* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Changes in left ventricular filling and left atrial function six
Survey
Document related concepts
Transcript
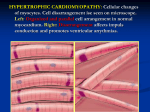
Journal of the American College of Cardiology © 1999 by the American College of Cardiology Published by Elsevier Science Inc. Vol. 34, No. 4, 1999 ISSN 0735-1097/99/$20.00 PII S0735-1097(99)00341-1 Changes in Left Ventricular Filling and Left Atrial Function Six Months After Nonsurgical Septal Reduction Therapy for Hypertrophic Obstructive Cardiomyopathy Sherif F. Nagueh, MD, FACC, Nasser M. Lakkis, MD, FACC, Katherine J. Middleton, RCT, Donna Killip, RN, William A. Zoghbi, MD, FACC, Miguel A. Quiñones, MD, FACC, William H. Spencer III, MD, FACC Houston, Texas OBJECTIVES The purpose of this study was to evaluate changes in left ventricular (LV) filling, left atrial (LA) volumes and function six months after nonsurgical septal reduction therapy (NSRT) for hypertrophic obstructive cardiomyopathy (HOCM). BACKGROUND Patients with HOCM frequently have enlarged left atria, which predisposes them to atrial fibrillation. Nonsurgical septal reduction therapy results in significant reduction in left ventricular outflow tract (LVOT) obstruction and symptomatic improvement. However, its effect on LV passive filling volume, LA volumes and function is not yet known. METHODS Thirty patients with HOCM underwent treadmill exercise testing as well as 2-dimensional and Doppler echocardiography before and six months after NSRT. Data included clinical status, exercise duration, LVOT gradient, mitral regurgitant (MR) volume, LV pre-A pressure and LA volumes. Left atrial ejection force and kinetic energy (KE) were computed noninvasively and were compared with 12 age-matched, normal subjects. RESULTS New York Heart Association (NYHA) class was lower and exercise duration was longer (p ⬍ 0.05) six months after NSRT. The LVOT gradient, MR volume and LV pre-A pressure were all significantly reduced. HOCM patients had larger atria, which had a higher ejection force and KE, compared with normal subjects (p ⬍ 0.01). After NSRT, LV passive filling volume increased (p ⬍ 0.01), whereas LA volumes, ejection force and KE decreased (p ⬍ 0.01). Reduction in LA maximal volume was positively related to changes in LV pre-A pressure (r ⫽ 0.8, p ⬍ 0.05) and MR volume (0.4, p ⬍ 0.05). Changes in LA ejection force were positively related to changes in LA pre-A volume (r ⫽ 0.7, p ⬍ 0.01) and KE (r ⫽ 0.81, p ⬍ 0.01). The increase in exercise duration paralleled the increase in LV passive filling volume (r ⫽ 0.85, p ⬍ 0.05). CONCLUSIONS Nonsurgical septal reduction therapy results in an increase in LV passive filling volume and a reduction in LA size, ejection force and KE. (J Am Coll Cardiol 1999;34:1123– 8) © 1999 by the American College of Cardiology Nonsurgical septal reduction therapy (NSRT) is a promising new treatment for hypertrophic obstructive cardiomyopathy (HOCM) that has been shown to improve both symptoms and exercise tolerance in patients with left ventricular outflow tract (LVOT) obstruction (1– 4). We have also recently observed an improvement in left ventricular (LV) diastolic dysfunction (pre-A pressure, tau and LV compliance) after NSRT, which accounted for some of the From the Cardiology Section, Department of Medicine, Baylor College of Medicine, Houston, Texas. Supported by grants from the T.L.L. Temple Foundation, Lufkin; Dunn Foundation; and Methodist Hospital Foundation, Houston, Texas. Manuscript received December 31, 1998; revised manuscript received March 31, 1999, accepted June 23, 1999. improvement in symptoms and exercise tolerance, six months after the procedure (5). However, it is currently unknown what effects NSRT has on LV filling and left atrial (LA) size and function. The purpose of this study was, therefore, to evaluate these changes. METHODS The