* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Genital herpes: neurologic complications
Survey
Document related concepts
Public health genomics wikipedia , lookup
2015–16 Zika virus epidemic wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Viral phylodynamics wikipedia , lookup
Transmission and infection of H5N1 wikipedia , lookup
Focal infection theory wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Infection control wikipedia , lookup
Canine distemper wikipedia , lookup
Marburg virus disease wikipedia , lookup
Transcript
Genital herpes: neurologic complications
By Marylou V Solbrig MD (Dr. Solbrig of the University of Manitoba has no relevant financial relationships to disclose.)
Originally released June 27, 1995; last updated June 5, 2016; expires June 5, 2019
Introduction
Overview
The author reviews the neurologic complications of genital herpes and herpes simplex virus type 2 (HSV-2) infections
in adults and neonates and summarizes the recommendations for acute and suppressive treatments from the
International Herpes Management Forum (IHMF). Illustrative cases of HSV-2 lumbosacral radiculomyelitis in an
immunocompromised patient and HSV-2 meningoencephalitis in a child are presented. This updated article discusses
the shifting epidemiology of genital herpes to reflect that herpes simplex virus type 1 (HSV-1) has become the most
common cause, current guidelines for the treatment of neonatal disease, and previously unrecognized surgical and
medical settings of reactivation in adults.
Key points
• HSV-1 and HSV-2 are prevalent viruses with the capacity to establish lifelong infections and
episodic reactivation. As all herpesviruses, HSV-2 performs 2 distinct genetic programs, lytic
replication, and latency, to produce primary and recurrent infections.
• The clinical presentation of HSV-2 infection of the CNS in adults is mainly meningitis, but
encephalitis, myelitis, and lumbosacral radiculitis occur.
• In immunocompetent adults, about 10% of cases of herpes simplex encephalitis are caused
by HSV-2, with the rest due to type 1.
• Recurring lymphocytic meningitis is most often a reactivation of HSV-2.
• HSV-2 infection is common. Currently HSV-2 seroprevalence is 17% in the United States (Xu
et al 2006). Annual crude incidence rate of HSV-2 CNS disease is 0.26 per 100,000 in
Denmark (Omland et al 2008) and of HSV-2 meningitis is 0.37 per 100,000 in Finland (Kupila
et al 2006).
• Within the past few years, HSV-1 has become the most common cause of genital herpes,
shifting the cause of neonatal herpes to HSV-1 in many parts of the world.
Historical note and terminology
“Throughout nature, infection without disease is the rule rather than the exception” (Dubos 1980) is a statement
appropriate to certain primary, latent, and recurrent herpetic syndromes. The term "herpes" was first used in ancient
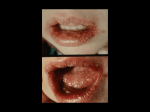
Greece for migratory (creeping or crawling) skin lesions. The ancient Greek historian Herodotus labeled mouth and lip
ulcers during fever "herpes febrilis" (Mettler 1947; Whitley and Schlitt 1991). Shakespeare referred to recurrent herpes
simplex virus labial lesions in Romeo and Juliet (Wildy 1973). In the 1700s, the French royal court physician introduced
genital herpes infections in the medical literature (Astruc 1736).
Transmissibility was established by passage of material from human lip and genital lesions to cornea or abraded skin
of rabbits. CNS transmission was demonstrated when Goodpasture showed that material from herpes labialis lesions
inoculated onto scarified rabbit cornea produced encephalitis (Goodpasture 1925). Herpes simplex virus was isolated
from a case of encephalitis in 1941 (Smith et al 1941), and 2 antigenic types of herpes simplex virus (HSV) were
recognized in 1968 (Nahmias and Dowdle 1968). Viral typing distinguished herpes simplex virus type 1 (HSV-1), which
is mainly responsible for infections “above the belt,” from herpes simplex virus type 2 (HSV-2), which is primarily
responsible for infections “below the belt,” although later studies have shown either virus can infect the mouth, genital
tract, or brain.
At present, 8 herpesviruses are known causes of human disease. They are herpes simplex virus types 1 and 2,
varicella-zoster virus, cytomegalovirus, human herpesvirus-6 and -7, Epstein-Barr virus, and Kaposi sarcoma virus
(human herpesvirus-8). HSV-2 causes a lifelong genital viral infection characterized by high rates of clinical and
subclinical reactivation in genital mucosa and risk of sexual transmission. Although HSV-2-associated syndromes such
as meningitis may have been under-recognized in the past, now widespread use of polymerase chain reaction
amplification of herpes simplex virus DNA has expanded recognized spectrum of HSV-2-related infections of the CNS.
Advances in laboratory detection, together with epidemiologic and pathogenesis studies, have enhanced
understanding of acquisition of infection, natural history of disease, and strategies for prevention.
Clinical manifestations
Presentation and course
Herpes simplex virus has biological properties - neurovirulence and latency - that affect human disease. The virus can
cause disease by direct CNS invasion and replication. Alternatively, this virus can infect sensory ganglia, become
latent, and provide a reservoir for reactivation with recurrent disease. A balanced household survey from 1999 to 2004
estimated that 17% of U.S. adults were infected with HSV-2 (Xu et al 2006).
Acute primary infection. Primary HSV-2 infections in adults, generally acquired through sexual contact, localizes
initially to the genital regions of both men and women (Corey and Spear 1986). Infection is associated with pain,
itching, dysuria, vaginal or urethral discharge, and tender inguinal adenopathy; vesicular, ulcerative, and then crusting
lesions develop over the external genitalia. Symptoms including fever, headache, or malaise, or signs of meningitis,
encephalitis, or lumbosacral radiculitis can accompany infection.
Meningitis. Symptoms of meningitis during primary genital herpes are reported in 36% of women and 11% of men
(Corey et al 1983). The average time between the development of genital lesions and hospitalization for meningitis is
9.3 days (Corey et al 1983). Although patients have fever, meningeal signs, and lymphocytic pleocytosis, the course of
meningitis is usually benign. Exceptions are cases of meningoencephalitis, chronic meningitis, and recurrent aseptic
meningitis.
The history and association of genital lesions and HSV-2 meningitis has varied across studies. Early studies showed a
history of genital lesions in about 40% of patients with HSV-2 meningitis and concurrent outbreaks in up to 86% (Tyler
and Adler 1983; Bergstrom et al 1990), and studies show approximately 4% of patients having genital outbreaks at the
time of meningitis (O'Sullivan et al 2003; Omland et al 2008; Miller et al 2013).
Meningoencephalitis. In immunocompetent adults, about 10% of cases of herpes simplex encephalitis are caused by
HSV-2, with the rest due to type 1 (Aurelius 1993). Cases of HSV-2 encephalitis more likely reflect primary infection
rather than reactivation (Aurelius 1993), with host immunity playing a role in disease expression. Studies have revised
downward percentages of HSV-2 encephalitis cases, with estimates of 2% to 6.5% of cases of herpes simplex
encephalitis due to HSV-2 in western countries (Berger and Houff 2008; Fernandez-Gerlinger et al 2015). A number of
cases of adult HSV-2 meningoencephalitis have been reported in patients with AIDS. In the immunosuppressed host,
the presentation of herpes encephalitis may be atypical as severe meningitis (Mommeja-Marin et al 2003) with
subacute but progressively worsening course (Schiff and Rosenblum 1998), with behavior abnormalities, and without
fever, headache, or CSF pleocytosis (Grover et al 2004). Risk factors in cancer patients are recent craniotomy,
radiotherapy to brain, chemotherapy, and corticosteroid use (Graber et al 2011). However, brainstem encephalitis
caused by primary HSV-2 infection can occur in immunocompetent adults (Tang et al 2003). In one review, an
estimated 21% of HSV brainstem encephalitis cases were caused by HSV-2 and 79% by HSV-1; the most common
clinical features were neuro-ophthalmologic and cranial nerve abnormalities (Livorsi et al 2010). Immunocompromise
by HIV, bone marrow transplant, rheumatoid arthritis treatment, or TNF-alpha inhibitors, was noted in a minority of
such patients. Traditionally, herpes simplex encephalitis as temporal lobe encephalitis has not been overly associated
with immunodeficiency, but some pediatric herpes simplex encephalitis cases have been linked to interferon-signaling
defects (Zhang et al 2007) and HSV-2 herpes simplex encephalitis to TNF-alpha inhibitor use in adults (Bradford et al
2009). The case of HSV-1 reactivation and encephalitis in a 67 year old with pneumococcal meningitis provides an
example of CNS bacterial-herpes coinfection (Ericsdotter et al 2015). The report raises the possibility that HSV-2
coinfections include more than cutaneous manifestations of coinfection, and CNS disease could be included in addition
to herpes labialis described in adults with acute bacterial meningitis (Weerkamp et al 2008).
HSV-2 encephalitis has also been noted after craniotomy, with most cases involving surgeries in frontotemporal areas,
pituitary gland in proximity to the trigeminal ganglion, or fifth cranial nerve decompression (Berger et al 2016). Other
risk factors are perioperative stress and steroids and factors contributing to immunosuppression.
As more patients with herpes simplex encephalitis are hospitalized early in their course, atypical presentations, such
as HSV-2 vasculitis-induced thalamic hemorrhage in a 72-year-old man, have been noted (Zepper et al 2012). A 57year-old woman with HSV-2 meningitis and vasculopathy producing hemorrhagic and ischemic stroke improved after
treatment with acyclovir and steroids (Snider et al 2014). Several cases of HSV-2 meningitis or encephalitis
complicated by ischemic stroke have been reported (Zis et al 2016).
Chronic meningoencephalitis. A single case of chronic, active granulomatous HSV-2 encephalitis was reported in an
immunocompetent 8-year-old who had been infected as a neonate (Brown et al 2010).
Lumbosacral radiculitis. HSV-2 sacral radiculitis (Elsberg syndrome) is characterized by acute urinary retention and
lumbosacral radicular dysfunction: constipation, erectile dysfunction, anogenital pain, paresthesias, loss of sensation,
or flaccid paresis of leg muscles. HSV-2 has been so frequently implicated as causing a self-limited syndrome of acute
urinary retention, root or cord dysfunction, and CSF pleocytosis that matches the descriptions of Elsberg more than 70
years ago (Elsberg 1931), that now HSV-2 sacral radiculitis and Elsberg syndrome have become almost synonymous.
