysics 21 > SUPERNOVAE Thermonuclear supernovae occur in binary systems when one of the stars is a white dwarf. The matter from the first star falls on to the white dwarf, increasing its mass to 1.4 times that of the Sun. It collapses and explodes. All the matter is dispersed into space, and nothing remains at the center of the supernova. GRAVITATIONAL SUPERNOVAE A gravitational supernova occurs when a star explodes at the end of its life. It explains the formation of the heaviest elements in the Uni- > SUPERNOVAE verse. The same amount of energy is released in a single day as the Sun has released in the last three million years. It ejects vast quantities of gas and dust. When the core of a massive star implodes and it immediately sheds its outer layer, a fantastic amount of energy is released, essentially in the form of neutrinos. Only one ten thousandth of the total energy appears in the form of visible light. Depending on the initial mass of the star that exploded, the implosion of the iron core of a © Hubble Heritage Team/W. Blair/D. Malin/Nasa © Dr C. Burrows/ESA/STScI/Nasa Shockwave generated by a supernova. The science of the stars and the cosmos succession of neutron captures and disintegrations. The two types of supernova do not produce different elements in the same proportions, nor do they explode at the same rate (one thermonuclear supernova appears for every five gravitational ones). Gravitational supernovae effectively produce a number of elements between carbon and calcium, oxygen being the most abundant, while thermonuclear supernovae provide iron and its neighboring elements. According to estimates, approximately 50% of iron comes from this type of supernova. massive star leaves behind a dense object that can be identified as either a neutron star or a black hole. SUPERNOVAE, THE SOURCE OF HEAVY ELEMENTS SN1987A, THE SUPERNOVA OF THE CENTURY A supernova can be visible to the naked eye, from Earth, if it explodes within the perimeter of our own galaxy or in the Magellanic Clouds, our satellite galaxies. The last time this happened was on 24 February 1987, when the supernova named SN1987A appeared in the Large Magellanic Cloud. Because of its proximity, it meant that a vast array of scientific results could be collected. Observatories and satellites all over the world immediately pointed their instruments and detectors in its direction. Several types of radiation it emitted could be observed: visible light, radio waves, ultraviolet and infrared. And for the first time, a neutrino flux could be detected and measured. It was a gravitational supernova. “The study of supernovae tells us more about the rate of formation of stars at all stages in the evolution of the Universe.” 11 > Nuclear astrophysics At the center of massive stars that are set to become supernovae, the density is such that protons change into neutrons by capturing an electron. The ball of neutrons measuring about thirty kilometers in diameter that remains after the explosion, in place of the supernova, is a neutron star. The matter projected into space during the explosion is subject to a very large flux of neutrons escaping from the neutron star. The heaviest nuclei in nature (up to uranium) are formed in this way, through the rapid capture of neutrons by the nuclei originating in the different phases of combustion of the star, in the external layers of the supernova that is exploding. This phenomenon is known as explosive nucleosynthesis. For example, studies have shown how gold was produced in the Universe by a The science of the stars and the cosmos © J. Hugues/Nasa/CXC/SOA 22 Emission of X-rays from the Cassiopeia supernova. 11 > Nuclear astrophysics 23 25 > SUPERNOVAE THE INTEGRAL SATELLITE OPENS UP A NEW GOLDEN AGE OF NUCLEAR ASTROPHYSICS. Life cycle of stars Birth of a star Cosmic radioactivity seen by the Integral satellite Red giant Nebula White dwarf Supernova Neutron star Black dwarf Pulsar Black hole SUPERNOVAE, THE SOURCE OF COSMIC RADIATION The shock waves produced by supernovae stir, shake and heat the interstellar environment. As they pass they accelerate atomic nuclei and The science of the stars and the cosmos electrons and are the source of cosmic radiation, which, through the nuclear reactions it triggers as it passes, is itself responsible for generating light nuclei, lithium, beryllium and boron. 11 > Nuclear astrophysics © CEA 24 The science of the stars and the cosmos 11 > Nuclear astrophysics 26 > COSMIC RADIOACTIVITY SEEN BY THE INTEGRAL SATELLITE The hot radioactive remains of star explosions emit X-rays and gamma rays. This is what astronomers observe, because the most energetic part of the electromagnetic spectrum, known as gamma, provides the clearest indicators of the synthesis of atomic nuclei in the Universe. The study of radioactivity from the Milky Way and from neighboring galaxies by the Integral satellite (International Gamma-Ray Astrophysics Laboratory) has opened up a new golden age for nuclear astrophysics. The satellite is the result of a European collaboration run by ESA (European Space Agency). Launched in October 2002 at the Baikonur space center in Kazakhstan, using a Proton rocket, the satellite is in its data acquisition phase. The study of the radioactivity of the remains of supernovae and of stellar winds, using Integral, should enable us to fine-tune our star models and gain a better understanding of the dynamic processes that govern their evaporation and explosion. The aim of this scientific > COSMIC RADIOACTIVITY SEEN BY THE INTEGRAL SATELLITE mission is essentially to detect the gamma rays emitted within stars by short-lived radioactive elements such as aluminium-26, medium-lived radioactive elements like titanium-44 and longlived radioactive elements such as cobalt-56. It also provides an opportunity to locate the places in the galaxy where the greatest amount of nucleosynthesis is occurring. Measuring these should enable the identification of the isotopes emitting radiation, the estimation of their abundance and the physical conditions of their source environment. “CEA is using X- and gamma ray astronomy to study the explosions of stars and their radioactive remains.” NUCLEAR EVOLUTION © CEA © CEA Our galaxy is still evolving. Indicators of recent nucleosynthesis have been obtained by the observation of radioactivity in the disc of our galaxy. Detection of the disintegration of aluminium-26, in different directions, has allowed us to create a map of the galaxy. Aluminium-26 is a radioactive nucleus with a lifespan of 1 million years (whilst our galaxy has a lifespan of approximately 10 billion years). So nucleosynthesis has been analyzed to study with accuracy the mechanisms that, in particular, lead to the formation of aluminium-26. The aim of this study is to understand how this isotope can be produced by stars and ejected into the interstellar environment before it disintegrates, i.e. in less than a million years. It seems that its main sources are the Wolf-Rayet massive stars and the supernovae. Spectrometer during ground-based scientific calibration. The science of the stars and the cosmos 11 > Nuclear astrophysics The science of the stars and the cosmos 11 > Nuclear astrophysics 27