protocol was approved by the institutional review board of Methodist Hospital and Baylor College of Medicine, and all patients gave written informed consent before participation. The group comprised 30 HOCM patients enrolled for ethanol septal reduction therapy (4). Patients had asymmetric septal hypertrophy with septal thickness ⱖ1.5 cm, and 1124 Nagueh et al. LV Filling and LA Size and Function After NSRT Abbreviations and Acronyms HCM ⫽ hypertrophic cardiomyopathy HOCM ⫽ hypertrophic obstructive cardiomyopathy KE ⫽ kinetic energy LA ⫽ left atrial LV ⫽ left ventricular LVOT ⫽ left ventricular outflow tract MR ⫽ mitral regurgitant NSRT ⫽ nonsurgical septal reduction therapy NYHA ⫽ New York Heart Association RVOT ⫽ right ventricular outflow tract TD ⫽ tissue Doppler septum/posterior wall thickness ⱖ1.3. All patients had a dynamic LVOT gradient ⱖ40 mm Hg at rest or ⱖ60 mm Hg during dobutamine (at baseline) (3 patients). Patients underwent treadmill exercise testing (Bruce protocol) at baseline and six months. A group of 12 normal age-matched adults (mean age: 53 ⫾ 16 years; 7 women) served as control subjects, for the purpose of comparing LA volumes and function. None of these individuals had evidence of cardiovascular disease by clinical and echocardiographic assessment. In addition, they were not receiving medications. Echocardiographic studies. Patients were imaged with an Acuson XP-128 or a Hewlett-Packard Sonos 2000 ultrasound system equipped with a multifrequency transducer (2.5 and 3.5 MHz) and tissue Doppler (TD) program. Parasternal and apical views (four- and two-chamber) were acquired first. Depth and gain settings were optimized to allow recording of LA volumes. Right ventricular outflow tract (RVOT) diameter in systole was measured in the parasternal short axis view at the base of the heart, with subsequent acquisition of systolic flow through the RVOT using pulse Doppler. From the four-chamber apical view, the sample volume was placed at mitral valve annulus then at tips, and 5 to 10 cardiac cycles were recorded with pulse Doppler at each site during normal respiration. With color Doppler guidance, the LVOT gradient was recorded with continuous-wave Doppler (6). Tissue Doppler program was applied in pulse-wave mode (⫺30 to 30 cm/s) with gains adjusted to minimize background noise. From the fourchamber view, a 5-mm sample volume was placed at the lateral border of the mitral annulus (7) for recording 5 to 10 cycles. Studies were stored on 1⁄2-inch VHS videotape for later playback and analysis. Echocardiographic analysis. All measurements were performed by an observer blinded to clinical data on an off line station equipped with 2-dimensional (2-D) and Doppler analysis software (Digisonics 500). Using frame-by-frame analysis, LA volumes were calculated at several points during the cardiac cycle, with the method of multiple discs (8). The following volumes were measured: maximal LA volume (LA max., taken before mitral valve opening), atrial JACC Vol. 34, No. 4, 1999 October 1999:1123–8 volume just before atrial contraction (LA pre-A) and minimal left atrial volume (LA min., measured after atrial contraction). Left atrial stroke volume was calculated as the difference between LA pre-A and LA min. volumes. The LV passive filling volume (rapid and mid-diastolic LV filling) was computed as the difference between LA max. volume and LA pre-A volume. The mitral annulus diastolic diameter was measured in the apical views, and mitral annulus area was then derived (9). Subsequently, LA ejection force was calculated using the equation: 0.5 ⫻ 1.06 ⫻ mitral annulus area ⫻ (peak A velocity2) in kdyne (10). Left atrial kinetic energy was calculated using the equation: 0.5 ⫻ 1.06 ⫻ LA stroke volume ⫻ (A velocity2) in kerg (11). In the calculation of LA kinetic energy, the absolute A velocity (peak A ⫺ velocity at onset of A) was used when this velocity