Lumbosacral radiculitis during primary genital herpes infection is reported in 2% of patients (Corey et al 1983). Signs
referable to conus medullaris or lower thoracic cord may accompany infection. Diabetes, HIV infection, or malignancy
increase risks of developing ascending myelitis (Wiley et al 1987).
Reactivation and recurrent disease. Recurrent HSV-2 infection due to latent genital infection with reactivation from
sacral ganglia sites of HSV-2 latency can be either symptomatic or asymptomatic. The estimated rate of recurrence of
HSV-2 infection is an average of 0.3 to 0.4 times per month (Corey et al 1983), and several studies have reported
frequency of recurrence as high as 60% (Chang et al 1974; Adam et al 1979). Recurrence may be associated with a
shorter duration of viral shedding and fewer lesions.
Patients can have concurrent or separate episodes of mucocutaneous and neurologic symptoms, have only neurologic
recurrences, or have only mucocutaneous recurrences. Associated genital herpes is found in a majority of patients with
primary meningitis yet absent in nearly all patients with secondary meningitis, perhaps leading to underdiagnoses. An
estimated 60% of patients with polymerase chain reaction-confirmed recurrent aseptic herpes simplex virus meningitis
had no previous history of genital herpes (Tyler 2004).
The more severe the primary HSV-2 infection, the more likely and frequent the recurrent episodes of the disease
(Corey et al 1983; Langenberg et al 1999). Neurologic syndromes that accompany reactivation of latent herpesvirus
can be similar to those that developed during initial infection in that individual. An estimated 20% of patients with an
initial episode of HSV-2 meningitis will have recurrences (Bergstrom et al 1990). Recurrences, such as meningitis or
self-limited seizures, cranial nerve palsies, or pathological reflexes, tend to disappear after several episodes over a
period of years, but recurrence has been documented up to 28 years afterwards (Tyler and Adler 1983). Most cases of
recurrent aseptic meningitis, previously designated Mollaret meningitis (episodes of fever, headache, and stiff neck
lasting 2 to 5 days), are caused by HSV-2. PCR is most sensitive in detecting HSV-2 DNA from CSF if sampled 2 to 5
days after symptom onset (Kallio-Laine et al 2009). Now, diagnosis of Mollaret meningitis is used for cases of recurrent
aseptic meningitis of unknown etiology.
Lumbosacral radicular dysfunction with acute or subacute urinary retention can develop not only with initial genital
herpes but also due to reactivation of latent HSV-2 virus. Skin lesions need not be present for HSV-2-induced acute
urinary retention.
Up to 50% of patients with recurrent genital herpes infections can have a prodrome consisting of local hyperesthesias
and dysesthesias, which precedes appearance of genital vesicles by several hours or days, or pain radiating down into
the buttocks and hips, known as “sacral dermatomal neuralgia” (Corey et al 1983). Lumbosacral HSV-2 syndromes
include an acute sensory neuropathy with delayed sensory nerve conduction velocities (Siebert and Seals 1984),
stocking-glove distribution numbness and paresthesias, or unilateral sciatica (Morris and Peters 1974). Remission is
mostly complete in milder cases after one to several weeks, and antiviral treatment may shorten the symptomatic
period. Additionally, lumbosacral root involvement may complicate or follow recurrent HSV-2 meningitis (Bergstrom et
al 1990). In HIV-1-positive patients, herpetic lumbosacral radiculitis can be seen fairly early after AIDS onset, can occur
as an immune reconstitution syndrome, or can even lead to a new diagnosis of AIDS (Yoritaka et al 2005). Over 40% of
septic patients develop viremia with multiple viruses that include herpes family viruses released from latency (Walton
et al 2014). Investigation of herpesvirus DNA in CSF of patients with tick-borne encephalitis or enteroviral
meningoencephalitis revealed 2 instances of HSV-2 with enterovirus meningitis, interpreted as bystander reactivation
(Labska et al 2015).
In paraneoplastic “encephalitis” (ie, CNS inflammation caused by anti-Ri (ANNA-2) antibodies associated with small cell
cancer of the lung), HSV-2 DNA was found in CSF. In this case, HSV-2 was judged as reactivation and interpreted an
epiphenomenon of the inflammatory disease process (Novy et al 2009). An expanding literature associates HSV
encephalitis with NMDA receptor antibody encephalitis (Desena et al 2014; Venkatesan and Benavides 2015).
Genital herpes simplex virus type 1 in adults. Although most genital infections had been caused by HSV-2, a larger
proportion is now occurring due to HSV-1 (James and Kimberlin 2015). Genital HSV-1 infections tend to be less severe
and less likely to recur. A single case of recurrent myelitis attributed to herpes simplex virus infection was found to be
caused by type 1 (Shyu et al 1993).
Neonatal disease. Neonatal herpes simplex virus infection most often occurs during delivery, although infection can
also be acquired in utero or postpartum during the first 4 weeks of life. Acquisition of infection in late pregnancy,
prolonged rupture of membranes (greater than 6 hours), or use of fetal scalp monitors increase risk of transmission.
HSV-2 infection in neonates infected during or after birth may have skin, eye, and mouth disease only; CNS disease
with or without skin, eye, and mouth disease involvement; or disseminated disease.
HSV-2 disease occurs as skin, eye, and mouth disease (vesicles and keratoconjunctivitis); tremors; poor feeding;
temperature instability; bulging fontanelle; and pyramidal signs. Two weeks of age is the most likely time for neonatal
HSV encephalitis. Untreated with antivirals, 75% of babies with skin, eye, and mouth infection progress to CNS or
disseminated disease (Whitley et al 1980; Kimberlin 2005). Cutaneous vesicles are absent at presentation in about
40% of CNS cases. A history of genital lesions in the mother or her sexual partner is important in implicating HSV-2 as
a cause of encephalitis in the newborn.
Acute retinal necrosis (ARN). Acute retinal necrosis caused by HSV-2 may accompany or occur many years after
neonatal HSV-2 encephalitis or meningitis. Alternatively, acute retinal necrosis may follow subclinical infections. The
eye is red, vision is blurred, and confluent white patches in the peripheral retina are seen on funduscopic exam. The
opposite eye is affected in about one third of patients, but disease in the second eye may lag by months to several
years (Landry et al 2005). Specific diagnostic criteria include 1 or more foci of retinal necrosis, rapid progression,
circumferential spread, occlusive vasculopathy, and inflammation in the vitreous and anterior chamber (Gupta et al
2010). Early antiviral therapy limits extent of necrosis and decreases risk of retinal detachment and involvement of the
opposite eye.
Prognosis and complications
Although an episode of HSV-2 meningitis is usually self-limited, almost half of 40 consecutive patients had verified or
suspected neurologic recurrences during the first year after HSV-2 meningitis (Aurelius et al 2002). Recurrent episodes
of aseptic meningitis are also self-limited and resolve without long-term neurologic sequelae. In the series of patients
followed after their initial episode of HSV-2 meningitis, symptoms of urinary retention, dysesthesias, paresthesias,
headaches, and concentration difficulties, although common after the acute infection, had uniformly resolved within 6
months of the original illness (Bergstrom and Alestig 1990). The natural history of lumbosacral sensory and autonomic
symptoms that may accompany genital and anorectal HSV-2 infections is also resolution over a period of several days
to several weeks (Corey et al 1983; Goodell et al 1983), although most cases encountered today receive antiviral
treatment (Yamanishi et al 1998; Eberhardt et al 2004; Yoritaka et al 2005). Ascending myelitis has a variable course
and prognosis. Typically, patients have longer periods of disability, and although a few have recovered, others have
been fatal (Wiley et al 1987; Folpe et al 1994; Nakajima et al 1998). The factors responsible for variation in HSV-2
severity in apparently immunocompetent persons are incompletely understood. Host polymorphisms in innate immune
pathways have been associated with severe or fatal HSV-1 encephalitis [eg, NEMO NFKappaB essential modulator
deficiency (Niehues et al 2004), UNC-93B deficiency, a gene encoding an ER transmembrane involved in toll signaling
(Casrouge et al 2006), TLR3 deficiency (Zhang et al 2007), and others, reviewed by Koelle and Corey (Koelle and Corey
2008)]. These host genetic results raise the possibility of a role for manipulations of innate immunity in infection
control. The role of viral polymorphisms in disease severity is largely unexplored.
Maternal genital herpes infection may transmit disease to the baby. Neonatal herpes encephalitis may be acquired in
utero (5%), during delivery (85%), or during the first 4 weeks of life (10%) (James and Kimberlin 2015). All babies,
regardless of disease classification, should be considered at risk for CNS complications. Untreated, or receiving
ineffective antivirals (idoxuridine or cytosine arabinoside), neonates with CNS disease have a mortality rate of 50%,
with disseminated disease a mortality of 80%. With treatment, mortality for skin, encephalitis, and disseminated
disease is 0%, 5%, and 30% 24 months after treatment, and long-term neurodevelopment outcomes after CNS disease
remain poor (Whitley 2015). Prematurity is associated with higher mortality from CNS disease. Relapse may occur in a
small percentage of cases.
Recurrent disease may be associated with interferon pathway genetic defects and require lifelong suppressive
therapy. HSV-2 infection, as a genital ulcerative disease, is associated with acquisition of both HIV-1 and human T-cell
lymphotrophic virus type 1 (Nahmias et al 1990; Hook et al 1992). Infection with HSV-2 is associated with increased
genital shedding of HIV-1 RNA and HIV-1 transmissibility (Wald and Link 2002; Corey et al 2004).
IRIS, immune reconstitution inflammatory syndrome, has been documented with several other herpes family viruses:
varicella zoster virus, Kaposi sarcoma–associated herpes virus (HHV-8), and cytomegalovirus (French 2009; Muller et al
2010). IRIS is suggested as a mechanism of HSV-2 reactivation, shedding, and genital ulcer disease recognized in
Ugandan women initiating antiretroviral therapy (Tobian et al 2013). Like IRIS and more common among individuals
with higher viral loads, HSV-2 shedding was most common among women with the highest HIV load prior to
antiretroviral therapy.
