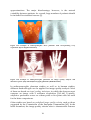
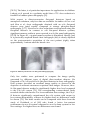
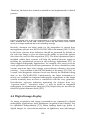
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Evaluation of image quality and patient radiation dose in digital
Survey
Document related concepts
Transcript
Faculty of Medicine and Health Sciences Department of Human Anatomy, Embryology, Histology and Medical Physics Evaluation of image quality and patient radiation dose in digital radiology Klaus Bacher 2006 Promotor: Prof. Dr. H. Thierens Co-promotor: Prof. Dr. K. Verstraete Faculty of Medicine and Health Sciences Department of Human Anatomy, Embryology, Histology and Medical Physics Evaluation of image quality and patient radiation dose in digital radiology Analyse van beeldkwaliteit en patiëntdosis bij digitale radiologie Klaus Bacher Promotor: Prof. Dr. H. Thierens Co-promotor: Prof. Dr. K. Verstraete 2006 Thesis submitted in fulfillment of the requirements for the degree of Doctor in Medical Sciences Promotor: Prof. Dr. H. Thierens Universiteit Gent Co-promotor: Prof. Dr. K. Verstraete Universiteit Gent Begeleidingscommissie: Prof. Dr. H. Thierens Prof. Dr. K. Verstraete Prof. Dr. E. Achten Dr. P. Smeets Dr. N. Reynaert Universiteit Gent Universiteit Gent Universiteit Gent UZ Gent Universiteit Gent Voorzitter Examencommissie: Prof. Dr. Ir. C. de Wagter Universiteit Gent Examencommissie: Prof. Dr. E. Achten Prof. Dr. H. Bosmans Prof. Dr. K. D’Herde Prof. Dr. I. Lemahieu Prof. Dr. C. Schaefer-Prokop Prof. Dr. H. Thierens Dr. M. Thijssen Prof. Dr. K. Versraete Universiteit Gent UZ Leuven Universiteit Gent Universiteit Gent AKH Wien, AMC Amsterdam Universiteit Gent Alysis Zorggroep Universiteit Gent Decaan van de Faculteit Geneeskunde en Gezondheidswetenschappen: Prof. Dr. J.-L. Pannier In honour of my grandparents Woord vooraf Januari 2001 – Dr Peter Smeets vertelt mij over de nieuw geïnstalleerde digitale radiografie zaal in het UZ Gent. De beeldkwaliteit zou er verbluffend zijn en dit zelfs bij een lage stralingsdosis. “Of ik dat eens even kon bekijken”, vroeg hij. Wel, ik heb dat even gedaan, met dit proefschrift als gevolg… Uiteraard kon dit werk niet tot stand komen zonder de hulp van heel wat mensen. Vooreerst wens ik mijn promotor, Prof. Dr. H. Thierens, en copromotor, Prof. Dr. K. Verstraete, van ganser harte te danken voor het vertrouwen, de steun en de vele constructieve bijdragen tijdens het uitvoeren van de diverse studies gebundeld in dit werk. De uitstekende samenwerking met de dienst Radiologie van het UZ Gent was een grote stimulans voor mij. Ik zou dan ook alle stafleden, verpleegkundigen en medische beeldvormers willen bedanken voor de ondersteuning. Zij behoorden tot de gelukkigen die massa’s “bolletjes” mochten (moesten) tellen op de contrast-detail beelden, en ondanks dat werd ik er als een goede collega ontvangen! Een speciaal dank u wel wil ik richten tot Dr. Smeets, die immer enthousiast met nieuwe en klinisch relevante ideeën kwam aankloppen. Technisch-logistieke problemen werden uiterst efficiënt aangepakt door de groep van Prof. Dr. Duyck. Tony en Pieter, zonder jullie zouden vele digitale beelden nog steeds “rondzwerven” op het netwerk… Inderdaad, één van de belangrijkste vereisten voor het welslagen van een doctoraat ligt in een aangename werkomgeving. Die vond ik uiteraard niet alleen op de dienst Radiologie, maar eveneens bij alle collega’s van de Medische Fysica. Bedankt iedereen voor het “medeleven” in wat moeilijker momenten. En hoe kon het ook anders, ook hier heb ik heel wat slachtoffers gevonden voor het tellen van de beruchte bolletjes… An, bedankt voor de actieve medewerking en het vele praktische werk dat je verricht hebt. Voor een belangrijk deel van deze studie kon ik ook terecht kon bij de collega’s van het Heilig Hart Ziekenhuis van Roeselare. Ludo, dank u voor de samenwerking. Klassiek komen de naaste familieleden en vrienden op het einde van een dankwoord. Onterecht eigenlijk. Vooral mijn ouders wil ik in het bijzonder bedanken voor de steun en het geduld… Zonder jullie had ik nooit dit werk kunnen voltooien. Kim, ook jij bent een hele tijd op de tweede plaats gekomen. Bedankt voor je begrip. Nu jij aan je thesis aan het schrijven bent, zal ik nu klaarstaan voor jou! Klaus mei 2006 Table of contents Table of contents................................................................................................... i List of abbreviations...........................................................................................iii Summary............................................................................................................... v Samenvatting......................................................................................................vii Résumé................................................................................................................. ix Chapter 1: Introduction ..................................................................................... 1 1.1 The digital (r)evolution in projection imaging................................. 1 1.2 Digital detector technology ................................................................. 3 1.2.1 Classification of digital acquisition systems ............................. 3 1.2.2 Computed radiography (CR) ...................................................... 4 1.2.3 Charge coupled devices (CCD)................................................... 6 1.2.4 Thin-film transistor flat-panel technology ................................ 8 1.3 Image quality in projection imaging................................................ 10 1.3.1 Contrast, spatial resolution and noise...................................... 10 1.3.2 Assessment of image quality ..................................................... 12 1.3.3 Image quality versus patient radiation dose........................... 17 1.4 Image presentation in digital radiography..................................... 18 1.5 References ............................................................................................ 19 Chapter 2: Aim and outline of the thesis ...................................................... 27 2.1 Aim ....................................................................................................... 27 2.2 Outline.................................................................................................. 28 2.3 References ............................................................................................ 29 i Chapter 3: Original research: results..............................................................33 3.1 Part I ......................................................................................................33 Dose reduction in patients undergoing chest imaging: digital amorphous silicon flat-panel detector radiography versus conventional film-screen radiography and phosphor-based computed radiography .............................................................................33 3.2 Part II.....................................................................................................49 Image quality and radiation dose in digital chest imaging: comparison of an amorphous silicon and an amorphous selenium flat-panel system ........................................................................................49 3.3 Part III....................................................................................................65 Analysis of image quality in digital chest imaging...............................65 3.4 Part IV ...................................................................................................75 Image quality performance of liquid crystal display systems: influence of display resolution, magnification and window settings on contrast-detail detection ......................................................................75 Chapter 4: General discussion and conclusions ...........................................93 4.1 Digital x-ray acquisition .....................................................................93 4.2 Image quality analysis in digital radiography................................99 4.3 Patient dosimetry and dose optimization .....................................103 4.4 Digital image display........................................................................106 4.5 Future Prospects ................................................................................109 4.5.1 Evolution of digital technology ...............................................109 4.5.2 Challenges for the medical physics expert.............................111 4.6 Conclusions ........................................................................................112 4.7 References...........................................................................................113 Curriculum Vitae .............................................................................................129 ii List of abbreviations AAPM: American Association of Physicists in Medicine AEC: Automatic Exposure Control ALARA: As Low As Reasonably Achievable ANOVA: Analysis of Variance a-Se: Amorphous Selenium a-Si: Amorphophous Silicon BMI: Body Mass Index CAD: Computer Aided Detection CCD: Charge Coupled Device CNR: Contrast-to-Noise Ratio CR: Computed Radiography CRT: Cathode Ray Tube CT: Computed Tomography DICOM: Digital Imaging and Communications in Medicine DQE: Detective Quantum Efficiency FDA: Food and Drug Administration HC: Hard-copy HIS: Hospital Information System ICRP: International Commission on Radiological Protection IEC: International Electrotechnical Commission iii IQFinv: Inverse Image Quality Figure LAT: Lateral LCD: Liquid Crystal Display MRI: Magnetic Resonance Imaging MTF: Modulation Transfer Function NPS: Noise Power Spectrum PA: Posteroanterior PACS: Picture Archiving and Communication System PMMA: Polymethylmethacrylate PPU: Per Pixel Uniformity RIS: Radiology Information System ROC: Receiver Operating Characteristics SC: Soft-copy SNR: Signal-to-Noise Ratio TFT: Thin-film Transistor TLD: Thermoluminescent dosemeter iv Summary X-ray projection