did not start from baseline (11). Mitral regurgitant (MR) volume was derived as the difference between diastolic mitral inflow (area of mitral annulus ⫻ velocity time integral of mitral annular flow) and pulmonic outflow (RVOT area ⫻ velocity time integral of RVOT systolic flow) (9). Peak LVOT gradient was derived with the modified Bernoulli equation as: LVOT gradient ⫽ 4v2, where v ⫽ peak velocity in LVOT by continuous-wave Doppler (6). Left ventricular pre-A pressure, which relates well to and has the same Doppler correlates as the pulmonary capillary wedge pressure (12), was estimated using the equation LV pre-A pressure ⫽ 3.2 ⫹ [1.1(E/Ea)], where Ea is the annular early diastolic velocity at the mitral annulus lateral corner (13). All echocardiographic measurements were ascertained at baseline and six months. Statistics. Data are presented as mean ⫾ SD. Unpaired t testing was used to compare LA size and function between the normal group and HOCM patients. Paired t testing was applied in the evaluation of LA changes in size and function six months after NSRT. Regression (linear or nonlinear) analysis was used to relate changes in LA volumes, ejection force, kinetic energy (KE), exercise duration, LVOT gradient, MR volume and LV pre-A pressure to one another. Significance was set at a p value ⱕ 0.05. RESULTS Mean age was 53 ⫾ 15 (range 29 to 83) years (14 women). At baseline before NSRT, 5 patients were in New York Heart Association (NYHA) class IV with 21 patients in class III, and 4 patients in class II (patients in class II could not tolerate maximum medications) despite maximal medical therapy. All but three patients had a resting LVOT gradient (52 ⫾ 33 [20 to 120] mm Hg, three had dobutamine-provocable gradients). Patients had hyperdynamic ventricles, with 23 patients having mild, and 7 patients having moderate, mitral regurgitation (as assessed by pulse Doppler of mitral inflow and RVOT outflow). Satisfactory 2-D apical views, pulse Doppler of mitral JACC Vol. 34, No. 4, 1999 October 1999:1123–8 Nagueh et al. LV Filling and LA Size and Function After NSRT 1125 Table 1. Left Atrial Volumes, Ejection Force and Kinetic Energy in HCM Patients Before and Six Months After NSRT HCM at Baseline LA max. volume (ml) LA min. volume (ml) LA pre-A volume (ml) LV passive filling volume (ml) LA stroke volume (ml) LA ejection force (kdyne) LA kinetic energy (kerg) Figure 1. Left atrial (LA) stroke volume (SV) in the control group, and in the HOCM group pre- and post-NSRT (comparing controls with HOCM patients pre-NSRT: p ⬍ 0.001, 95% confidence intervals for difference of means: 10 –22; comparing controls with HOCM patients post-NSRT: p ⬍ 0.001, 95% confidence intervals: 4.1–14.4). inflow and RVOT as well as TD of mitral annulus were obtained in all patients. Changes in functional status, MR volume and pre-A pressure six months after NSRT. After NSRT, patients experienced a significant improvement in symptoms. Five patients were in NYHA class II, 24 in class I and 1 in class III (p ⬍ 0.01). Exercise tolerance was improved, with exercise duration increasing from 270 ⫾ 147 to 412 ⫾ 177 s (p ⬍ 0.05). The LVOT gradient was significantly lower at six months (52 ⫾ 33 to 9 ⫾ 19 mm Hg, p ⬍ 0.01). The MR severity was likewise reduced after the procedure, with 20 patients having none or trivial MR and 10 patients having mild MR (23 patients had mild MR, and 7 patients had moderate MR before NSRT). The MR volume decreased from 16 ⫾ 12 ml before to 8 ⫾ 8 ml after NSRT (p ⬍ 0.05). Also, the LV filling pressures were significantly lower (19 ⫾ 6 to 14 ⫾ 5 mm Hg, p ⬍ 0.05). HCM Six Months Post-NSRT 89 ⫾ 36 34 ⫾ 22 60 ⫾ 17 29 ⫾ 12 66 ⫾ 21† (16–37.5) 14 ⫾ 15† (2.8–26.2) 28 ⫾ 18† (4.7–37.5) 38 ⫾ 14† (3–19.4) 26 ⫾ 10 23 ⫾ 14 14 ⫾ 9† (3.4–20) 15.8 ⫾ 10.25* (5.1–10) 58.4 ⫾ 25 36.1 ⫾ 22.6* (15–29.4) *p ⬍ 0.01 versus baseline; numbers between parentheses refer to the 95% confidence