Clinical vignette
A 43-year-old man with HIV presented with low back pain radiating to the legs, accompanied by progressive bilateral
leg weakness, for 2 weeks. He denied sensory loss and experienced 2 episodes of bowel incontinence. General
examination revealed an emaciated man with generalized decreased muscle bulk, and a resolving herpes lesion was
present near the anus. Motor exam and reflexes were normal in the arms. Tone was decreased in the legs with
moderate proximal and mild distal weakness, areflexia, and flexor plantar responses. Rectal tone was decreased.
Sensory exam was normal. He was unable to walk, even with assistance. MRI of the spine and MR neurogram of the
lumbosacral roots with contrast were normal. EMG and nerve conduction study showed evidence for bilateral
polyradiculopathy versus motor neuropathy. Laboratory investigations revealed mild anemia, normal creatine kinase,
erythrocyte sedimentation rate of 37 mm per hour, cytomegalovirus antigen negative, rapid plasma reagin
nonreactive, CD4 count 18 cells/mm3, and HIV viral load 62,000 copies/mL. CSF studies showed a mixed lymphocytic
and monocytotic pleocytosis with elevated protein (white blood cell count 498 cells/mm3 with 54% lymphocytes, 37%
monocytes, 3% neutrophils; red blood cell count 6 cells/mm3; protein 143 mg/dL; glucose 48 mg/dL). Ganciclovir was
started for possible cytomegalovirus or herpes simplex virus radiculopathy. CSF cytology; cytomegalovirus,
enterovirus, varicella-zoster virus, and herpes simplex virus-1 polymerase chain reaction tests; venereal disease
research laboratory test; West Nile antibody; and fungal and bacterial cultures were negative or nonreactive. HSV-2
polymerase chain reaction of CSF was positive. The patient's pain and weakness improved within days of intravenous
ganciclovir treatment (5 mg/kg IV over 12 hours). Treatment was modified to a 3-week course of intravenous acyclovir
(10 mg/kg IV over 8 hours) following results of CSF HSV-2 infection. Within 1 week, the patient walked with the
assistance of a walker. At follow-up 1 month later, on continued oral acyclovir (400 mg by mouth, twice daily), he
walked with a cane without pain and demonstrated marked improvement in leg strength.
Comment. This case demonstrates a pure motor lumbosacral polyradiculopathy associated with HSV-2 in a patient with
advanced HIV and may represent a more extensive version of the Elsberg variant of herpes simplex virus (lumbosacral
polyradiculopathy associated with genital herpes) (Eberhardt et al 2004). This clinical presentation illustrates overlap
with cytomegalovirus polyradiculitis associated with HIV. The final diagnosis relies on viral polymerase chain reaction
or culture. However, a CSF profile of lymphocytic (more common in herpes simplex virus) versus neutrophilic
predominance (more common in cytomegalovirus) (Miller et al 1996) may help to distinguish the viral infections but is
not pathognomonic. Finally, it is critical to exclude other infections, such as tuberculosis, neurosyphilis, and lymphoma.
The optimal type and duration of intravenous antiviral treatment for herpes simplex virus-associated polyradiculopathy
is not clearly established but depends on clinical response, possible antiviral resistance, severity of symptoms, and
drug tolerance (Yoritaka et al 2005). Our patient was treated with 3 weeks of intravenous acyclovir based on persistent
but slow recovery during this time and good tolerance to the drug. He continues on lifelong oral acyclovir given his
immunocompromised state.
Biological basis
Etiology and pathogenesis
Clinical syndromes are caused by infection with HSV-2, a double-stranded DNA virus. Latent genital infection with
reactivation is the largest reservoir for transmission of HSV-2, and most HSV-2 transmissions occur as a result of
asymptomatic shedding (Mertz et al 1988).
Genital route transmission is the usual way HSV-2 is acquired by adults. The virus replicates in the vaginal tract or on
penile skin with seeding of the sacral ganglia via virion transport by retrograde axonal flow. In most cases, after
another round of viral replication in ganglia, latency is established.
All herpesviruses have the ability to become latent, persist in inactive state for variable time periods, and be
reactivated by provocative stimuli. Latent virus has been recovered from trigeminal, sacral, and vagal ganglia of
humans by explant co-cultivation (Baringer and Swovland 1973; Baringer 1974). The sacral ganglia are the main but
not exclusive sites of HSV-2 latency. HSV-2 DNA at low copy number has been detected in ganglia throughout the
neuraxis (Berger and Houff 2008). Thus, HSV-2 viral DNA can be found in neuronal tissue in the absence of rash or
cutaneous lesions (Obara et al 1997). Aseptic meningitis may complicate either primary or recurrent disease, and HSV2 DNA can be identified in CSF of many patients with recurrent aseptic meningitis (Mollaret meningitis) (Tyler 2004).
Alternatively, replication during primary infection can produce CNS disease or systemic infection. The pathogenesis of
meningitic, radicular, myelitic, and encephalitis syndromes, especially as it relates to neuronal or hematogenous
mechanisms of the spread of virus, has been addressed in case reports, studies, reviews (Steiner 2011), and animal
studies (Allavena et al 2011).
Intra-axonal spread into spinal cord through the dorsal roots, and segmental spread by direct neuronal extension, was
suggested based on the absence of viral antigen or immunoreactivity in vessel walls of severe myelitis cases (Wiley et
al 1987). Spinal cord disease may be limited to a few segments and followed by recovery or, in immunosuppressed
patients, an ascending necrotizing myelopathy with coagulative necrosis of cord, inflammation, intraparenchymal viral
particles, and poor recovery is seen (Wiley et al 1987). The variable natural history and outcomes are consistent with a
role for host immunity in pathogenesis. Because transmission of HSV-2 infection through renal transplantation has
been reported (Dummer et al 1987), dissemination of competent virus by hematogenous spread also occurs.
Neonatal encephalitic disease yields examples supporting viral spread by neuronal and hematogenous routes.
Neuronal transmission would explain the focal CNS encephalitic disease without distal organ infection seen in some
neonates. On the other hand, hematogenous spread of virus is consistent with finding systemic, disseminated disease
and diffuse brain involvement. Any part of the brain may be affected, including grey and white matter of cerebrum,
cerebellum, or brainstem. Necrotizing encephalitis is accompanied by lymphocytic and monocytic infiltrates of
meninges and parenchyma. Nuclear inclusions, viral antigen, and DNA can be found during early infection, in the first
week.
A swollen, congested brain with or without parenchymal or ventricular hemorrhage is associated with acute
encephalitis. Long-term survivors show changes of cystic
encephalomalacia.{embed="pagecomponents/media_embed" entry_id="10109"}
Epidemiology"
The appearance of HSV-2 antibodies reflects acquisition of infection and correlates with onset of sexual activity. Latent
genital infection with subsequent reactivation is the largest reservoir for transmission of HSV-2. Reactivation of
infection may appear as skin vesicles or mucosal ulcers. However, subclinical reactivation in genital mucosa is also
associated with viral proliferation, shedding, and risk of sexual transmission.
Infection with HSV-2 is common. There are an estimated 417 million people aged 15 to 49 living with HSV-2 worldwide
(11.3% global prevalence), and 19.2 million aged 15 to 49 years are newly infected (0.5% of all individuals globally)
(Looker et al 2015). An estimated 45 million people in the United States have genital herpes infection, and new
infections occur at a rate of approximately 1 million per year. Roughly 85% to 90% of infections are unrecognized and,
therefore, undiagnosed (Leone 2005). Women, particularly those with multiple sex partners, have the highest rates of
infection. The highest prevalence of antibodies to HSV-2 in the United States was identified in female prostitutes (75%)
(Nahmias et al 1990). Factors that influence acquisition of HSV-2 include gender (greater for women than men), race
(more frequent in African Americans than whites), marital status (more for divorced than single or married), and
residence (more in cities than suburbs) (Whitley 2001). Meningitis is more common in women (36%) than men (13%)
after primary genital HSV-2 infection (Corey et al 1983), whereas lumbosacral involvement is more common in
homosexual men with HSV-2 proctitis (Goodell et al 1983). Previous herpes simplex virus-1 infection does not reduce
the rate of HSV-2 infection, but it does increase the likelihood of asymptomatic seroconversion, as compared to
symptomatic seroconversion (Langenberg et al 1999). Across several studies, no consistent relation between genital
herpes and HSV-2 meningitis was found (Miller et al 2013).
HSV-2 proved to be the second leading cause of aseptic meningitis cases in adults in a study from Finland. HSV-2
infection accounted for 17% of cases, second after the enterovirus family (26% of cases) (Kupila et al 2006).
A second Finnish study has shown high prevalence of HLA-DRB1*01 allele and low plasma immunoglobulin G1
concentration in patients with HSV-2-associated recurrent lymphocytic meningitis, suggesting these heritable factors
may predispose to recurrent meningitis (Kallio-Laine et al 2010).
The mechanisms of susceptibility and severity of HSV-2 infection are incompletely understood, but appear to involve
both innate and adaptive immune responses. Toll-like receptor 3 (TLR3) recognizes dsRNA and activates antiviral
immune responses by producing inflammatory cytokines and type I interferons. Now, 2 single nucleotide
polymorphism variations of TLR3 (enabling higher levels of TLR3 mRNA expression in response to HSV-2 stimulation)
have been found to be associated with reduced incidence of HSV-2 infection (Svensson et al 2012). The same
protective allele rs3775291 is also thought to confer natural resistance to tick-borne encephalitis virus (Kindberg et al
2011) and HIV-1 (Sironi et al 2012).
A synergy between HSV-2 and HIV-1 has been shown in clinical and epidemiological studies (Van de Perre et al 2008).
In HIV-1-infected persons, high rates of HSV-2 infection are also common, ranging from 50% to 90% in studies of HIVinfected populations around the world. Genital herpes in persons with HIV infection is associated with more severe and
chronic lesions, as well as increased rates of genital shedding of virus (Strick et al 2006). Biological mechanisms for
the HIV/HSV-2 comorbidity or epidemiological synergy have mainly focused on HSV-2-mediated inflammation. HSV-2
reactivation in an HIV-negative individual increases numbers of CD4+ cells recruited to genital HSV-2 lesions to serve
as targets for HIV (Zhu et al 2009), and HSV can stimulate HIV replication through cytokines released from HSVinfected cells (Palu et al 2001). In addition, HSV immediate-early gene products can increase HIV-1 transcription in
vitro (Lingappa and Celum 2007). Providing anti-HSV treatment for 3 months to co-infected persons not on antiretroviral therapy lowers the mean plasma HIV-1 RNA level by 0.53 log10, which could postpone the need for antiretroviral therapy (Nagot et al 2007), and reduces HIV-1 disease progression, as measured by a fall in CD4 counts
below 350 cells/microL (Lingappa et al 2010).