radiographs are the most frequently obtained images in radiology. Until recently, most of them were still acquired with conventional screen-film techniques. The last years, however, digital radiography detectors are gaining importance as medical imaging departments are moving towards a completely integrated digital environment where the digital images are centrally stored and the patient management is organized using complex database systems. The first implementation of digital radiography came in the mid eighties with the introduction of photostimulable phosphor plates. In computed radiography systems manual cassette manipulations are needed to produce digitalized images in a dedicated readout unit using laser light. Only a few years ago, flat-panel detectors with integrated read-out mechanisms became commercially available for implementation in digital radiography applications. The latter detectors provide an instant image display and use a thin-film transistor array for signal transport. Conversion of x-rays into a collectable charge is achieved either directly, by using an amorphous selenium photoconductor, or indirectly, by using a scintillator and an amorphous silicon photodiode. When introducing these new detectors into clinical practice, they should perform at least as good as conventional screen-film radiography systems with respect to image quality and patient radiation dose. In the first part of present thesis, the image quality and dose performance of different digital acquisition systems for chest radiography were compared. For the assessment of the image quality, patient chest images were scored according to the European Guidelines on Quality Criteria for Diagnostic Radiographic Images. In addition, CDRAD 2.0 contrast-detail phantom acquisitions were used for a more objective measure of the lowcontrast performance. The image quality for current digital chest radiography systems was found to be superior compared to conventional screen-film systems, especially for low-contrast regions. Flat-panel detectors based on cesium iodide and amorphous silicon, resulted in the best overall performance, v combining an excellent contrast-detail detectability with the lowest patient radiation doses. Compared to a conventional screen-film system, the latter detector achieved a dose reduction of more than 60%. In flatpanel systems based on amorphous selenium, on the other hand, similar dose levels as measured in state-of-the-art screen-film chest radiography must be applied. Similarly, no significant dose reductions were found for storage phosphor plate chest imaging. Image quality analysis based on patient images correlated very well with the more objective and less time-consuming contrast-detail analysis. As contrast-detail phantoms can be used for the image quality assessment and optimization of the complete digital imaging chain, including image acquisition and display, the latter methodology may be an interesting tool for implementation in a quality assurance program for digital radiography. In the second part of this thesis, digital image presentation was studied using digital CDRAD 2.0 and CDMAM 3.4 contrast-detail phantom acquisitions. It was shown that soft-copy reading can significantly improve the contrast-detail detectability compared to hard-copy presentation. In fact, with the interactive adjustment of brightness and contrast the wide dynamic range of digital acquisition systems are optimally used. The low-contrast performance of medical grade LCD devices of different matrix resolutions was compared with that of a state-of-the-art greyscale CRT monitor. For digital radiography, a LCD monitor with a matrix resolution of 2-megapixel can provide an equivalent contrast-detail detectability compared to a 5-megapixel CRT device when the digital images are magnified to full resolution. As the latter adjustment will cause a significant reduction of the throughput, monitors with a resolution of 3-megapixel or higher are recommended for the application in digital chest radiography. For the digital mammography setting, the CDMAM 3.4 contrast-detail analysis revealed that for digital mammography applications, a 5-megapixel monitor should be the first choice. A 3-megapixel monitor can only be used in digital mammography, when using a very time-consuming magnification. In general, interactive adjustment of brightness and contrast of digital images significantly improved the image quality in soft-copy reading without affecting the throughput. Therefore, the use of this functionality should be strongly recommended, especially for applications were subtle low-contrast details must be visualized. vi Samenvatting Radiografie vormt één van de belangrijkste en meest toegepaste technieken binnen de medische beeldvorming. Tot voor kort werden deze beelden op een conventionele manier opgenomen met klassieke schermfilm systemen. De laatste jaren wordt echter steeds vaker de overgang gemaakt naar digitale radiografie systemen omdat de medische beeldvorming naar een volledige digitalisatie streeft. Hierbij worden de beelden centraal gestockeerd en zijn deze na protocollering overal beschikbaar in het ziekenhuis. Bovendien evolueert men naar een digitaal patiëntenmanagement. De eerste stap naar digitale radiografie werd gezet door het gebruik van foto-stimuleerbare fosforplaten. Deze computed radiography systemen zijn compatibel met de bestaande conventionele installaties en vergen dezelfde manuele handelingen met cassettes. Er wordt evenwel gebruik gemaakt van een digitale uitleeseenheid die werkt op basis van laserlicht. Enkele jaren geleden kwam een nieuw type van digitale detectoren op de markt. Deze flat-panel detectoren bezitten geïntegreerde schakelingen die ogenblikkelijke beeldvorming toelaten. De x-stralen conversie gebeurt hierbij ofwel rechtstreeks, met een amorf selenium laag, ofwel onrechtstreeks door de combinatie van een scintillator en een amorf silicium laag. Bij de introductie van deze nieuwe systemen is het van groot belang dat zowel de beeldkwaliteit als de patiëntdosis onderzocht wordt in vergelijking met conventionele scherm-film systemen. In het eerste deel van deze thesis werd de beeldkwaliteit en de hierbij benodigde stralingsdosis van diverse digitale systemen voor thoraxradiografie bestudeerd. Voor de beeldkwaliteitanalyse scoorden een groep radiologen thoraxbeelden van patiënten volgens de Europese richtlijnen voor kwaliteitscriteria binnen de radiografie. Aansluitend werd eveneens een meer objectieve contrast-detail studie opgezet gebruik makende van het CDRAD 2.0 fantoom. Algemeen werd een uitstekende beeldkwaliteit gevonden in de digitale thoraxradiografie. Vooral in laag-contrast regio’s scoorden deze systemen beter dan de conventionele scherm-film systemen. Flat-panel detectoren vii gebaseerd op een cesium jodide scintillator en een amorf silicium laag, gaven algemeen de beste resultaten. In vergelijking met een conventioneel systeem, konden hiermee thoraxbeelden opgenomen worden met een patiëntdosis die meer dan 60% lager lag. Er werd echter vastgesteld dat dit niet het geval was voor alle flat-panel detectoren. Bij deze gebaseerd op amorf selenium, waren gelijkaardige doses nodig als voor conventionele thoraxradiografie. Ook bij fosforplaten werd geen significante dosisreductie gevonden. De resultaten van de beeldkwaliteitanalyse gebaseerd op het contrastdetail fantoom correleerden uitstekend met de analyse van de patiëntenbeelden. Toepassing van deze contrast-detail fantoom techniek, maakt een objectievere en snellere kwaliteitsanalyse van de gehele digitale beeldvormingketen mogelijk. In het tweede deel van dit werk werd de presentatie van digitale beelden bestudeerd. Hierbij werd opnieuw gebruik gemaakt van een contrastdetail analyse. De CDRAD 2.0 beeldpresentatie op een hoogwaardige monitor bleek superieur te zijn aan de afgedrukte versie van de fantoomopname. Inderdaad, met een digitale beeldanalyse op een monitor wordt een groot dynamisch bereik van deze acquisities optimaal benut. In een verdere studie werden LCD monitoren met diverse resoluties geanalyseerd met zowel een CDRAD 2.0 als met een CDMAM 3.4 contrast-detail fantoom. Dit laatste fantoom geeft subtiele contrasten weer, typisch voor mammografie. Algemeen scoorden de LCD monitoren zeer goed voor wat betreft de contrast-detail weergave. Voor digitale radiografie werd echter besloten dat een 2-megapixel monitor enkel kan gebruikt worden, wanneer de digitale beelden op volledige resolutie worden weergegeven. Het op deze manier vergroten van beelden, neemt echter bijzonder veel tijd in beslag. Hierdoor wordt minstens een 3megapixel monitor aanbevolen voor analyse van thoraxbeelden. Voor wat betreft digitale mammografie, werden de CDMAM 3.4 uitlezingen enkel aanvaardbaar gescoord op een 3- en 5-megapixel monitor. Bij de 3megapixel monitor moet men evenwel intensief gebruik maken van de zoom-functie. Dit laatste neemt, zoals reeds vermeld, veel tijd in beslag. Algemeen werd eveneens vastgesteld dat de interactieve aanpassing van de helderheid- en contrastinstellingen de laag-contrast analyse significant beïnvloedde. Aangezien dit zeer efficiënt en nagenoeg zonder tijdsverlies kan gebeuren, wordt dit dan ook sterk aanbevolen. viii Résumé La radiographie est une des techniques les plus importantes et les plus appliquées dans l’imagerie médicale. Jusqu’à présent les images étaient prises utilisant le couple écran-film conventionnel. Ces dernières années, les systèmes numériques radiographiques ont gagné en importance car les départements d’imagerie médicale visent une digitalisation complète. Ainsi, les images sont stockées centralement et sont disponibles partout à l’hôpital après en avoir fait le protocole. En outre, on progresse en utilisant des bases de données complexes pour la gestion des données des patients. Le premier pas vers la radiographie numérique est fait avec l’introduction de plaques au phosphore photostimulables. Ces systèmes de radiographie numérique sont compatibles avec les installations conventionnelles existantes et nécessitent les mêmes manipulations avec les cassettes