intervals for difference of means. although it was still more than that of the control group (Fig. 3). Relation of changes in LA size and function to LV hemodynamics and clinical status. There were significant relations between changes in LA volumes and LA function. As LA volume before its contraction (LA pre-A volume) decreased, LA ejection force decreased (r ⫽ 0.72, p ⬍ 0.001, Fig. 4). Changes in LA ejection force were positively related to the reduction in LA stroke volume (r ⫽ 0.66, p ⬍ 0.01). Likewise, changes in LA KE were positively related to changes in LA stroke volume (r ⫽ 0.77, p ⬍ 0.001, Fig. 5) and LA ejection force (r ⫽ 0.81, p ⬍ 0.001, Fig. 6). There was a weak, albeit significant, relation between LA maximal volume reduction and MR volume reduction (r ⫽ 0.4, p ⬍ 0.05). A stronger relation was present, however, with changes in LV pre-A pressure (r ⫽ 0.8, p ⬍ 0.01, Fig. 7). Regarding changes in LV passive filling volume, these Changes in LV passive filling volume and LA size six months after NSRT. The volumes in HOCM patients were significantly larger than age-matched controls. Six months after NSRT, LA volumes (max., min., pre-A and stroke volume) were significantly reduced in comparison with baseline values, although still larger than LA volumes of the control group (Fig. 1). However, LV passive filling volume increased after NSRT (Table 1; p ⬍ 0.05). Changes in LA function six months after NSRT. Mean LA ejection force in the control group was 8.4 ⫾ 3.4 kdyne. Mean LA ejection force in HCM patients was significantly larger. Six months after NSRT, mean LA ejection force was lower although still more than that of the control group (Fig. 2). Mean LA KE of the control group was 13.6 ⫾ 4 kerg. Mean LA KE in HOCM patients before NSRT was significantly larger and decreased six months after NSRT Figure 2. Left atrial (LA) ejection force in the control group, and in the HOCM group pre- and post-NSRT (comparing controls with HOCM patients pre-NSRT: p ⬍ 0.001, 95% confidence intervals: 6.46 –23.5; comparing controls with HOCM patients post-NSRT: p ⫽ 0.019, 95% confidence intervals: 1.3–13.6). 1126 Nagueh et al. LV Filling and LA Size and Function After NSRT Figure 3. Left atrial (LA) kinetic energy (KE) in the control group, and in the HOCM group pre- and post-NSRT (comparing controls with HOCM patients pre-NSRT: p ⬍ 0.001, 95% confidence intervals: 30 –59.6; comparing controls with HOCM patients post-NSRT: p ⫽ 0.001, 95% confidence intervals: 9.3– 36). were positively related to reduction in LVOT gradient (r ⫽ 0.5, p ⫽ 0.05), and to prolongation of exercise duration at six months (r ⫽ 0.85, p ⬍ 0.01, Fig. 8). DISCUSSION In comparison with age-matched controls, HOCM patients as shown in this study have increased LA volumes as well as increased LA ejection force and kinetic energy. After NSRT, LA volumes, ejection force and KE are reduced concomitant with an increase in LV filling during early diastole, and a statistically significant prolongation in exercise duration. Figure 4. Regression plot of ⌬ LA pre-A volume versus ⌬ LA ejection force (pre- and post-NSRT). JACC Vol. 34, No. 4, 1999 October 1999:1123–8 Figure 5. Regression plot of ⌬ LA KE versus ⌬ LA SV (pre- and post-NSRT). Changes in LV passive filling volume six months after NSRT. HOCM patients have enlarged LA due to impaired LV relaxation, and mitral regurgitation (14). Impaired LV relaxation leads to higher early diastolic LV pressures, and thus lower transmitral pressure gradients. Accordingly, HOCM patients usually have reduced LV passive filling volume as demonstrated by radionuclide and Doppler echocardiographic techniques (15–17). Nonsurgical septal reduction therapy improves LV relaxation (5), at least in part through relief of LVOT obstruction and increase in loads that aid relaxation and filling. It also appears to improve LV compliance (5) through a reduction in