Several additional randomized trials are testing whether HSV-2 treatment can limit the spread of HIV. The results are
mixed. Although treatment with acyclovir 400 mg twice daily does not reduce HIV incidence or transmission,
suppressive acyclovir and valacyclovir reduces HIV levels in plasma, seminal fluids, and genital and rectal tracts
(Delany-Moretlwe et al 2009; Celum et al 2010). Although the demonstration of direct inhibitory action of
phosphorylated acyclovir on HIV-1 reverse transcriptase in a cell-free system offers new insights into analysis of these
results (Lisco et al 2008), future clinical trials will need to consider characteristics of acyclovir activity: (1) that the
direct effect of acyclovir on HIV-1 depends on the ability of HSV-2 to produce sufficient phosphorylated acyclovir to
suppress HIV reverse transcriptase, (2) host enzymes may differentially affect amounts of phosho-acyclovir, and (3)
different doses of acyclovir would produce different amounts of phospho-drug (Lisco et al 2009).
Neonatal herpes encephalitis is acquired in utero, during delivery, or during the first 4 weeks of life. The incidence,
previously estimated as approximately 1 in 3500 to 5000 live births, with about three quarters of neonatal herpes
simplex virus infections due to HSV-2, has dropped to an estimated 1 in 3200 live births in the U.S. (Whitley 1980;
Whitley 2015; Love and Wiley 2002). The per-delivery risk of neonatal infection is higher in maternal primary infection
in which IgG antibody is lacking than in maternal chronic infection, in which a mature IgG response can cross the
placenta. The difference implies protection by passively transferred antibody (Brown et al 2005).
The epidemiology of genital herpes infections has been changing, with increased proportions of primary infections (up
to 80% in some populations of young women) being HSV-1, which may translate to more babies with HSV-1 infections
(James and Kimberlin 2015; Whitley 2015).
Prevention
Preventing the neurologic complications of HSV-2 begins with preventing person-to-person transmission of the virus.
Suppressive antiviral therapy is valuable in managing some complications of recurrent genital herpes. The
International Herpes Management Forum (IHMF) now recommends that physicians offer suppressive valacyclovir
therapy (500 mg by mouth once daily) to immunocompetent individuals concerned about transmitting genital herpes
to a heterosexual partner and advises safer sex behavior (Corey and Ashley 2004). The U.S. Food and Drug
Administration has approved valacyclovir for prevention of HSV-2 transmission.
Because peak HSV replication occurs rapidly after reactivation of latent virus, there is a narrow window of opportunity
to prevent replication using an antiviral agent. A single-day, high-dose, patient-initiated therapy regimen of famciclovir
(1500 mg single dose or 750 mg twice daily, single day) is reportedly effective in the treatment of recurrent genital
herpes in immunocompetent adults and now is licensed (Patel et al 2007). Three-day treatments with valacyclovir, 2day courses with acyclovir, and 1- or 2-day courses with famciclovir can all be used in treating recurrences of genital
herpes (Corey et al 2007).
Acyclovir, valacyclovir, and famciclovir effectively suppress HSV-2 reactivation in persons co-infected with HIV-1 and
HSV-2 (Warren et al 2004; Strick et al 2006). The use of suppressive acyclovir to decrease HIV-1 transmission or
improve the clinical course of HIV-1 infection has become an additional important reason for early diagnosis and
treatment of HSV-2 infection in HIV-infected persons. To date, despite increasing use of suppressive acyclovir therapy,
there has been little increase in the detection of acyclovir-resistant HSV-2 isolates (Strick et al 2006).
Use of topical microbicides such as resiquimod, a toll-like receptor 7 and 8 agonist that induces interferon-alpha, is
under investigation for treating mucosal infection and prevention of HSV-2 infection (Mark et al 2007).
Strategies to prevent neonatal acquisition of HSV-2 infection include caesarean section delivery and use of suppressive
therapy in the seropositive partner of a seronegative gravid woman. For pregnant women with active genital herpes,
caesarian section performed within 4 hours of membrane rupture reduces the rate of neonatal herpes simplex virus
infection from 7.2% to 1.5% in comparison to vaginal delivery (Brown et al 2003). Recommendations for latepregnancy use of acyclovir or valacyclovir in the Obstetrics literature have shown that acyclovir administered from 36
weeks of gestation through delivery decreases the incidence of outbreaks of HSV-2, viral shedding at delivery, and
need for Caesarian section (Scott et al 2001; Sheffield et al 2006). Current American Academy of Pediatrics guidelines
state that if the mother has a primary infection in the third trimester, 14-day prophylactic treatment is administered
(American Academy of Pediatrics 2015). If the mother has a history of genital herpes, surface cultures are performed
within 24 hours of delivery. If positive, the infant is treated for 14 days for skin and 21 days for organ involvement. In
the United Kingdom, the Royal College of Obstetricians and Gynaecologists recommends caesarian section only for
women with a first episode of genital herpes at the time of delivery, and not for women with a first episode of genital
herpes in the first or second trimester. Elective caesarian is considered at term for women with a first episode of
genital herpes within 6 weeks of the expected date or onset of preterm labor (Royal College of Obstetricians and
Gynaecologists 2002; Steiner et al 2007).
Vaccination. Although HSV-2 infections can be controlled by the use of orally bioavailable antiviral drugs, these
agents do not cure the individual. Antiviral drugs treat actively replicating virus, limit subclinical shedding, and prevent
transmission of HSV-2 within couples. As such, antivirals do not affect latent virus and are not completely effective.
Because HSV-2 can cause severe recurrent disease and establish lifelong infection, and because there may be linkage
of HSV-2 and HIV-1 control (Nagot et al 2007), HSV-2 vaccines are in development (Johnston et al 2016).
The objective of a prophylactic vaccine will be to induce sterilizing immunity effective at all portals of HSV entry
(genital mucosa, facial mucosa, eye). HSV in genital lesions and vaginal/cervical secretions exists as a cell-free virus,
so that vaccine induction of high levels of neutralizing antibody would be required. A difficulty is that a vaccine that
provides effective immunity against HSV-2 must produce an immune response, such as a strong neutralizing antibody
response, that exceeds the response produced with natural infection. One vaccine that has entered clinical trials is a
recombinant glycoprotein vaccine, an alum and monophosphoryl lipid A-adjuvanted subunit glycoprotein D2 vaccine
(gD-alum-MPL) containing truncated gD2 in a novel lipid adjuvant. Activity in the prevention of HSV-2 infection and
disease in HSV-2-uninfected women was investigated in a phase III clinical trial (Stanberry 2004; Koelle 2006). Early
results showed the glycoprotein D vaccine had efficacy against genital herpes in women who were seronegative for
both HSV-1 and HSV-2 at baseline, but not in those who were seropositive for HSV-1 and negative for HSV-2. The
vaccine elicited antibody and CD4 T cell responses but had no efficacy in men, regardless of their HSV sero-status
(Stanberry et al 2002). A large follow-up study in a population representative of the general population of HSV-1 and
HSV-2 seronegative women concluded that prophylactic vaccine failed to prevent HSV-2 infection and disease. The
investigational vaccine was effective in preventing HSV-1 genital disease and infection but not in preventing HSV-2
disease or infection (Belshe et al 2012).
The objectives of a therapeutic vaccine will be to prevent recurrences or minimize their severity and duration. Virus
reactivation in the ganglion should be prevented, or virus replication after leaving the nerve should be limited. To
accomplish this, the vaccine should enhance the host's specific immune responses. Probably the therapeutic vaccine
will have to boost different immune responses than that of a prophylactic vaccine. The success of prophylactic
vaccines has not been observed in therapeutic vaccine trials when the same prophylactic vaccines were used
(Ramachandran and Kinchington 2007).
Early results from ongoing trials of postexposure therapeutic vaccines against genital HSV-2 infection show less HSV-2
shedding in vaccine recipients compared to placebo (Looker et al 2015). These are preliminary results for GEN-003
(Genocea), which is based on viral antigens ICP4 and gD2, and HerpV (Agenus), which contains recombinant HSP-70
and 32 HSV-2 antigens.
Other vaccine formats, including attenuated live or replication-incompetent HSV-2 strains and technologies that target
virus-specific CD8 T-cell responses, are being developed (Koelle 2006). Vaccines that elicit both effector/protective
antibodies and adaptive T cell responses probably offer the best hope for developing effective immunogens. Some of
the strategies for HIV-1 vaccines, such as inclusion of fusion intermediates as targets for antibodies and the use of viral
vectors to elicit CD8 T cell responses, may be adapted for HSV vaccine development (Koelle and Corey 2008).
Optimistic estimates for an HSV-2 vaccine are in 10 years' time. Over 20 years in most model scenarios, fewer than
100 prophylactic vaccinations against HSV-2 would be required to avert 1 HIV infection (Freeman et al 2009).
Transfusion. Although HSV-2 transfer by transfusion has been considered very rare, HSV-2 is joining cytomegalovirus
and HHV-8 as herpes viruses of concern in transfusion medicine. Due to the ability to detect herpes simplex virus DNA
in plasma of patients with primary herpes genitalis, deferral of blood donations from individuals with primary herpes
infections is recommended (Juhl et al 2009). The current consensus is that blood donors with recurrent herpes simplex
virus infection are probably not at risk of transmitting herpes simplex virus.
Differential diagnosis
The differential diagnosis is of a meningitic, encephalitic, lumbosacral radicular, or myelitic syndrome and
inflammatory CSF with a mild-to-moderate lymphocytic pleocytosis (10 to 500 WBC/µl), mildly elevated protein (less
than 100 mg/dl), and normal or depressed glucose. CSF glucose may be depressed in patients with HSV-2, mumps,
varicella-zoster virus, and lymphocytic choriomeningitis virus; CSF glucose levels below 25 mg/dl should prompt a
search for bacteria, fungi, sarcoid, or carcinomatous meningitis.