écranfilm. Les détecteurs flat-panel, avec lecteurs intégrés ont été lancés sur le marché que depuis quelques années. La conversion des rayons X a lieu, soit directement, en utilisant un semi-conducteur au sélénium amorphe, soit indirectement, en combinant un scintillateur avec silicium amorphe. En introduisant ces nouveaux systèmes il est indispensable d’investiguer la qualité d’image et la dose des patients en comparaison avec le couple écran-film. Dans la première partie de cette thèse, la qualité d’image et la dose nécessaire à cet effet de systèmes digitaux divers pour la radiographie thoracique est étudiée. Pour l’analyse de la qualité d’image, un groupe de radiologues a marqué les images de thorax selon les directives européennes concernant les critères de qualité en radiologie. En outre, une étude contraste -détail plus objective a été effectuée utilisant le fantôme CDRAD 2.0. Généralement, une qualité d’image exceptionnelle est trouvée dans la radiographie thoracique digitale. Particulièrement dans les régions à ix faible contraste, ces systèmes sont meilleurs que les systèmes conventionnelles écran-film. Les détecteurs flat-panel, combinant un scintillateur à iodure de césium et le silicium amorphe, donnent les meilleurs résultats. En comparaison à un système conventionnel, il est possible de prendre des images de thorax avec une dose moins élevée de plus de 60 %. Toutefois le constat est fait ce n’est pas le cas pour tous les détecteurs flat-panel. Pour ceux fonctionnant au sélénium amorphe, des doses semblables étaient nécessaires que lors d’une radiographie thoracique conventionnelle. Les plaques au phosphore n’apportent non plus de réduction significative de dose. Les résultats de l’analyse la qualité d’image avec le fantôme contrastedétail corrèlent excellemment avec l’analyse des images de patients. L’application de cette dernière technique rend plus objective et plus rapide l’analyse de qualité de la chaîne de la création d’image. Dans la deuxième partie de cette thèse, la présentation d’images numérique est étudiée. A cet effet, l’analyse contraste-détail est réutilisée. La présentation de l’image CDRAD 2.0 sur un moniteur de haute qualité semble être supérieure à la version imprimée. En effet, avec l’ajustement interactif de la clarté et du contraste, une grande gamme de ces acquisitions sont mit à profit de façon optimale. Ensuite les moniteurs LCD de différentes résolutions sont analysés avec un CDRAD 2.0 et un CDMAM 3.4 fantôme contraste-détail. Ce dernier fantôme désigne de subtils contrastes, caractéristique pour la mammographie. Généralement les moniteurs LCD marquent très bien en ce qui concerne l’image contraste-detail. Pour la radiographie numérique est décidé d’utiliser un moniteur composé de 2 méga pixels uniquement quand les images numériques paraissent avec une résolution complète. L’agrandissement des images de cette façon, prend beaucoup de temps. De ce fait, un moniteur composé de 3 méga pixels est recommandé pour l’analyse des images de thorax. En ce qui concerne la mammographie numérique, l’analyse contraste- détail CDMAM 3.4 révèle qu’un moniteur composé de 5 méga pixels devrait être le premier choix. L’utilisation d’un moniteur composé de 3 méga pixels est seulement acceptable en faisant un agrandissement qui, comme mentionné avant, prend beaucoup de temps. En général, l’ajustement interactif de la clarté et du contraste influence significativement l’analyse à faible contraste. Etant donné que ça se passe très efficacement et sans perte de temps, ces ajustements sont fortement recommandés. x Chapter 1 Introduction 1.1 The digital (r)evolution in projection imaging Only a few months after their discovery by Wilhelm Röntgen (November 8, 1895), the first applications of X-rays in medicine were reported [1]. Hereby, a two-dimensional projection image of the patient’s threedimensional anatomy was created on a glass photographic plate using the specific attenuation properties of X-rays within tissues. Ever since, the latter radiography technique evolved into an essential component of medical care. Over the past hundred years, radiography has been optimized and the technology has been vastly improved. Rapid acquisition, low risk, low cost and a high diagnostic value are the major features why X-ray projection imaging represents the bulk of all diagnostic imaging studies [1]. Radiographs of the chest, limbs and joints, represent about 50% of all diagnostic X-ray examinations [2,3]. The majority of the radiographs are still acquired with conventional screen-film systems because of their good image quality, high spatial resolution and general low costs [1,4,5]. The film has a three-fold function as the medium for image acquisition, presentation and storage, making it impossible to optimize any of these functions independently. Moreover, typical disadvantages of screen-film techniques are the limited exposure range (Figure 1.1), a rather high retake rate and the inflexibility of image display and film management [1,4]. Since computer technology and storage capacity have developed rapidly during the last years, Picture Archiving and Communication Systems (PACS) are gaining more importance [7]. With a PACS, digital images are centrally archived and can be rapidly distributed within the entire hospital. This allows the radiologist to view the images and make the 1 report from a display screen, whereas the clinician can view the images and consult the report very shortly after these images have been acquired [8]. The different post-processing tools, the possibility for multi-modality image display and the use of Computer Aided Detection (CAD) software are just some examples of the additional possibilities of a digital image management in a PACS. Nowadays, PACS are being integrated in Hospital or Radiology Information Systems (HIS/RIS), resulting in an alldigital environment. It is obvious that such an evolution only can be successful in combination with full-digital image acquisition systems. Most of them (such as computed tomography (CT), magnetic resonance imaging (MRI), ultrasound and nuclear medicine imaging) comply with this requirement. Hence, projection radiography is the last modality to make the transition to digital acquisition [1]. The last decade, digital image receptors (discussed in 1.2) are gradually replacing screen-film cassettes. In general, digital radiography systems offer an instant image display, a wide dynamic range and a linear signal response (Figure 1.1) [9,10]. Moreover, digital images are very flexible in processing and archiving, thereby providing a solution to the major disadvantages of screen-film systems. Variability in image quality in conventional radiography, due to the developing procedure of the X-ray films, vanishes with the introduction of digital radiography. In addition, radiological reporting from the display screen spares the cost of film material and X-ray film archiving. Figure 1.1: Schematic representation of the dynamic range of a screen-film and a digital (computed) radiography system. Screen-film systems can typically only operate in a very limited exposure range [6]. 2 1.2 Digital detector technology1 1.2.1 Classification of digital acquisition systems Today, a large variety of digital acquisition systems for projection imaging is available on the market. In general, digital detectors can be classified into direct readout and indirect readout systems. Indirect readout detectors do not contain integrated readout mechanisms [9,11]. The digital image is obtained after a (manual) development step of the latent image acquired on the detector. Storage phosphor screens (discussed in 1.2.2) used in Computed Radiography (CR) can be classified in this group. Here, the detector area is divided into pixels during the readout procedure [7]. In direct readout systems, on the other hand, the digital radiograph is immediately available after the X-ray exposure [9]. These detectors primarily consist of pixel elements with independent readout [7,10]. Depending on the conversion method of X-rays into an electronic signal, direct readout detectors can be subsequently divided into two classes (Figure 1.2). Figure 1.2: Classification of direct readout detectors used in projection radiography [9]. Selenium drum radiography systems are excluded from this discussion as they are not commercially available any more. 1 3 Direct-conversion detectors have an X-ray photoconductor, such as amorphous selenium, that directly converts X-ray photons into an electric charge [1,9]. Afterwards, the electric charge pattern is sensed by an electronic readout mechanism, such as a Thin Film Transistor (TFT) array, and analog-to-digital conversion is performed to produce the digital image. Indirect-conversion detectors have a two-step process for X-ray detection [1,7,9,10]. In the latter systems, a scintillator is the primary material for Xray interaction in which the X-ray energy is converted into visible light, and that light is then converted into an electric charge by means of photodetectors such as amorphous silicon photodiode arrays or Charge Coupled Devices (CCD). After analog-to-digital conversion of the charge distribution (either in a TFT array or internally in the CCD chip), a digital image is formed. 1.2.2 Computed radiography (CR) Computed radiography systems were introduced in the beginning of the 80s as the first digital detector device for projection radiography [7,11]. CR systems use photostimulable storage phosphor imaging plates replacing the traditional film-screen cassettes. Typical imaging plates consist of BaFBr:Eu and BaFI:Eu. The small quantity of europium creates defects in the BaFBr and BaFI crystals which will allow to trap electrons in an efficient way [1,7]. Figure 1.3: Operation principle of a photostimulable phosphor [1]. 