mass/volume ratio (4) as well as improvement in LV relaxation. Accordingly, LV diastolic pressures are lower, leading to an increase in LV passive filling volume. Although reducing MR volume would be expected to lower early diastolic LV filling through a reduction in LA ‘v’ wave pressure, this was not observed in our patient popu- Figure 6. Regression plot of ⌬ LA KE versus ⌬ LA ejection force (pre- and post-NSRT). JACC Vol. 34, No. 4, 1999 October 1999:1123–8 Figure 7. Regression plot of ⌬ LA maximal volume versus ⌬ LV pre-A pressure (pre- and post-NSRT). lation. Most of this HOCM cohort had only mild MR, which minimized the influence of this lesion on LV filling. These changes in LV filling are particularly important because HOCM patients usually have reduced LV filling with exercise that results in a lower end-diastolic volume, stroke volume (18), and, accordingly, reduced exercise tolerance. Similar to observations with verapamil (16), we observed an improvement in exercise duration that was closely associated with the increase in LV passive filling (Fig. 8). LA size and function in HOCM patients and the effects of NSRT. Left atrial contribution to LV filling is increased in HOCM patients secondary to impaired LV relaxation. As shown in the present study, LA volumes are much larger in HOCM patients compared with control subjects. After NSRT, LV diastolic pressures and MR volume are lower, leading to significantly reduced LA volumes. Nagueh et al. LV Filling and LA Size and Function After NSRT 1127 Likewise, LA ejection force in HOCM patients is much larger than in normal subjects secondary to increased pre-A volume. The atria follow the Frank-Starling mechanism (19 –21), leading to augmented contractility with increases in LA preload. As noted previously, after NSRT the LV passive filling volume increases and LA pre-A volume decreases. Therefore, LA preload is reduced and accordingly ejection force and stroke volume. Likewise, LA KE is higher in HOCM patients because ejection force and stroke volume are increased in comparison with normal individuals. After NSRT, LA ejection force and stroke volume are reduced and therefore LA work. It is interesting to note that this cohort of 30 HOCM patients included a 32-year-old woman with frequent episodes of severely symptomatic atrial fibrillation. After NSRT, her LA maximal volume was down from 132 ml to 45 ml, with no further episodes of atrial fibrillation as assessed by history and Holter monitoring. Further observations will be needed to determine whether these changes will affect the incidence of atrial fibrillation. Study limitations. It would have been ideal had LA pressure and stroke work been assessed invasively (11,22). This would have necessitated transeptal LA catheterization, with its possible risks in these very sick patients. Furthermore, the noninvasive assessment of LA kinetic energy has been well validated against invasive standards with an excellent correlation (r ⫽ 0.95) (11). Although the noninvasive methods used to derive LV filling pressures and parameters of LA function may each have imperfections and sources of error, the changes observed after NSRT (with patients as their own control) were all in the same direction. Acknowledgment The authors thank Ms. Maria Frias for her expert secretarial assistance. Reprint requests and correspondence: Sherif F. Nagueh, Cardiology Section, Baylor College of Medicine, 6550 Fannin, SM1246, Houston, Texas 77030. E-mail: [email protected]. REFERENCES Figure 8. Relation of changes in exercise duration to those in left ventricular passive filling volume. 1. Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 1995;346:211– 4. 2. Knight C, Kurbaan AS, Seggewiss H, et al. Non-surgical septal reduction for hypertrophic obstructive cardiomyopathy: outcome in the first series of patients. Circulation 1997;95:2075– 81. 