The differential diagnosis of a localized viral exanthem and aseptic meningitis includes varicella-zoster virus, and more
generalized rashes with meningitis include measles, rubella, enteroviruses (particularly Echo 9), B19 parvovirus, and
dengue. Other agents producing rash and aseptic meningitis are syphilis, Lyme borreliosis, leptospirosis, and rickettsial
diseases.
HSV-2 meningitis is distinguished from aseptic meningitis caused by other viruses, or by spirochetes, Chlamydia,
Rickettsia, mycoplasma, Brucella, Ehrlichiae, partially treated bacterial meningitis, parameningeal infection,
tuberculosis, fungal meningitis, endocarditis, postviral or vaccination meningeal reaction, drugs, leaking epidermoid
cyst, posttraumatic skull defects, collagen vascular diseases, and subarachnoid hemorrhage based on CSF, serum, and
neuroimaging studies.
Recurrent HSV-2 meningitis is distinguished from chronic meningeal processes with recurrent episodes of symptomatic
meningitis (spirochetes, brucella, fungi), idiopathic inflammatory conditions (sarcoid, Behçet disease, Vogt-Koyanag-Harada syndrome), chemical meningitis, or drug reaction (nonsteroidal anti-inflammatories, trimethoprimsulfamethoxazole) by history, CSF, serum, and neuroimaging studies.
Acute encephalitis in adults due to herpes simplex virus type 1 and type 2 may have similar focal or multifocal
patterns. Therefore, polymerase chain reaction procedures capable of detecting both herpes simplex virus types 1 and
2 in CSF are used. Instead of the single polymerase chain reaction tests, the multiplex or consensus-herpes
polymerase chain reaction for simultaneous detection of a number of human herpesviruses has been gaining ground in
diagnostics (Tenorio et al 1993; Calvario et al 2002). The tests are particularly useful in evaluating HIV-infected
patients with neurologic disorders related to human herpesviruses (Quereda et al 2000).
Besides HSV-2, viral causes of lumbosacral radiculomyelitis include herpes simplex virus-1, cytomegalovirus, EpsteinBarr virus, varicella-zoster virus, human T-cell lymphotropic virus type 1, West Nile virus, and tick-borne encephalitis
viruses; bacterial causes include syphilis and tuberculosis. Lumbosacral root hyperintensity on T2-weighted MRI
images or contrast enhancement is not specific for viral radiculitis, as it also occurs with Guillain-Barré, lymphoma, and
metastatic malignant disease.
The differential diagnosis of the newborn with CSF pleocytosis and elevated protein includes CSF examination for
herpes simplex virus types 1 and 2, as well as other bacterial and viral infections. Infants with HSV-2 encephalitis show
diffuse bilateral disease or periventricular white matter lesions and meningeal enhancement using MRI (KleinschmidtDeMasters et al 2001). A history of genital lesions in the mother or her sexual partner can be helpful.
Viral causes of acute retinal necrosis in childhood include varicella-zoster virus and cytomegalovirus, as well as HSV-2.
Many cases of acute retinal necrosis caused by HSV-2 have been reported in children, teenagers, and young adults as
a result of reactivation of congenital or neonatal infections, which may have been subclinical.
HSV-1 or -2 encephalitis in glioma patients may be suspected in patients with hyperthermia, rapid change in mental
status to coma, or appearance of new epileptic foci not corresponding to the site of the primary tumor. Diffusionweighted MRI and CSF HSV polymerase chain reaction will aid in the differential diagnosis (Berzero et al 2015).
Diagnostic workup
For adult meningitis, encephalitis, myelitis, or radiculitis cases, diagnostic evaluation is directed toward confirmation of
viral etiology by polymerase chain reaction analysis of CSF for HSV-2 DNA and exclusion of other causes of
lymphocytic or monocytic CSF pleocytosis. Detection of polymerase chain reaction-amplified viral nucleic acid in the
CSF is supported by virus isolation or serology (such a rise in serum HSV-2 immunoglobulin G or proof of intrathecal
HSV-2 immunoglobulin G synthesis). HSV-2 can be isolated from the CSF of many patients with primary HSV-2-induced
meningitis using standard virological techniques but rarely during recurrent meningitis (Bergstrom and Alestig 1990).
Neuroimaging and EMG nerve conduction velocity studies are performed as needed.
Herpes simplex virus polymerase chain reaction of genital lesions is more sensitive than viral culture in determining
etiology of genital ulcer disease. For CNS tissue specimens, the absence of inclusions by light microscopy does not
exclude HSV-2. HSV-2 myelitis and encephalitis have occurred without demonstrating inclusions in biopsy or
postmortem material (Wiley et al 1987).
Neonatal herpes may occur in the absence of skin lesions, so if the infection is suspected, swabs of the oropharynx,
conjunctiva, rectum, skin lesions, mucosal lesions, and urine should be taken and sent for virus culture. CSF should be
sent for polymerase chain reaction detection of herpes simplex virus DNA. Evidence for disseminated infection
includes liver function tests, complete blood count, CSF, and chest x-ray (Kimberlin 2005). The genomes of HSV-1 and
HSV-2 have approximately 50% homology. HSV type differentiation can be by type-specific glycoprotein antibody
response, restriction endonuclease fingerprinting, and DNA sequencing (James and Kimberlin 2015).
Management
Management is symptomatic, along with empiric antiviral and antibiotic treatment, until a diagnosis is available.
Adjunctive steroid therapy is used in select brainstem encephalitis cases (Livorsi et al 2010) or in edema cases with
risk of herniation or CSF block.
Anecdotal evidence has suggested that acyclovir can be effective in the treatment of HSV-2 meningitis, whereas more
recent reviews report lack of efficacy of suppressive antiviral treatment of Mollaret meningitis (Whitley 2015).
Treatment of primary or recurrent meningitis in the presence of HSV-2 genital lesions with intravenous acyclovir (5 to
10 mg/kg 3 times daily) for 7 to 10 days can shorten the symptomatic period, improving signs and symptoms of
meningitis within 72 hours. Oral agents may also work in such cases (Tyler 2004). Meningitic doses are lower doses
than recommended by the IHMF for herpes simplex encephalitis (acyclovir 10 mg/kg every 8 hours for 14 to 21 days)
outside the newborn period. Differentiation of HSV-2 from type 1 in adult herpes simplex virus encephalitis may lead to
prolonged acyclovir treatment, potentially followed by prophylaxis to prevent symptomatic relapse with HSV-2. At the
close of encephalitis treatment, polymerase chain reaction assessment of CSF is recommended to verify response to
treatment and elimination of replicating virus (Tyler 2004).
Intravenous acyclovir 10 mg/kg every 8 hours for 10 to 14 days has been the consensus treatment for patients with
severe or progressive herpetic radiculomyelitis or for immunocompromised patients (Eberhardt et al 2004). However,
there has been no evidence of improvement from controlled clinical trials. If response to therapy is poor and the
isolate is found to be resistant to acyclovir, intravenous foscarnet (a non-thymidine-kinase dependent agent) is
indicated because all acyclovir-resistant strains are resistant to valacyclovir and most are also resistant to famciclovir
(Strick et al 2006). Valacyclovir is biotransformed to acyclovir and L-valine by first-pass intestinal or hepatic
metabolism. Famciclovir, a synthetic acyclic guanine derivative, is the prodrug of the active antiviral penciclovir.
Resistance to nucleoside analogues is most commonly due to deletion of the HSV thymidine kinase (tk) gene. Less
often, resistance is due to HSV-tk gene or viral DNA polymerase mutations (Field 1989). Infrequent case reports of
resistance to acyclovir link resistance to very high viral loads (105 or greater) in CSF (Whitley 2015). HSV clinical
isolates are quasispecies composed of acyclovir-sensitive and -resistant variants, with resistant variants presumably
achieving critical numbers in these cases.
Foscarnet, a structural mimic of the anion pyrophosphate that inhibits the pyrophosphate binding site of viral DNA
polymerase, is not activated by HSV-tk. Foscarnet 40 mg/kg IV every 8 hours is added to acyclovir in encephalitis
cases of suspected acyclovir resistance (Schulte et al 2010). Resistance to nucleoside analogues previously estimated
as rare (less than 5%), without significant increase since acyclovir was introduced more than 20 years ago (Lingappa
and Celum 2007), could ultimately be higher in immunocompromised patients receiving long-term prophylactic
treatment with acyclovir (Chen et al 2000). Cidofovir, another nucleoside analogue, phosphorylated to its active form
by cellular enzymes, is another second-line drug (Naesens and de Clercq 2001). HSV-1 deficient strains are thought to
be less neurovirulent (Schulte et al 2010).
The IHMF recommends that neonates with suspected herpes simplex virus infection be treated with intravenous
acyclovir (20 mg/kg) every 8 hours for 21 days. If the disease is localized to skin, eyes, and mouth, treatment is for 14
days, but neuroimaging is indicated even with mild (skin, eye, or mouth) presentations of disease (Whitley 2015). The
neutrophil count for children receiving intravenous acyclovir should be monitored, and decreasing the dose or
administering granulocyte colony stimulating factor should be considered for absolute neutrophil counts below
500/mm3. At the end of therapy in CNS and disseminated disease, polymerase chain reaction assessment of the CSF is
recommended and treatment continued if the child remains polymerase chain reaction positive at this site (Kimberlin
2005). Following IV acyclovir treatment, oral acyclovir treatment (300 mg/m2 orally every 8 hours) should be
administered for at least 6 months, treating persistent low-level replication of HSV in the young brain (Kimberlin et al
2011). In suspected or proven HSV disease in neonates, when acyclovir is not available, it is recommended that the
patient should be treated with intravenous ganciclovir (6 mg/kg) every 12 hours, and intravenous foscarnet (60 mg/kg)
every 12 hours can be used as second-line therapy (Wang and Smith 2014).
Shorter episodes or resolution of Mollaret meningitis with antiherpes treatment have been reported. However, therapy
does not affect the viral reservoir in dorsal root ganglia.