4 In Figure 1.3, the principle of photostimulable phosphors is schematically presented. During X-ray exposure, the incident radiation excites electrons from the valence band to the conduction band. A substantial amount of these electrons is trapped at a meta-stable energy level (F-centers). Hence, after the X-ray exposure of the phosphor imaging plate, a latent image is created as a spatially distribution of electrons trapped in these highenergy states [1,6]. During readout, He-Ne laser light is used to stimulate the trapped electrons back to their original energy state, while emitting light. The photostimulated luminescence is proportional to the absorbed x-ray energy. Figure 1.4: Practical implementation of a computed radiography system [6]. In practice, a phosphor plate is translated along the readout stage in the vertical direction (y-direction) and a flying spot He-Ne laser beam will scan the plate horizontally (x-direction) (Figure 1.4). The resulting twodimensional pattern of luminescence is caught by a photomultiplier tube or an array of photodiodes, is logarithmically amplified, and subsequently digitized by an analog-to-digital converter with 8- to 14-bit resolution. For digital chest radiography typically 10-12 bit per pixel are minimally required in corresponance with the large variety of contrast levels included in a chest radiograph [1]. Because only a fraction of the emitted light can be detected by the photomultiplier tubes, CR systems will have a limited X-ray quantum conversion efficiency, resulting in only moderate dose reductions compared to screen-film systems [1,7,10]. Thicker phosphor layers will result in a better quantum efficiency but suffer from less spatial resolution than the high resolution screens that are characterized by thinner layers but less quantum efficiency. As CR systems require cassette handling and transport to a cassette reader, there is only marginal workflow improvement over a conventional screen-film unit [1,7,12]. Despite these 5 limitations, CR systems are widely used due to compatibility with existing radiographic equipment, the generally low costs and their image quality that is comparable with conventional screen-film combinations [7,8]. Moreover, CR detector plates are portable and are therefore a digital alternative for bedside radiography. Recently introduced technical innovations in CR, including dual-reading CR, dual-screen CR and new linear laser diode readout technologies, have shown promising results and underline the fact that CR will continue to play an important role in projection radiography [7,13-16]. 1.2.3 Charge coupled devices (CCD) Charge coupled devices (CCDs) were introduced some 30 years ago [7,9,17]. A CCD is a silicon detector chip, composed of several million independent pixels (metal-oxide-semiconductor capacitors) [7]. The silicon surface of a CCD chip is photosensitive: as visible light interacts with a pixel, electrons are liberated and build up in the pixel [1]. More electrons are produced in pixels that receive greater light intensity. The first applications of CCDs in medical imaging date from the mid 80s [18-21], thereby representing the first direct readout detector in radiology [7]. CCD-based radiography systems use a scintillating phosphor as an absorption medium for x-rays (Figure 1.2). Afterwards, the visible light is transmitted to the CCD chips and a digital image can be processed. The major disadvantage of CCDs with respect to digital radiography is the fact that they are physically small (typically a few cm²), which is much smaller than typical projected x-ray areas [1,7,9]. Because of this, CCDbased radiographic systems must include some means of optical coupling to reduce the size of the projected visible light image and transfer the image to the face of one or more CCDs [9]. Some CCD-based systems have an image intensifier that reduces the large x-ray field to the size of one CCD (Figure 1.5A). Other systems are based on a group of CCDs, each of which is coupled to a scintillator by a fiberoptic taper (Figure 1.5B) or a lens system (Figure 1.5C). In general, lens optical coupling in CCD radiography substantially reduces the number of photons that reach the CCD [1,9]. As a result, only very modest detective quantum efficiencies (DQE, discussed in 1.3.2) are reported in large-field CCD radiography [7,22,23]. In addition, optical coupling with lenses can introduce geometric distortions, optical scatter, 6 and reduced spatial resolution [1,9,22]. On the other hand, imperfections in optical fibers can introduce structure artifacts on the image of CCD systems with fiberoptic tapers. Figure 1.5: Different designs of CCD-based x-ray imaging detectors: CCD coupled with lenses to an image intensifier (A), CCD coupled with fiber optics to a scintillating phosphor screen (B), CCD coupled with lens systems to a scintillating phosphor screen (C) [1]. Because of these disadvantages, CCDs are mainly used in fields where demagnification can be minimized, such as dental radiography [24-26], digital mammography [27-29] and in digital image-intensifier systems used in fluoroscopy and cineangiography [19,21,27]. The last years, digital CCD detectors are gaining importance due to the introduction of slot-scan designs (Figure 1.6) [23,27,31,32]. In the latter technology, the x-rays are collimated into a narrow, horizontally oriented, fan-shaped beam that matches a rectangular CCD array [23]. During the exposure, the x-ray beam moves across the surface and the detector follows the beam. Advantages of the slot-scan concept include doseefficient scatter rejection (no need for an anti-scatter grid) and the possibility of using small detectors to image large areas without demagnification [31]. 7 Figure 1.6: Illustration of the operation of a slot-scan digital radiography system [33]. 1.2.4 Thin-film transistor flat-panel technology Recent developments in technology have made possible a new generation of large-area, flat-panel detectors with integrated, thin-film transistor (TFT) direct readout mechanisms. Unlike CCD-based detectors that require optical coupling and image demagnification, TFT–based, flatpanel systems are constructed such that the pixel charge collection and readout electronics for each pixel are immediately adjacent to the site of the x-ray interactions [9]. Depending on the x-ray detection medium, two types of flat-panel detectors can be distinguished. Digital signals can be generated either directly, using a photoconductor, or indirectly, using a scintillator and an amorphous silicon photodiode (Figure 1.2) [1,7,9,10]. Indirect-conversion TFT detector systems use an amorphous silicon photodiode circuitry for the detection of light emitted by the scintillator layer of the detector (Figure 1.2). The latter scintillator will emit visible light proportional to the incident x-ray energy. Visible light photons are then converted into an electric charge by the photodiode array, and the charge collected at each photodiode is converted into a digital value by using the underlying TFT readout electronics. The scintillators used in indirect-conversion detectors can be either structured or unstructured (Figure 1.7) [9,34]. 8 Figure 1.7: Lay-out of an indirect conversion TFT flat-panel detector based on a scintillator (structured or unstructured) and an amorphous silicon photodiode [9]. With an unstructured scintillator, such as Gd2O2S, visible light that is emitted in the material can spread to adjacent pixels and thereby reduce spatial resolution [1,9,34]. To reduce this problem, a structured scintillator that consists of small, column-like cesium iodide crystals can be used (Figure 1.8) [1,7,9,10,34]. The latter specific crystal structure avoids lateral diffusion of the emitted light [1,9,34]. As a result, even thick scintillator layers can be realized, resulting in a high detective quantum efficiency and still a high spatial resolution [9,34]. Figure 1.8: Scanning electron micrograph illustrates the typical CsI:Tl crystal structure. The “needles” are approximately 5-10µm wide [1]. Direct-conversion systems typically use an amorphous selenium x-ray photoconductor as the top layer of the TFT electronics (Figure 1.9) [1,7,9,34]. Before the exposure, an electric field is applied across the photoconductor through a bias electrode on the top surface of the selenium. As x-rays are absorbed in the detector, electrons and holes are released within the selenium layer. Due to the electric field, the electric charges are drawn directly to the charge-collecting electrodes, thereby eliminating the problem of light scatter of indirect-conversion systems. Hence, very high intrinsic spatial resolutions can be achieved, which is of 9 interest especially in mammography [1,7,9,34,35]. On the other hand, compared to gadolinium or cesium, selenium has less favorable x-ray absorption characteristics at diagnostic energies > 40 keV. Making the selenium layer thicker will compensate for this. Figure 1.9: Lay-out of a direct-conversion TFT flat-panel detector, using amorphous selenium to convert x-rays into charges [9]. Direct readout flat-panel detector systems were FDA-approved in 1997 and 1998 and are now increasingly implemented in medical imaging departments [7]. Unlike CR systems, these detectors allow an optimized working procedure due to the instant image display and the elimination of the use of cassettes [8,12]. Moreover, indirect-conversion system can be used for real-time display for fluoroscopy and cineangiography [7,36,37]. 1.3 Image quality in projection imaging 1.3.1 Contrast, spatial resolution and noise In medical imaging, a good image quality is of major importance to assure an accurate diagnosis. In general, image quality is determined by contrast, spatial resolution and noise [1,34]. Contrast is generated by the differential attenuation of x-rays in tissues. The latter radiation (or subject) contrast is transformed by the detector into differences in optical density in the radiograph or differences in brightness on the monitor (image contrast) [1]. The capability to convert subtle differences in the patient’s tissue into image information is called contrast resolution and is an important characteristic of the imaging system [34]. The final image contrast is affected by the applied x-ray energy spectrum and the contrast resolution capabilities of both detector and display system. 