3. Seggewiss H, Gleichmann U, Faber L, Fassbender D, Schmidt H, Strick S. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results and 3-month follow-up in 25 patients. J Am Coll Cardiol 1998;31:252– 8. 4. Lakkis NM, Nagueh SF, Kleiman NS, et al. Echocardiography guided ethanol septal reduction for hypertrophic obstructive cardiomyopathy. Circulation 1998;98:1750 –5. 5. Nagueh SF, Lakkis NM, Middleton KJ, et al. Changes in left ventricular diastolic function six months after nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy. Circulation 1999;99:344 –7. 6. Sasson Z, Yock PG, Hatle LK, Alderman EL, Popp RL. Doppler 1128 7. 8. 9. 10. 11. 12. 13. 14. 15. Nagueh et al. LV Filling and LA Size and Function After NSRT echocardiographic determination of the pressure gradient in hypertrophic cardiomyopathy. J Am Coll Cardiol 1988;11:752– 6. Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA: DTI: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997;30:1527–33. Kircher B, Abbott JA, Pau S, et al. Left atrial volume determination by biplane two-dimensional echocardiography: validation by cinecomputed tomography. Am Heart J 1991;121:864 –71. Rokey R, Sterling LL, Zoghbi WA, et al. Determination of regurgitant fraction in isolated mitral or aortic regurgitation by pulsed Doppler two-dimensional echocardiography. J Am Coll Cardiol 1986; 7:1273– 8. Manning WJ, Silverman DI, Katz SE, Douglas PS. Atrial ejection force: a noninvasive assessment of atrial systolic function. J Am Coll Cardiol 1993;22:221–5. Stefanadis C, Dernellis J, Lambrou S, Toutouzas P. Left atrial energy in normal subjects, in patients with symptomatic mitral stenosis, and in patients with advanced heart failure. Am J Cardiol 1998;82:1220 –3. Appleton CP, Galloway JM, Gonzalez MS, Graballa M, Basnight MA. Estimation of left ventricular filling pressures using twodimensional and Doppler echocardiography in adult patients with cardiac disease. J Am Coll Cardiol 1993;22:1972– 82. Nagueh SF, Lakkis NM, Middleton KJ, et al. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation 1999;99:254 – 61. Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy: the importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis 1985;28:1– 83. Bonow RO, Ostrow HG, Rosing DR, et al. Effects of verapamil on JACC Vol. 34, No. 4, 1999 October 1999:1123–8 16. 17. 18. 19. 20. 21. 22. left ventricular systolic and diastolic function in patients with hypertrophic cardiomyopathy: pressure-volume analysis with a nonimaging scintillation probe. Circulation 1983;68:1062–73. Bonow RO, Dilsizian V, Rosing DR, Maron BJ, Bacharach SL, Green MV. Verapamil-induced improvement in left ventricular diastolic filling and increased exercise tolerance in patients with hypertrophic cardiomyopathy: short- and long-term effects. Circulation 1985;72: 853– 64. Maron BJ, Spirito P, Green KJ, Wesley YE, Bonow RO, Arce J. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1987;10:733– 42. Lele SS, Thomson HL, Seo H, Belenkie I, McKenna WJ, Frenneaux MP. Exercise capacity in hypertrophic cardiomyopathy: role of stroke volume limitation, heart rate, and diastolic filling characteristics. Circulation 1995;92:2886 –94. Braunwald E, Frahm CJ. Studies on Starling’s law of the heart: IV. observations on the hemodynamic functions of the left atrium in man. Circulation 1961;24:633– 42. Matsuda Y, Toma Y, Ogawa H, et al. Importance of left atrial function in patients with myocardial infarction. Circulation 1983;67: 566 –71. Sigwart U, Grbic M, Goy JJ, Kappenberger L. Left atrial function in acute transient left ventricular ischemia produced during percutaneous transluminal coronary angioplasty of the left anterior descending coronary artery. Am J Cardiol 1990;65:282– 6. Hoit BD, Shao Y, Gabel M, Walsh RA. In vivo assessment of left atrial contractile performance in normal and pathological conditions using a time-varying elastance model. Circulation 1994;89:1829 –38.