Mostly, treatment of herpes simplex infections has relied on nucleoside analogues with similar mechanisms of action
that inhibit the HSV DNA polymerase after phosphorylation by viral thymidine kinase. These were developed 30 years
ago. Thiazolylamide pritelivir (BAY 57-1293) is the first of a new class of antiherpes viral agents that inhibit viral
replication by targeting the viral helicase-primase enzyme complex, and shows efficacy in suppressing viral shedding
and lesion development in patients with genital herpes (Wald et al 2014). Preclinical studies also are showing
synergistic activity of another helicase-primase inhibitor amenamevir (ASP2151) with nucleoside analogues against
HSV-2, raising the possibility of combination therapy for treating severe disease and infections suspected to be caused
by nucleoside analogue-resistant viral variants (Chono et al 2013). The potential for combination therapy for those
with life-threatening HSV infections (beyond foscarnet and cidofovir) may now be closer.
Mostly, treatment of herpes simplex infections has relied on nucleoside analogues with similar mechanisms of action,
which inhibit the HSV DNA polymerase after phosphorylation by viral thymidine kinase. These were developed 30
years ago. The thiazolylamide pritelivir (BAY 57-1293) is the first of a new class of antiherpes viral agents that inhibit
viral replication by targeting the viral helicase-primase enzyme complex and shows efficacy in suppressing viral
shedding and lesion development in patients with genital herpes (Wald et al 2014). In the case of genital infections,
resistance may be suspected when lesions last for more than 1 week after initiating antiviral treatment, or new
satellite lesions appear during treatment.
Preclinical studies also are showing synergistic activity of another helicase-primase inhibitor amenamevir (ASP2151)
with nucleoside analogues against HSV-2, raising the possibility of combination therapy for treating severe disease and
infections suspected to be caused by nucleoside analogue-resistant viral variants (Chono et al 2013).
Although the potential for combination therapy for patients with life-threatening HSV infections (beyond foscarnet and
cidofovir) is approaching, combination therapy with agents with matched pharmacodynamics and different molecular
targets also will require strategies to manage herpesvirus infections in immunosuppressed hosts requiring long-term
treatments. Potential regimens consist of a DNA polymerase inhibitor supplemented with a helicase-primase inhibitor
and potentially a third target inhibitor (James and Prichard 2014). For example, HIV integrase inhibitors block
replication of alpha, beta, and gamma herpesviruses in vitro (Yan et al 2014). Because immunocompromised patients
are at risk for multiple herpesvirus infections, a broad-spectrum agent, such as brincidofovir, would also be considered
in prophylactic regimens (James and Prichard 2014).
Special considerations
Pregnancy
Clinical manifestations of recurrent genital herpes infections are similar in pregnant and nonpregnant women (Corey et
al 1983). Differences in the frequency of neurologic manifestations from HSV-2 infection during pregnancy are not
known. Use of acyclovir suppression to prevent clinical recurrences and use of acyclovir or valacyclovir prophylaxis
during late pregnancy (after 36 weeks of gestation) to prevent recurrent herpes at delivery are evaluated in several
manuscripts (Scott 1999; Scott et al 2001; Sheffield et al 2006) and presented in the other sections of this article.
Based on combined data of acyclovir and valacyclovir, there does not appear to be any major risk to the fetus from
acyclovir or valacyclovir (Briggs et al 2002). The manufacturer of famciclovir maintains a pregnancy registry to monitor
maternal-fetal outcomes of women exposed to famciclovir during pregnancy. Maternal genital infection may be
asymptomatic or subtle, accounting for the nearly 80% of women who vertically transmit HSV to the newborn without
a history of genital herpes lesions (James and Kimberlin 2015).
References cited
Adam E, Kaufman RH, Mirkovic RR, Melnick JL. Persistence of virus shedding in asymptomatic women after recovery
from herpes genitalis. Obstet Gynecol 1979;54:171-3. PMID 223095
Allavena RE, Desai B, Goodwin D, Khodai T, Bright H. Pathologic and virologic characterization of neuroinvasion by
HSV-2 in a mouse encephalitis model. J Neuropathol Exp Neurol 2011;70(8):724-34. PMID 21760533
American Academy of Pediatrics AAP Committee on Infectious Diseases. Red book. 29th ed. Elk Grove Village IL:
American Academy of Pediatrics, 2015.
Astruc J. De morbis venereis libri sex. Paris: G Cavelier, 1736:361.
Aurelius E. Herpes simplex encephalitis. Early diagnosis and immune activation in the acute stage and during longterm follow-up. Scand J Infect Dis Suppl 1993;89:3-62. PMID 8235453
Aurelius E, Forsgren M, Gille E, Skoldenberg B. Neurologic morbidity after herpes simplex virus type 2 meningitis: a
retrospective study of 40 patients. Scand J Infect Dis 2002;34:278-83. PMID 12064691
Baringer JR. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med 1974;291:828-30. PMID
4371760
Baringer JR, Swovland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med
1973;288:648-50. PMID 4347057
Belshe RB, Leone PA, Bernstein DI, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med
2012;366(1):34-43. PMID 22216840
Berger JR, Houff S. Neurological complications of herpes simplex virus type 2 infection. Arch Neurol 2008;65(5):596600. PMID 18474734
Berger AR, Shahar T, Margalit N. Herpes simplex type 2 encephalitis after craniotomy: case report and literature
review. World Neurosurg 2016;88:691.e9-691.e12. PMID 26724620
Bergstrom T, Alestig K. Treatment of primary and recurrent herpes simplex virus type 2 induced meningitis with
acyclovir. Scand J Infect Dis 1990;22:239-40. PMID 2162558
Bergstrom T, Vahlne A, Alestig K, Jeansson S, Forsgren M, Lycke E. Primary and recurrent herpes simplex virus type 2induced meningitis. J Infect Dis 1990;162:322-30. PMID 2165105
Berzero G, Di Stefano AL, Dehais C, et al. Herpes simplex encephalitis in glioma patients: a challenging diagnosis. J
Neurol Neurosurg Psychiatry 2015;86(4):374-7. PMID 24876188
Bradford RD, Pettit AC, Wright PW, et al. Herpes simplex encephalitis during treatment with tumor necrosis-alpha
inhibitors. Clin Infect Dis 2009;49:924-7. PMID 19681709
Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. A Reference Guide to Fetal and Neonatal Risk
(sixth edition). Philadelphia: Lippincott Williams and Wilkins, 2002.
Brown WD, Bearer EL, Donahue JE. Chronic active herpes simplex type 2 encephalitis in an asymptomatic
immunocompetent child. J Child Neurol 2010;25:901-8. PMID 20179002
Brown ZA, Gardella C, Wald A, Morrow RA, Corey L. Genital herpes complicating pregnancy. Obstet Gynecol
2005;106(4):845-56. PMID 16199646
Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission
rates of herpes simplex virus from mother to infant. JAMA 2003;289(2):203-9. PMID 12517231
Calvario A, Bozzi A, Scarasciulli M, et al. Herpes consensus PCR test: a useful diagnostic approach to the screening of
viral diseases of the central nervous system. J Clin Virol 2002;25 Suppl 1:S71-8. PMID 12091084
Casrouge A, Zhang SY, Eidenschenk C, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science
2006;314(5797):308-12. PMID 16973841
Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N
Engl J Med 2010;362:427-39. PMID 20089951
Chang TW, Fiumara NJ, Weinstein L. Genital herpes. Some clinical and laboratory observations. JAMA 1974;229:544-5.
PMID 4365597
Chen Y, Scieux C, Garrait V, et al. Resistant herpes simplex virus type 1 infection: an emerging concern after
allogeneic stem cell transplantation. Clin Infect Dis 2000;31(4):927-35. PMID 11049772
Chono K, Katasumata K, Suzuki H, Shiraki K. Synergistic activity of amenamevir (ASP2151) with nucleoside analogs
against herpes simplex virus types 1 and 2 and varicella-zoster virus. Antiviral Res 2013;97(2):154-60. PMID 23261844
Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and
complications. Ann Int Med 1983;98:958-72. PMID 6344712
Corey L, Ashley R; Valaciclovir HSV Transmission Study Group. Prevention of herpes simplex virus type 2 transmission
with antiviral therapy. Herpes 2004;11 Suppl 3:170A-4A. PMID 15319087
Corey L, Bodsworth N, Mindel A, Patel R, Schacker T, Stanberry L. An update on short-course episodic and prevention
therapies for herpes genitalis. Herpes 2007;14 Suppl 1:5A-11A. PMID 17877886
Corey L, Spear PG. Infections with herpes simplex viruses (2). N Engl J Med 1986;314:749-57 PMID 3005859
Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a
review of two overlapping epidemics. J Acquir Immune Defic Syndr 2004;35:435-45. PMID 15021308
Delany-Moretlwe S, Lingappa JR, Celum C. New insights on interactions between HIV-1 and HSV-2. Curr Infect Dis Rep
2009;11:135-42. PMID 19239804
Desena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-methy-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol 2014;71(3):344-6. PMID 24473671
Dubos RJ. Man Adapting. New Haven: Yale University Press, 1980:179.
Dummer JS, Armstrong J, Somers J, et al. Transmission of infection with herpes simplex virus by renal transplantation. J
Infect Dis 1987;155:202-6. PMID 3027191
Eberhardt O, Kuker W, Dichgans J, Weller M. HSV-2 sacral radiculitis (Elsberg syndrome). Neurology 2004;63:758-9.
PMID 15326269
Elsberg CA. Experiences in spinal surgery. Observations upon 60 laminectomies for spinal disease. Surg Gynecol
Obstet 1931;16:117-35.
Ericsdotter AC, Brink M, Studahl M, Bengner M. Reactivation of herpes simplex type 1 in pneumococcal meningitis. J
Clin Virol 2015;66:100-2. PMID 25866347
Fernandez-Gerlinger M, Greffe S, Meffre A, et al. HSV-2 meningoencephalitis in an immunocompetent young man: what
is the pathogenesis and what is the treatment? J Clin Virol 2015;69:40-3. PMID 26209376
Field HJ. Persistent herpes simplex virus infection and mechanisms of virus drug resistance. Eur J Clin MIcrobiol Infect
Dis 1989;8:671-80. PMID 2550235
Folpe A, Lapham LW, Smith HC. Herpes simplex myelitis as a cause of acute necrotizing myelitis syndrome. Neurology
1994;44:1955-7. PMID 7936255
Freeman EE, White RG, Bakker R, et al. Population-level effect of potential HSV2 prophylactic vaccines on HIV
incidence in sub-Saharan Africa. Vaccine 2009;27:940-6. PMID 19071187
French MA. HIV/AIDS: Immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis 2009;48(1):101-7.