10 In digital imaging technology, contrast is mostly described as the contrastto-noise ratio (CNR). The CNR is defined as the signal intensity differences between two image regions A and B with different attenuation divided by the image noise σ (Figure 1.10) [1]: CNR = ( A − B) σ . Figure 1.10: Illustration of the parameter (A-B) in the definition of the contrast-to-noise ratio. Spatial resolution refers to the ability of an imaging system to accurately depict small objects in an image. This is frequently described in visually discernible line pairs per mm (lp/mm) [7]. However, a more objective and accurate presentation of spatial resolution is the concept of the modulation transfer function (MTF) [1,7,34] as it takes into account the relation between spatial resolution and structural contrast [38]. In fact the MTF indicates the contrast which can be transmitted at best by the imaging system to the viewing station, depending on the spatial frequency [34]. The better the contrast transfer at high spatial frequencies is, the smaller are the details that can be recognized separately in the image. In general, the maximum spatial resolution is limited by the pixel size and pixel spacing [1,7]. However, systems with smaller pixel sizes do not necessarily have a higher spatial resolution because other factors such as x-ray scatter and light scatter within the detector contribute to degradation of spatial resolution [7,39,40]. In all imaging modalities, a level of noise is present as a random or stochastic component into the image. Different sources of noise can be distinguished. First of all, the number of x-ray quanta absorbed by the image receptor determines the quantum noise [34]. When an average number of x-rays in a pixel is N, the noise σ in this pixel will be: σ= N. In addition, the total noise is affected and increased by the system noise, i.e. the statistical fluctuations within the imaging system. In digital systems, system noise includes electrical noise and quantization noise [1,34]. Moreover, normal tissue anatomy can act to mask subtle lesions 11 and therefore can reduce the contrast resolution [1]. The latter effect is called anatomic noise. When comparing signal and noise properties of imaging systems, it is useful to analyze the noise versus spatial frequency. These spatial characteristics of the noise fluctuations can be described by the noise power spectrum (NPS) or Wiener spectrum [34,41]: ∫ NPS (ν )dν = σ 2 tot and σ tot2 = ∑ σ i2 i In the latter equation, σ tot denotes the total image noise, whereas σ i represents the individual noise components. The presence of noise significantly influences the contrast detectability of detector systems. The difference between signal and noise must therefore be as high as possible. The latter can be expressed in the quantity signalto-noise ratio (SNR) [34]: SNR = N σ tot ∝ N The SNR will increase with N , as the signal N increases (i.e. when higher detector dose levels are applied). As a result, low-contrast objects will become more perceptible. Previous theoretical studies have shown that the SNR should be at least 3-5, so that an image detail can be recognized by the eye with sufficient reliability [1,34,42]. 1.3.2 Assessment of image quality As in medical imaging a correct diagnosis relies on a good image quality, the latter should be quantified as accurately as possible. This quantification can be done in several ways and might encompass the acquisition part of the (digital) imaging system or the entire system. Physical fundamental characteristics such as contrast, spatial resolution and noise can be independently analyzed using quantities such as CNR, MTF and NPS. However, the latter quantities are abstract measures and a direct link with diagnostic image quality is rather difficult to make. In addition, quantitative results may considerably vary depending on the methodology (experimental setup and calculations) used for the measurement of those quantities, especially for the determination of the MTF [34,38,43-47]. 12 In another approach, image quality is expressed as the detective quantum efficiency (DQE), which incorporates MTF, noise and exposure level. DQE is defined as the ratio of the square of SNR at the output of the system to that at the input of the system and plotted as a function of the spatial frequency [1,34,41]: 2 SNRout DQE = SNRin2 Hence, this quantity describes how efficiently an imaging system will transfer absorbed x-ray energy into useful image information [7]. DQE is dependent on the x-ray spectrum, the radiation dose and the detector medium [1,7,34,38]. In general, DQE is regarded as the best single fundamental indicator to describe the performance of digital radiographic systems [1,7,9,34,38,41]. Unfortunately DQE is difficult to measure in clinical practice [46]. Moreover, the calculation of the DQE is very sensitive to small variations in the measurement setup [47]. Previous discussed objective parameters focus on the performance of the x-ray detector, but do not include the observer in the process of medical image quality analysis. In fact, a number of studies have shown the important influence of psychophysical factors related with the observer in the imaging chain [48-50]. With the use of a contrast-detail phantom, a test of the observer’s perception is possible on a semi objective basis, as the difference between interpretation of the radiologist and the true distribution of test objects can be calculated [48,51]. These phantoms contain test objects of different size and contrast. After image acquisition of the phantom, the observer(s) must indicate the borderline visibility in the radiograph of the phantom, resulting in a contrast-detail curve. In the contrast-detail score, a combined effect of image noise, contrast and resolution is included, thereby providing a straightforward measure for the overall performance [1,34]. 13 Figure 1.11: Digital acquisition of the CDRAD 2.0 phantom. The CDRAD 2.0 phantom consists of a Plexiglas plate (26.5 x 26.5 x 1 cm³) with a 15x15 array of 225 square cell regions in which circular holes are drilled. The latter holes are logarithmically sized from 0.3 to 8.0 mm in both diameter and depth. For 4 mm and smaller objects, the phantom contains an additional hole of matching diameter and depth, placed at random in one of the four cell corners. Today, different contrast-detail phantoms are commercially available. In most of them, however, a potential bias in the phantom image reading can be introduced due to a priori knowledge of the presence of a lowcontrast object [52]. In recent contrast-detail phantom designs – CDRAD 2.0 (Figure 1.11) and CDMAM 3.4 (Figure 1.12) for image quality in radiography and mammography respectively – a four-alternative forcedchoice experiment is set up [53]. These phantoms are subdivided in a large number of cell regions, each of which contains a low-contrast object in the center and another one at a randomly chosen corner (Figure 1.11 and 1.12). After acquisition, the observer must select the location of the low-contrast objects among the four possible corners [54]. 14 Figure 1.12: Sample image of the CDMAM 3.4 phantom. The CDMAM 3.4 phantom, designed for image quality evaluation in mammography, consists of an aluminum base with gold disks of various thicknesses and diameters, attached to a Plexiglas cover (18 x 24 x 0.3 cm³). The discs are arranged in a 16x16 matrix divided into 205 squares cell regions. Within a row, the disc thickness logarithmically increases from 0.03 to 2 µm, while the diameter is constant; within a column, the disc diameter logarithmically increases from 0.06 to 2.00 mm, while the thickness is constant. Each of the 205 cell regions of the phantom contains one gold disk in the centre and another one at a randomly chosen corner. Contrast-detail studies are particularly of interest as the detection of subtle (low-contrast) details is one of the most important aspects in medical imaging [55]. Moreover, the contrast-detail methodology allows an image quality assessment of the complete digital imaging chain, including the image display, whereas DQE measurements concentrate only on the acquisition part. Despite the involvement of human observers in the analysis, contrast-detail studies provide a more objective and less time consuming image quality measure as compared to patient studies [56]. Differences in image quality between imaging systems or between different acquisition settings can be visualized easily, even when the differences are very small [56]. For a better approximation of the clinical reality with respect to human anatomy, acquisitions of anthropomorphic phantoms (Figure 1.13 and 1.14) can be used. They lend themselves to subjective evaluation of image quality in idealized circumstances. In addition, different structures (low and high-contrast objects) can be superimposed over the phantom on well defined positions, simulating different pathologies [57-60]. Obviously, detailed assessments of patient images are the most realistic simulations for image quality evaluation. They have the advantage of being the very same images that are being used in clinical practice without any 15 approximations. The major disadvantage, however, is the natural variability between patients. As a result, large numbers of patients should be included for statistical reasons [1]. Figure 1.13: Example of anthropomorphic chest phantom with corresponding x-ray acquisition (Kyoto Kagaku Phantoms). Figure 1.14: Example of anthropomorphic phantoms for image quality analysis and education: pelvis (left) and hand/wrist (right) phantoms (RSD Phantoms). In anthropomorphic phantom studies as well as in human studies, different methodologies can be applied for image quality analysis. Most of them are based on visual grading techniques, in which the observer must compare an image with a reference acquisition [5,61-64]. Typically, subjective preference scores on a three-point or five-point scale are used for the latter comparisons. Other studies are based on predefined image quality criteria, such as those suggested by the Commission of the European Communities [65]. In the latter document, the image quality criteria refer to characteristic features 16 of imaged anatomical structures with a specific degree of visibility. In the Guidelines, three levels of visibility are defined: visualization (an anatomical feature is detectable but details are not fully reproduced), reproduction (details of anatomical structures are visible but not necessary clearly defined) and visually sharp reproduction (anatomical details are clearly defined) [66]. The observer has to score whether the latter criteria are fulfilled or not. Receiver operating characteristics (ROC) is a method based on signal detection theory where human observers have to score their level of confidence with respect to the presence of specific image details [67]. After data-analysis, the true-positive detection fraction is plotted versus the false-positive fraction, resulting in an ROC-curve [1]. An advantage of ROC studies is that they measure the ability of observers to detect (subtle) details in an image, thereby simulating the clinical practice. In general, the ROC technique is accepted to be the one with the highest accuracy for image quality analysis in a clinical setting [56,67]. Unfortunately, ROC studies are difficult to set up and are very time-consuming. 