PMID 19025493
Goodell SE, Quinn TC, Mkrtichian E, Schuffler MD, Holmes KK, Corey LC. Herpes simplex proctitis in homosexual men:
clinical, sigmoidoscopic, and histopathological features. N Engl J Med 1983;308:868-71. PMID 6300674
Goodpasture EW. The axis-cylinders of peripheral nerves as portals of entry to the central nervous system for the virus
of herpes simplex in experimentally infected rabbits. Am J Pathol 1925;1:11-28. PMID 19969628
Graber JJ, Rosenblum MK, DeAngelis LM. Herpes simplex encephalitis in patients with cancer. J Neuroncol
2011;105(2):415-21. PMID 21637964
Grover R, Newsholme W, Brink N, Manji H, Miller R. Herpes simplex virus infection of the central nervous system in
human immunodeficiency virus-type 1-infected patients. Int J STD AIDS 2004;15(9):597-600. PMID 15339367
Gupta A, Rani PK, Bagga B, et al. Bilateral herpes simplex-2 acute retinal necrosis with encephalitis in premature twins.
J Am Assoc Ped Ophthal Strabismus 2010;14(6):541-3. PMID 21168079
Hook EW 3rd, Cannon RO, Nahmias AJ, et al. Herpes simplex virus infection as a risk factor for human
immunodeficiency virus infection in heterosexuals. J Infect Dis 1992;165:251-5. PMID 1309846
James SH, Kimberlin DW. Neonatal herpes simplex virus infection: epidemiology and treatment. Clin Perinatol
2015;42(1):47-59. PMID 25677996
James SH, Prichard MN. Current and future therapies for herpes simplex virus infections: mechanisms of action and
drug resistance. Curr Opin Virol 2014;8:54-61. PMID 25036916
Johnston C, Gottlieb SL, Wald A. Status of vaccine research and development of vaccines for herpes simplex virus.
Vaccine 2016;34(26):2948-52. PMID 26973067
Juhl D, Mosel C, Nawroth F, et al. Detection of herpes simplex virus DNA in plasma of patients with primary but not with
recurrent infection: implication for transfusion medicine. Transfusion Medicine 2009;19:1-10. PMID 19708895
Kallio-Laine K, Seppanen M, Aittoniemi J, et al. HLA-DRB101 allele and low plasma immunoglobulin G1 concentration
may predispose to herpes-associated recurrent lymphocytic meningitis. Human ZImmunol 2010;71:179-81. PMID
19879913
Kallio-Laine K, Seppanen M, Kautiainen H, et al. Recurrent lymphocytic meningitis positive for herpes simplex virus
type 2. Emerg Infect Dis 2009;15:1119-22. PMID 19624935
Kimberlin DW. Herpes simplex virus infections in neonates and early childhood. Semin Pediatr Infect Dis 2005;16:27181. PMID 16210107
Kimberlin DW, Whitley RJ, Wan W, et al. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N
Engl J Med 2011;365(14):1284-92. PMID 21991950
Kindberg E, Vene S, Mickiene A, Lundkvist A, Lindquist L, Svensson L. A functional Toll-like receptor 3 gene (TLR3) may
be a risk factor for tick-borne encephalitis virus (TBEV) infection. J Infect Dis 2011;203(4):523-8. PMID 21216866
Kleinschmidt-DeMasters BK, DeBiasi RL, Tyler KL. Polymerase chain reaction as a diagnostic adjunct in herpesvirus
infections of the nervous system. Brain Pathol 2001;11:452-64. PMID 11556691
Koelle DM. Vaccines for herpes simplex virus infections. Curr Opin Invest Drugs 2006;7:136-41. PMID 16499283
Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med 2008;59:381-95.
PMID 18186706
Kupila L, Vuorinen T, Vainionpaa R, Hukkanen V, Mattila RJ, Kotilainen P. Etiology of aseptic meningitis and encephalitis
in an adult population. Neurology 2006;66:75-80. PMID 16401850
Labska K, Roubalova K, Picha D, Maresova V. Presence of herpesvirus DNA in cerebrospinal fluid of patients with tickborne encephalitis and enteroviral meningoencephalitis. J Med Virol 2015;87(7):1235-40. PMID 25771938
Landry ML, Mullangi P, Nee P, Klein BR. Herpes simplex virus type 2 acute retinal necrosis 9 years after neonatal
herpes. J Pediatrics 2005;146:836-8. PMID 15973328
Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. A prospective study of new infections with herpes simplex
virus type 1 and type 2. Chiron HSV Vaccine Study Group. N Engl J Med 1999;341:1432-8. PMID 10547406
Leone P. Reducing the risk of transmitting genital herpes: advances in understanding and therapy. Curr Med Res Opin
2005;21:1577-82. PMID 16238897
Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1
and herpes simplex virus type 2: a randomized placebo-controlled trial. Lancet 2010;375:824-33. PMID 20153888
Lingappa JR, Celum C. Clinical and therapeutic issues for herpes simplex virus-2 and HIV co-infection. Drugs
2007;67:155-74. PMID 17284082
Lisco A, Vanpouille C, Margolis L. A missed point in deciphering the viral synergy between herpes simplex virus and
HIV. Lancet 2009;9:522-3. PMID 19695487
Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in
herpesvirus-infected human tissues. Cell Host Microbe 2008;4:260-70. PMID 18779052
Livorsi D, Anderson E, Qureshi S, Howard M, Wang YF, Franco-Paredes C. Brainstem encephalitis: an unusual
presentation of herpes simplex virus infection. J Neurol 2010;257:1432-7. PMID 20495814
Looker KJ, Magaret AS, Turner KME, et al. Global estimates of prevalent and incident herpes simplex virus type 2
infections in 2012. PLOS One 2015;10(1):e114989. PMID 25608026
Love S, Wiley CA. Viral diseases. In: Graham DI, Lantos PL, editors. Greenfield's Neuropathology. Seventh edition.
London: Arnold (Hodder Headline Group), 2002:24.
Mark KE, Corey L, Meng TC, et al. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital
shedding: a randomized, controlled trial. J Infect Dis 2007;195(9):1324-31. PMID 17397003
Mertz GJ, Coombs RW, Ashley R, et al. Transmission of genital herpes in couples with one symptomatic and one
asymptomatic partner: a prospective study. J Infect Dis 1988;157:1169-77. PMID 2836518
Mettler C. History of medicine. New York: McGraw-Hill (Blakiston), 1947:356.
Miller RF, Fox JD, Thomas P, et al. Acute lumbosacral polyradiculopathy due to cytomegalovirus in advanced HIV
disease: CSF findings in 17 patients. J Neurol Neurosurg Psychiatry 1996;61:456-60. PMID 8937337
Miller S, Mateen FJ, Aksamit AJ. Herpes simplex virus 2 meningitis: a retrospective cohort study. J Neurovirol
2013;19(2):166-71. PMID 23494382
Mommeja-Marin H, Lafaurie M, Scieux C, Galicier L, Oksenhendler E, Molina JM. Herpes simplex virus type 2 as a cause
of severe meningitis in immunocompromised adults. Clin Infect Dis 2003;37:1527-33. PMID 14614676
Morris HH, Peters BH. Recurrent sciatica associated with herpes simplex. J Neurosurg 1974;41:97-9. PMID 4366418
Muller M, Wandel S, Colebunders R, et al. Immune reconstitution inflammatory syndrome in patients starting
antiretroviral therapy for HIV infection: a systematic review and metaanalysis. Lancet Infect Dis 2010;10(4):251-61.
PMID 20334848
Naesens L, De Clercq E. Recent developments in herpesvirus therapy. Herpes 2001;8(1):12-16. PMID 11867011
Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex
virus. N Engl J Med 2007;356:790-9. PMID 17314338
Nahmias AJ, Dowdle WR. Antigenic and biologic differences in herpesvirus hominis. Prog Med Virol 1968;10:110-59.
PMID 4304588
Nahmias AJ, Lee FK, Bechman-Nahmias S. Sero-epidemiological and -sociological patterns of herpes simplex viral
infections in the world. Scand J Infect Dis Suppl 1990;69:19-36. PMID 2175939
Nakajima H, Furutama D, Kimura F, et al. Herpes simplex virus myelitis: clinical manifestations and diagnosis by the
polymerase chain reaction method. Eur Neurol 1998;39:163-7. PMID 9605393
Niehues T, Reichenbach J, Neubert J, et al. Nuclear factor kappaB essential modulator-deficient child with
immunodeficiency yet without anhidrotic ectodermal dysplasia. J Allergy Clin Immunol 2004;114(6):1456-62. PMID
15577852
Novy J, Carota A, Eggimann P, Pusztaszeri M, Rossetti AO, Du Pasquier R. Encephalitis with herpes simplex-2 in the
cerebrospinal fluid and anti-RI (ANNA-2) antibodies: an infectious or a paraneoplastic syndrome. BMJ Case Rep
2009;2009. PMID 21833342
Obara Y, Furuta Y, Takasu T, et al. Distribution of herpes simplex virus types 1 and 2 genomes in human spinal ganglia
studied by PCR and in situ hybridization. J Med Virol 1997;52:136-42. PMID 9179758
Omland LH, Vestergaard BF, Wandall JH. Herpes simplex virus type 2 infections of the central nervous system: a
retrospective study of 49 patients. Scand J Infect Dis 2008;40:59-62. PMID 17852910
O'Sullivan CE, Aksamit AJ, Harrington JR, Harmsen WS, Mitchell PS, Patel R. Clinical spectrum and laboratory
characteristics associated with detection of herpes simplex virus DNA in cerebrospinal fluid. Mayo Clin Proc
2003;78(11):1347-52. PMID 14601693
Palu G, Benetti L, Calistri A. Molecular basis of the interactions between herpes simplex viruses and HIV-1. Herpes
2001;8:50-5. PMID 11867019
Patel R, Stanberry L, Whitley RJ. Review of recent HSV recurrent-infection treatment studies. Herpes 2007;14(1):23-6.