1.3.3 Image quality versus patient radiation dose All radiological procedures involving x-rays deliver a radiation dose to the patient. The radiation exposure received in an x-ray examination is known to increase the risk for a radio-induced malignancy as well as, above a certain threshold dose, the probability of deterministic effects [1,6,68,69]. Therefore, patient radiation doses should be kept as low as possible (ALARA-principle). On the other hand, image quality will significantly decrease if too low exposure levels are being used. Hence, an ideal balance between patient dose and image quality should be achieved [70-72]. The latter optimization process is described in the recommendations of the International Commission on Radiological Protection (ICRP) [68] and is imposed by the European Union council directive 97/43/Euratom [73]. One interpretation of the ALARA principle, is that the exposure to the patient should be adjusted to obtain the required diagnostic information, not to get the best image quality possible [8,67,70,71]. The latter discussion is particularly relevant in the digital setting. Whereas an overexposed conventional film is easily recognized because the film becomes dark, the wider dynamic range of digital detectors together with the image processing software can accommodate overexposure [1,8]. As a result, overexposure is more difficult to detect in 17 digital radiography, while underexposure will significantly decrease contrast resolution because of the increased noise in the image [1,6,8,9,72]. When installed at a location, the x-ray equipment is usually adjusted by the manufacturer to give a good image quality. However, the latter settings are not automatically adapted to the lowest possible radiation dose [74]. Therefore, an effort by the users to reduce the radiation dose to a level that results in a diagnostically acceptable image quality without excessive radiation exposure is often beneficial [70-72,74]. In all attempts to reduce dose, image quality is crucial. The dose reduction may not be pursued too far, potentially jeopardizing the diagnostic outcome of the procedure. Consequently, it is vital to follow the image quality while optimizing a radiographic procedure. 1.4 Image presentation in digital radiography In the transition from the conventional screen-film methodology to digital radiography, the performance of the digital detector and the corresponding processing are not the only parameters in the (digital) imaging chain that should be studied. In fact, image acquisition and image presentation are separated in the digital setting, allowing an optimized image display when using digital images [7,8]. Until recently, hard-copy prints of digital radiographs were often used for analysis on conventional lightboxes. However, most advantages of the digital environment are lost when printing digital acquisitions on film [8]. Since the introduction of PACS in radiology, computer-based image interpretation is rapidly replacing the film-based evaluation [7,75-77]. Soft-copy reading on medical grade displays provides different advantages such as the possibility of interactively zooming and adjusting the contrast and brightness levels [1,7,8]. Moreover, workflow in a radiological department can considerably be improved by the direct network link between the acquisition console and the display units for reporting [7,12]. High-resolution grey-scale cathode ray tube (CRT) monitors are now widely accepted for primary image interpretation [7]. However, CRT monitors have some disadvantages, including a non-isotropic modulation transfer function (MTF) and a large vulnerability to reflections and ambient lighting conditions [78-80]. In addition, due to their curved faceplate surface, CRT displays can cause peripheral distortion artefacts 18 [78-80]. Furthermore, CRT displays are heavy and bulky and have high levels of power consumption [76,78]. A few years ago, high quality medical grade active matrix Liquid Crystal Display (LCD) devices with varying spatial resolutions became commercially available. LCDs have a nearly perfect MTF behaviour, have higher luminance and are less susceptible to light reflections compared to CRT monitors [75,78-80]. Moreover, LCDs are compact and energyefficient display systems [75,76]. Clinical experience with LCD is limited to date. However, published results show an equivalent diagnostic performance of LCD compared to CRT. In contrast to conventional lightboxes, display devices emit less light. Therefore, special attention has to be paid on the design of the digital reading room. As low-contrast detectability significantly reduces as ambient light is increased, surrounding illuminance should be as low as possible [7]. Moreover, monitors should be accurately calibrated and positioned to avoid light reflections. 1.5 References 1. Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. The essential physics of medical imaging, 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkens, 2002:145-173 2. UNSCEAR, 2000. Sources and effects of ionizing radiation – Vol I: Sources. Report to the General Assembly, United Nations, New York 3. Reiff KJ. Flat panel detectors – closing the (digital) gap in chest and skeletal radiology. Eur J Radiol 1999; 31:125-131 4. Rong XJ, Shaw CC, Liu X, Lemacks MR, Thompson SK. Comparison of an amorphous silicon/cesium iodide flat-panel digital chest radiography system with screen/film and computed radiography systems – A contrast-detail phantom study. Med Phys 2000; 28: 2328-2335 5. Garmer M, Hennings SP, Jäger HJ, et al. Digital radiography versus conventional radiography in chest imaging: diagnostic performance of a large-area flat-panel detector in a clinical CTcontrolled study. AJR 2000; 174: 75-80 19 6. Dowsett DJ, Kenny PA, Johnston RE. The physics of diagnostic imaging. London, UK: Chapman & Hall, 1998:149-174 7. Schaefer-Prokop C, Uffmann M, Eisenhuber E, Prokop M. Digital radiography of the chest: detector techniques and performance parameters. Journal of Thoracic Imaging 2003; 18:124-137 8. Verschakelen J, Bellon E, Deprez T. Digital chest radiography: quality assurance. Journal of Thoracic Imaging 2003; 18:169-177 9. Chotas HG, Dobbins JT, Ravin CE. Principles of digital radiography with large-area electronically readable detectors: a review of the basics. Radiology 1999; 210:595-599 10. Kotter E, Langer M. Digital radiography with large-area flat-panel detectors. Eur Radiology 2002; 12:2562-2570 11. Sonoda M, Takano M, Miyahara J, Kato H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology 1983: 148:833-838 12. May GA, Deer DD, Dackiewietz D et al. Impact of digital radiography on clinical workflow. J Digit Imaging 2000;13 (suppl):76-78 13. Fetterly KA, Schueler BA. Performance valuation of a "dual-side read" dedicated mammography computed radiography system. Med Phys 2003;30:1843-1854 14. Liu X, Shaw CC. Regional improvement of signal-to-noise and contrast-to-noise ratios in dual-screen CR chest imaging: a phantom study. Med Phys 2001;28:1293-1302 15. Schaetzing R, Fasbender R, Kersten P. New high-speed scanning technique for computed radiography. Proc SPIE 2002;4682:511-520 16. Uffmann M, Prokop M, Eisenhuber E, Fuchsjager M, Weber M, Schaefer-Prokop C. Computed radiography and direct radiography: influence of acquisition dose on detection of simulated lung lesions. Invest Radiol 2005;40:249-256 17. Boyle WS, Smith GE. Charge-coupled semiconductor devices. Bell Syst Tech 1970; 49:587-593 20 18. Herron J, Kennedy WH, Gur D. X-ray imaging with twodimensional charge-coupled device (CCD) arrays. Proc SPIE 1984; 486:141-145 19. Roehrig H, Ovitt TW, Dallas WJ, Vercillo R, McNeill KM. Development of a high resolution x-ray imaging device for use in coronary angiography. Proc SPIE 1987; 767:144-153 20. Shaber GS, Lockard C, Boone JM. High resolution digital radiography utilizing CCD planar array. Proc SPIE 1988; 914:262269 21. Atari NA. Performance characteristics of a real-time digital x-ray fluoroscopic system using an intensified charge-injection device camera and CsI:Na crystal. Med Phys 1989; 16:862-872 22. Bath M, Sund P, Mansson LG. Evaluation of the imaging properties of two generations of a CCD-based system for digital radiography. Med Phys 2002; 29:2286-2297 23. Kroft LJM, Geleijns J, Mertens BJA, Veldkamp WJH, Zonderland HM, de Roos A. Digital slot-scan charge-coupled device radiography versus AMBER and screen-film radiography for detection of simulated nodules and interstitial disease in a chest phantom. Radiology 2004; 231:156-163 24. Orava R. New detectors for radiology. Phys Medica 1999; 15:295300 25. Wenzel A, Gröndahl HG. Direct digital radiography in the dental office. Int Dent J 1995; 45:27-34 26. Welander U, Nelvig P, Tronje G, et al. Basic technical properties of system for direct acquisition of digital intraoral radiographs. Oral Surg Oral Med Oral Pathol 1993; 75:506-516 27. Tesic MM, Piccaro MF, Munier B. Full field digital mammography scanner. Eur J Radiol 1999; 31:2-17 28. Maidment AD, Yaffe MJ. Analysis of the spatial-frequencydependent DQE of optically coupled digital mammography detectors. Med Phys 1994; 21:721-729 21 29. Karellas A, Harris LJ, Liu H, Davis MA, D’Orsi CJ. Charge-coupled device detector: performance considerations and potential for small-field mammographic imaging applications. Med Phys 1992; 19:1015-1023 30. Liu H, Xu J, Halama G, McAdoo J. Digital fluoroscopy using an optically coupled CCD: preliminary investigations. Proc SPIE 1997; 2976:256-261 31. Veldkamp WJH, Kroft LJM, Mertens BJA, Geleijns J. Digital slotscan charge-coupled device radiography versus AMBER and bucky screen-film radiography: comparison of image quality in a phantom study. Radiology 2005; 235:857-866 32. Mainprize JG, Ford NL, Yin S, Tumer T, Yaffe MJ. A slot-scanned photodiode-array/CCD hybrid detector for digital mammography. Med Phys 2002; 29:214-225 33. Samei E, Saunders RS, Lo JY, et al. Fundamental imaging characteristics of a slot-scan digital chest radiographic system. Med Phys 2004; 31:2687-2698 34. Aichinger H, Dierker J, Joite-Barfuss S, Säbel M. Radiation exposure and image quality in x-ray diagnostic radiology. Berlin, Germany: Springer-Verlag, 2004:57-81 35. Que W, Rowlands JA. X-ray imaging using amorphous selenium: inherent spatial resolution. Med Phys 1995; 22:365-374 36. Ducourant T, Michel M, Päppler T, et al. Optimisation of key building blocks for a large area radiographic and fluoroscopic dynamic digital x-ray detector based on a a-Si:H/CsI:Tl flat panel technology. Proc SPIE 2000; 3977:14-25 37. Granfors PR, Aufrichtig R, Possin GE, et al. Performance of a 41x41 cm² amorphous silicon flat panel x-ray detector designed for angiographic and R&F imaging applications. Med Phys 2003; 30:2715-2726 38. Moy JP. Signal–to-noise ratio and spatial resolution in x-ray electronic imagers: is the MTF a relevant parameter? Med Phys 2000; 27:86-93 22 39. Flynn MJ, Samei E. Experimental comparison of noise and resolution for 2k and 4k storage phosphor radiography system. Med Phys 1999; 26:1612-1623 40. Miro SPM, Leung AN, Rubin GD, et al. Digital storage phosphor chest radiography: an ROC study of the effect of 2k versus 4k matrix size on observer performance. Radiology 2001; 218:527-532 41. International Commission on Radiation Units and Measurements (ICRU), Report 54, Medical imaging – the assessment of image quality. Bethesda, MD, 1996 42. Rose A. Vision: human and electronic. New York, NY: Plenum Press, 1973 43. Bradford CD, Peppler WW, Waidelich JM. Use of a slit camera for MTF measurements. Med Phys 1999; 26:2286-2294 44. Illers H, Buhr E, Günther-Kohfahl S, Neitzel U. Measurement of the modulation transfer function of digital x-ray detectors with an opaque edge-test device. Rad Prot Dosim 2005; 114:214-219 45. Greer PB, van Doorn T. Evaluation of an algorithm for the assessment of the MTF using an edge method. Med Phys 2000; 27:2048-2059 46. Borasi G, Nitrosi A, Ferrari P, Tassoni D. On site evaluation of three flat panel detectors for digital radiography. Med Phys 2003; 30: 1719-1731 47. Neitzel U, Günther-Kohfahl S, Borasi G, Samei E. Determination of the detective quantum efficiency of a digital x-ray detector: comparison of three evaluations using a common image data set. Med Phys 2004; 31:2205-2211 48. Peer S, Neitzel U, Giacomuzzi SM, et al. Comparison of lowcontrast detail perception on storage phosphor radiographs and digital flat panel detector images. IEEE Trans Med Imag 2001; 20:239-242 49. Tapiovaara MJ. Efficiency of low-contrast detail detectability in fluoroscopic imaging. Med Phys 1997; 24:655-664 50. Burgess AE. Comparison of receiver operating characteristic and forced choice observer performance measurement methods. Med Phys 1995;22:643-655 23 51. Kundel HL. Images, image quality and observer performance: new horizons in radiology lecture. Radiology 1979; 132:265-271 52. Rong XJ, Shaw CC, Liu X, Lemacks MR, Thompson SK. Comparison of an amorphous silicon/cesium iodide flat-panel digital chest radiography system with screen/film and computed radiography systems – A contrast-detail phantom study. Med Phys 2000; 28: 2328-2335 53. Thijssen MAO, Thijssen HOM, Merx JL, Lindeijer JM, Bijkerk KR. A definition of image quality: the image quality figure. In: Moores BM, Wall BF, Eriskat H, Schibilla H, editors. BIR Report 20: Optimization of image quality and patient exposure in diagnostic radiology. London: The British Institute of Radiology; 1989. p. 29-34 54. Aufrichtig R, Xue P. Dose efficiency and low-contrast detectability of an amorphous silicon x-ray detector for digital radiography. Phys Med Biol 2000; 45:2653-2669 55. Peer S, Giacomuzzi SM, Peer R, Gassner E, Steingruber I, Jaschke W. Resolution requirements for monitor viewing of digital flatpanel detector radiographs: a contrast-detail analysis. Eur Radiol 2003; 13:413-417 56. Geijer H, Beckman KW, Andersson T, Perslinden J. Image quality vs radiation dose for a flat-panel amorphous silicon detector: a phantom study. Eur Radiol 2001; 11:1704-1709 57. Strotzer M, Gmeinwieser J, Voelk M, Fruend R, Setz J, Feuerbach S. Detection of simulated chest lesions with normal and reduced radiation dose: comparison of conventional screen-film radiography and a flat-panel X-ray detector based on amorphous silicon. Invest Radiol 1998; 33:98–103 58. Rapp-Bernhardt U, Roehl FW, Gibbs RC, Schmidl H, Krause UW, Bernhardt TM. Flat-panel X-ray detector based on amorphous silicon versus asymmetric screen-film system: phantom study of dose reduction and depiction of simulated findings. Radiology 2003; 227:484-492 59. Bernhardt TM, Otto D, Reichel G, et al. Detection of simulated interstitial lung disease and catheters with selenium, storage phosphor, and film-based radiography. Radiology 1999; 213:445454 24 60. Kroft LJM, Geleijns J, Mertens BJA, Veldkamp WJH, Zonderland HM, de Roos A. Digital slot-scan charge-coupled device radiography versus AMBER and screen-film radiography for detection of simulated nodules and interstitial disease in a chest phantom. Radiology 2004; 231:156-163 61. Fink C, Hallscheidt PJ, Noeldge G, et al. Clinical comparative study with a large-area amorphous silicon flat-panel detector: image quality and visibility of anatomic structures on chest radiography. AJR 2002; 178:481–486 62. Strotzer M, Völk M, Fründ R, et al. Routine chest radiography using a flat-panel detector: image quality at standard detector dose and 33% dose reduction. AJR 2002; 178:169-171 63. Hosch C, Hallscheidt PJ, Noeldge G, et al. Radiation dose reduction in chest radiography using a flat-panel amorphous silicon detector. Clin Radiology 2002; 57:902-907 64. Herrmann KA, Bonel H, Stäbler A, et al. Chest imaging with flatpanel detector at low and standard doses: comparison with storage phosphor technology in normal patients. Eur Radiol 2002; 12:385390 65. Commission of the European Communities. European guidelines on quality criteria for diagnostic radiographic images. (EUR 16260). Brussels: CEC, 1996 66. Vaño E, Guibelalde E, Morillo A, Salvarez-Pedrosa CS, Fernandez JM. Evaluation of the European image quality criteria for chest examinations. Br J Radiol 1995; 68:1349-1355 67. Martin CJ, Sharp PF, Sutton DG. Measurement of image quality in diagnostic radiology. Appl Radiat Isot 1999; 50:21-38 68. ICRP, Publication 60, Recommendations of the International Commission on Radiological Protection, Pergamon Press. Oxford, 1991 69. Faulkner K, Broadheas DA, Harrison RM. Patient dosimetry measurement methods. Appl Radiat Isot 1999; 50:113-123 70. Martin CJ, Sutton DG, Sharp PF. Balancing patient dose and image quality. Appl Radiat Isot 1999; 50:1-19 25 71. ICRP, Publication 93, Managing patient dose in digital radiology, Elsevier. Oxford, 2004 72. Marshall NW. Optimisation of dose per image in digital imaging. Rad Prot Dosim 2001; 94:83-87 73. The Council of the European Union. Council Directive 97/43/Euratom. Official Journal of the European Communities 1997;L (180):22-27 74. Peters SE, Brennan PC. Digital radiography: are the manufacturers’ settings too high? Optimisation of the Kodak digital radiography system with aid of the computed radiography dose index. Eur Radiol 2002; 12:2381-2387 75. Balassy C, Prokop M, Weber M, Sailer J, Herold CJ, SchaeferProkop C. Flat-panel display (LCD) versus high-resolution gray-scale display (CRT) for chest radiography: an observer preference study. Am J Roentgenol 2005; 184:752-756 76. Hwang SA, Seo JB, Choi BK, et al. Liquid-crystal display monitors and cathode-ray tube monitors: a comparison of observer performance in the detection of small solitary pulmonary nodules. Korean J Radiol 2003; 4:153-156 77. Goo JM, Choi JY, Im JG, et al. Effect of monitor luminance and ambient light on observer performance in soft-copy reading of digital chest radiographs. Radiology 2004; 232: 762-766 78. Scharitzer M, Prokop M, Weber M, Fuchsjäger M, Oschatz E, Schaefer-Prokop C. Detectability of catheters on bedside chest radiographs: comparison between liquid crystal display and highresolution cathode-ray tube monitors. Radiology 2005; 234:611-616 79. Oschatz E, Prokop M, Scharitzer M, Weber M, Balassy C, SchaeferProkop C. Comparison of liquid crystal versus cathode ray tube display for the detection of simulated chest lesions. Eur Radiol 2005; 15:1472-1476 80. Krupinski AE, Johnson J, Roehrig H, Nafziger J, Fan J, Lubin J. Use of a human visual system model to predict observer performance with CRT vs LCD display of images. J Digit Imaging 2004; 17: 258263 26 Chapter 2 Aim and outline of the thesis 2.1 Aim The last years, a large variety of digital detectors became commercially available for implementation in digital radiography applications [1-4]. As the performance of these new detectors should be at least as good as in conventional screen-film radiography, one of the aims of the thesis was to assess both image quality and patient radiation dose in different digital acquisition