PMID 17848215
Quereda C, Corral I, Laguna F, et al. Diagnostic utility of a multiplex herpesvirus PCR assay performed with
cerebrospinal fluid from human immunodeficiency virus-infected patients with neurological disorders. J Clin Microbiol
2000;38:3061-7. PMID 10921978
Ramachandran S, Kinchington PR. Potential prophylactic and therapeutic vaccines for HSV infections. Curr
Pharmaceutical Design 2007;13:1965-73. PMID 17627530
Royal College of Obstetricians and Gynaecologists. Clinical Guidelines number 30: management of genital herpes in
pregnancy. London: Royal College of Obstetricians and Gynaecologists, 2002.
Schiff D, Rosenblum MK. Herpes simplex encephalitis (HSE) and the immunocompromised: a clinical and autopsy study
of HSE in the settings of cancer and human immunodeficiency virus-type 1 infection. Hum Pathol 1998;29:207-10.
PMID 9496822
Schulte EC, Sauerbrei A, Hoffmann D, Zimmer C, Hemmer B, Muhlau M. Acyclovir resistance in herpes simplex
encephalitis. Ann Neurol 2010;67(6):830-3. PMID 20517946
Scott LL. Prevention of perinatal herpes: prophylactic antiviral therapy. Clin Obstet Gynecol 1999;42:134-48. PMID
10073307
Scott LL, Hollier LM, McIntire D, Sanchez PJ, Jackson GL, Wendel GD Jr. Acyclovir suppression to prevent clinical
recurrences at delivery after first episode genital herpes in pregnancy: an open-label trial. Infect Dis Obstet Gynecol
2001;9:75-80. PMID 11495557
Sheffield JS, Hill JB, Hollier LM, et al. Valacyclovir prophylaxis to prevent recurrent herpes at delivery: a randomized
clinical trial. Obstet Gynecol 2006;108:141-7. PMID 16816068
Shyu WC, Lin JC, Chang BC, Harn HJ, Lee CC, Tsao WL. Recurrent ascending myelitis: an unusual presentation of herpes
simplex virus type 1 infection. Ann Neurol 1993;34:625-7. PMID 8215253
Siebert DG, Seals JE. Polyneuropathy after herpes simplex type 2 meningitis. South Med J 1984;77:1476. PMID
6494977
Sironi M, Biasin M, Cagliani R, et al. A common polymorphism in TLR3 confers natural resistance to HIV-1 infection. J
Immunol 2012;188(2):818-23. PMID 22174453
Smith MG, Lennette EH, Reames HR. Isolation of the virus of herpes simplex and the demonstration of intranuclear
inclusions in a case of acute encephalitis. Am J Pathol 1941;17:55-68. PMID 19970544
Snider SB, Jacobs CS, Scripko PS, Klein JP, Lyons JL. Hemorrhagic and ischemic stroke secondary to herpes simplex
virus type 2 meningitis and vasculopathy. J Neurovirol 2014;20(4):419-22. PMID 24806272
Stanberry LR. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes 2004;11 Suppl
3:161A-9A. PMID 15319086
Stanberry LR, Spruance SL, Cunningham AL; GlaxoSmithKline Herpes Vaccine Efficacy Study Group. Glycoprotien--adjuvant vaccine to prevent genital herpes. N Engl J Med 2002;347(21):1652-61. PMID 12444179
Steiner I. Herpes simplex virus encephalitis: new infection or reactivation. Curr Opin Neurol 2011;24(3):268-74. PMID
21483260
Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol
2007;6:1015-28. PMID 17945155
Strick LB, Wald A, Celum C. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin
Infect Dis 2006;43:347-56. PMID 16804851
Svensson A, Tunback P, Nordstrom I, Padyukov L, Eriksson K. Polymorphisms in Toll-like receptor 3 confer natural
resistance to human herpes simplex virus type 2 infection. J Gen Virol 2012;93(Pt 8):1717-24. PMID 22552940
Tang JW, Coward LJ, Davies NW, et al. Brain stem encephalitis caused by primary herpes simplex 2 infection in a young
woman. J Neurol Neurosurg Psychiatry 2003;74:1323-5. PMID 12933947
Tenorio A, Echevarria JE, Casas I, Echevarria JM, Tabares E. Detection and typing of human herpesviruses by multiplex
polymerase chain reaction. J Virol Methods 1993;44:261-9. PMID 8263120
Tobian AA, Grabowski MK, Serwadda D, et al. Reactivation of herpes simplex virus type 2 after initiation of
antiretroviral therapy. J Infect Dis 2013;208(5):839-46. PMID 23812240
Tyler KL. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including
Mollaret's. Herpes 2004;11 Suppl 2:57A-64A. PMID 15319091
Tyler KL, Adler D. Twenty-eight years of benign recurring Mollaret meningitis. Arch Neurol 1983;40(1):42-3. PMID
6848089
Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV: deciphering viral synergy. Lancet
2008;8:490-7. PMID 18652995
Venkatesan A, Benavides DR. Autoimmune encephalitis and its relation to infection. Curr Neurol Neurosci Rep
2015;15(3):3. PMID 25637289
Wald A, Corey L, Timmler B, et al. Helicase-primase inhibitor pritelivir for HSV-2 infection. N Engl J Med
2014;370(3):201-10. PMID 24428466
Walton AH, Muenzer JT, Rasche D, et al. Reactivation of multiple viruses in patients with sepsis. PLoS One
2014;9(2):e98819. PMID 24919177
Wang Y, Smith KP. Safety of alternative antiviral agents for neonatal herpes simplex virus encephalitis and
disseminated infection. J Pediatr Pharmacol Ther 2014;19(2):72-82. PMID 25024666
Warren T, Harris J, Brennan CA. Efficacy and safety of valacyclovir for the suppression and episodic treatment of
herpes simplex virus in patients with HIV. Clin Infect Dis 2004;39 Suppl 5:S258-66. PMID 15494897
Weerkamp N, van de Beek D, de Gans J, Koehler PJ. Herpes reactivation in patients with bacterial meningitis. J Infect
2008;57(6):493-4. PMID 19027168
Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Field's Virology. Philadelphia: Lippincott, Williams
and Wilkins, 2001:2461-509.
Whitley RJ, Herpes simplex virus infections of the central nervous system. Continuum (Minneap Minn)
2015;21(6):1704-13. PMID 26633784
Whitley RJ, Nahmias AJ, Visintine AM, Fleming CL, Alford CA. The natural history of herpes simplex virus infection of
mother and newborn. Pediatrics 1980;66:489-94. PMID 6253866
Whitley RJ, Schlitt M. Encephalitis caused by herpesviruses, including B virus. In: Scheld WM, Whitley RJ, Durack DT,
editors. Infections of the Central Nervous System. New York: Raven, 1991:41-86.
Wildy P. Herpes: history and classification. In: Kaplan AS, editor. The Herpesviruses. New York: Academic Press, 1973.
Wiley CA, VanPatten PD, Carpenter PM, Powell PM, Thal LJ. Acute ascending necrotizing myelopathy caused by herpes
simplex virus type 2. Neurology 1987;37:1791-4. PMID 3670617
Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United
States. JAMA 2006;296(8):964-73. PMID 16926356
Yamanishi T, Yasuda K, Sakakibara R, et al. Urinary retention due to herpes virus infections. Neurourol Urodyn
1998;17:613-9. PMID 9829425
Yan Z, Bryant KF, Gregory SM, et al. HIV integrase inhibitors block replication of alpha-, beta-, and
gammaherpesviruses. MBio 2014;5(4):e01318-14. PMID 24987091
Yoritaka A, Ohta K, Kishida S. Herpetic lumbosacral radiculoneuropathy in patients with human immunodeficiency virus
infection. Eur Neurol 2005;53:179-81. PMID 15942245
Zepper P, Wunderlich A, Forschler A, et al. Pearls and Oy-sters: cerebral HSV-2 vasculitis presenting as hemorrhagic
stroke followed by multifocal ischemia. Neurology 2012;78:e12-5. PMID 22249502
Zhang SY, Jouanguy E, Ugolini S, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science
2007;317(5844):1522-7. PMID 17872438
Zhu J, Hladik F, Woodward A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential
mechanism for increased HIV-1 acquisition. Nature Med 2009;15:886-92. PMID 19648930
Zis P, Stritsou P, Angelidakis P, Tavernarakis A. Herpes simplex virus type 2 encephalitis as a cause of ischemic stroke:
case report and systematic review of the literature. J Stroke Cerebrovasc Dis 2016;25(2):335-9. PMID 26542825
**References especially recommended by the author or editor for general reading.
Former authors
David Irani MD (original author), Dena Dubal MD PhD, and David D Brown MD
ICD and OMIM codes
ICD codes
ICD-9:
Genital herpes, unspecified: 054.10
Herpes simplex meningitis: 054.72
Congenital herpes simplex: 771.2
ICD-10:
Anogenital herpesviral infection, unspecified: A60.9
Herpesviral infection, unspecified: B00.9
Congenital herpesviral [herpes simplex] infection: P35.2
Profile
Age range of presentation
06-12 years
13-18 years
19-44 years
45-64 years
65+ years
Sex preponderance
female>male, >2:1
female>male, >1:1
Family history
none
Heredity
none
Population groups selectively affected
none selectively affected
Occupation groups selectively affected
none selectively affected
Differential diagnosis list
mumps
varicella-zoster virus
lymphocytic choriomeningitis
meningitis
measles
rubella
enterovirus (Echo 9)
B19 parvovirus
dengue
syphilis
Lyme borreliosis
leptospirosis
rickettsial diseases
idiopathic inflammatory conditions (sarcoid, Behçet disease, Vogt-Koyanagi-Harada syndrome)
chemical meningitis
drug reaction (nonsteroidal anti-inflammatories, trimethoprim-sulfamethoxazole)
HSV-1
cytomegalovirus
Epstein-Barr virus
human T-cell lymphotropic virus type 1
West Nile virus
tick-borne encephalitis viruses
tuberculosis
Guillain-Barré
lymphoma
metastatic malignant disease
hyperthermia
rapid change in mental status to coma
appearance of new epileptic foci not corresponding to the site of the primary tumor
Other topics to consider
Herpes simplex encephalitis
Molecular diagnosis of central nervous system infections
Neonatal herpes encephalitis
Recurrent meningitis
Copyright© 2001-2016 MedLink Corporation. All rights reserved.