systems. For the assessment of the image quality, patient chest images as well as contrast-detail phantom acquisitions were used. The latter methodology provides a more objective and less time consuming image quality measure [5-8]. In this thesis, both methods were compared and the feasibility of using contrast-details studies for image quality assessment was studied. One of the major advantages of digital radiographs is the flexible image display with the possibility of interactively adjusting the contrast and brightness levels [9,10,12]. These benefits can only be achieved in softcopy display reading. Moreover, soft-copy reading may significantly improve the workflow and cost-effectiveness in a digital medical imaging department [10,11,13]. Despite these advantages, digital radiographs are still printed in many departments and analyzed on conventional light boxes [10]. Therefore, the image quality performance of hard-copies was compared with a soft-copy image presentation. In addition, the influence of image magnification and the interactive window/level adjustment on the low-contrast performance in soft-copy reading was studied. In soft-copy reading, a high quality monitor system should be used. Liquid crystal displays are gradually replacing cathode-ray tubes for primary soft-copy reading [14-16]. As LCDs are available in a large 27 variety of different matrix resolutions, the last aim of the thesis was to assess the contrast-detail quality of primary liquid crystal devices of varying resolutions in comparison with a state-of-the-art high resolution cathode ray tube monitor. 2.2 Outline Chest radiography is a highly demanding imaging methodology because a wide range of tissue densities have to be projected on the detector [17,18]. Hence, the latter detector should have a high dynamic range and more specifically an excellent contrast resolution in order to be able to visualize all structures of the chest in detail [17,19]. Therefore, the quality of chest radiographs is frequently used as a reference for the image quality that can be obtained in new technologies in radiography. In the first study described in chapter three (part I, p 32), the image quality was scored using the “European Guidelines on Quality Criteria for Diagnostic Radiographic Images” [20] for chest radiographs acquired with a full-field digital amorphous silicon flat-panel detector, a state-ofthe-art screen-film system and a computed radiography system. Both entrance skin and effective doses were measured in a patient study including three hundred patients. In addition, a more objective image quality measure was performed, using a contrast-detail analysis. As our first study showed an excellent image quality and dose performance for the flat-panel detector system, two different flat-panel detector types based on the active matrix thin-film transistor technology were compared in part II (p 48). The analyzed detector systems comprised the previous studied amorphous silicon system and an amorphous selenium flat-panel detector. An extensive contrast-detail analysis was performed at different exposure levels and the latter results were compared with the subjective image quality analysis according to the European guidelines in a patient study. In the transition from the conventional screen-film methodology to digital radiography, the performance of the digital detector is not the only parameter in the (digital) imaging chain that should be studied. In fact, image acquisition and image presentation are separated in the digital setting [21-23]. In part III (p 64) different image reading methodologies were analyzed in a contrast-detail study. Hard-copy prints of digital radiographs were compared with a soft-copy reading on medical grade displays. 28 A more detailed study of soft-copy image reading was performed in part IV (p 73). In the latter part, liquid crystal displays of different resolutions were compared with a high resolution greyscale cathode ray tube monitor. Furthermore, the influence of interactive image magnification and adjustment of image brightness and contrast on the image quality and the reading efficiency was assessed. 2.3 References 1. Reiff KJ. Flat panel detectors – closing the (digital) gap in chest and skeletal radiology. Eur J Radiol 1997;31:125-131 2. Chotas HG, Dobbins JT, Ravin CE. Principles of digital radiography with large-area electronically readable detectors: a review of the basics. Radiology 1999; 210:595-599 3. Kotter E, Langer M. Digital radiography with large-area flat-panel detectors. Eur Radiology 2002; 12:2562-2570 4. Schaefer-Prokop C, Uffmann M, Eisenhuber E, Prokop M. Digital radiography of the chest: detector techniques and performance parameters. Journal of Thoracic Imaging 2003; 18:124-137 5. Peer S, Giacomuzzi SM, Peer R, Gassner E, Steingruber I, Jaschke W. Resolution requirements for monitor viewing of digital flatpanel detector radiographs: a contrast-detail analysis. Eur Radiol 2003; 13:413-417 6. Borasi G, Nitrosi A, Ferrari P, Tassoni D. On site evaluation of three flat panel detectors for digital radiography. Med Phys 2003; 30: 1719-1731 7. Rong XJ, Shaw CC, Liu X, Lemacks MR, Thompson SK. Comparison of an amorphous silicon/cesium iodide flat-panel digital chest radiography system with screen/film and computed radiography systems – A contrast-detail phantom study. Med Phys 2000; 28: 2328-2335 8. Chotas HG, Ravin CE. Digital chest radiography with a solid-state flat-panel X-ray detector: contrast-detail evaluation with processed images printed on film hard copy. Radiology 2001; 218:679-682 29 9. Balassy C, Prokop M, Weber M, Sailer J, Herold CJ, SchaeferProkop C. Flat-panel display (LCD) versus high-resolution gray-scale display (CRT) for chest radiography: an observer preference study. Am J Roentgenol 2005; 184:752-756 10. Scharitzer M, Prokop M, Weber M, Fuchsjäger M, Oschatz E, Schaefer-Prokop C. Detectability of catheters on bedside chest radiographs: comparison between liquid crystal display and highresolution cathode-ray tube monitors. Radiology 2005; 234:611-616 11. Fuchsjäger MH, Schaefer-Prokop CM, Eisenhuber E, et al. Impact of ambient light and window settings on the detectability of catheters on soft-copy display of chest radiographs at bedside. Am J Roentgenol 2003; 181:1415-1421 12. Goo JM, Choi JY, Im JG, et al. Effect of monitor luminance and ambient light on observer performance in soft-copy reading of digital chest radiographs. Radiology 2004; 232: 762-766 13. Graf B, Simon U, Eickmeyer F, Fiedler V. Bushberg JT, Seibert JA, 1K versus 2K monitor: a clinical alternative free-response receiver operating characteristic study of observer performance using pulmonary nodules. AJR 2000;174:1067-1074 14. Averbukh AN, Channin DS, Homhual P. Comparison of human observer performance of contrast-detail detection across multiple liquid crystal displays. J Digit Imaging 2005; 18: 66-77 15. Krupinski AE, Johnson J, Roehrig H, Nafziger J, Fan J, Lubin J. Use of a human visual system model to predict observer performance with CRT vs LCD display of images. J Digit Imaging 2004; 17: 258263 16. Oschatz E, Prokop M, Scharitzer M, Weber M, Balassy C, SchaeferProkop C. Comparison of liquid crystal versus cathode ray tube display for the detection of simulated chest lesions. Eur Radiol 2005; 15:1472-1476 17. Garmer M, Hennigs SP, Jäger HJ, et al. Digital radiography versus conventional radiography in chest imaging: diagnostic performance of a large-area flat-panel detector in a clinical CTcontrolled study. AJR 2000; 174:75–80 30 18. Kroft LJM, Geleijns J, Mertens BJA, Veldkamp WJH, Zonderland HM, de Roos A. Digital slot-scan charge-coupled device radiography versus AMBER and screen-film radiography for detection of simulated nodules and interstitial disease in a chest phantom. Radiology 2004; 231:156-163 19. Rapp-Bernhardt U, Roehl FW, Gibbs RC, Schmidl H, Krause UW, Bernhardt TM. Flat-panel X-ray detector based on amorphous silicon versus asymmetric screen-film system: phantom study of dose reduction and depiction of simulated findings. Radiology 2003; 227:484-492 20. Commission of the European Communities. European guidelines on quality criteria for diagnostic radiographic images. Brussels: Commission of the European Communities, 1996. Publication EUR16260 21. Bonardi R, Ambrogetti D, Ciatto S, et al. Conventional versus digital mammography in the analysis of screen-detected lesions with low positive predictive value. Eur J Radiol 2005; 55: 258-263 22. Hamers S, Freyschmidt J, Neitzel U. Digital radiography with a large-scale electronic flat-panel detector vs screen-film radiography: observer preference in clinical skeletal diagnostics. Eur Radiol 2001; 11:1753-1759 23. Honey ID, MacKenzie A, Evans DS. Investigation of optimum energies for chest imaging using film-screen and computed radiography. Br J Radiol 2005; 78:422-427 31 Chapter 3 Original research: results 3.1 Part I Dose reduction in patients undergoing chest imaging: digital amorphous silicon flat-panel detector radiography versus conventional film-screen radiography and phosphor-based computed radiography Klaus Bacher1 – Peter Smeets2 – Kris Bonnarens1 – An De Hauwere1 – Koenraad Verstraete2 – Hubert Thierens1 1Department of Medical Physics and Proeftuinstraat 86, Gent B-9000, Belgium. 2Department Belgium. Radiation Protection, Ghent University, of Radiology, Ghent University Hospital, De Pintelaan 185, Gent B-9000, Reprint from AJR 2003; 181:923–929 Abstract We sought to compare the radiation dose delivered to patients undergoing clinical chest imaging on a full-field digital amorphous silicon flat-panel detector radiography system with the doses delivered by a state-of-the-art conventional film-screen radiography system and a storage phosphor-based computed radiography system. Image quality was evaluated to ensure that the potential reduction in radiation dose did not result in decreased image acuity. Three groups of 100 patients each were examined using the amorphous silicon flat-panel detector, film-screen, or computed radiography systems. All patient groups were matched for body mass index, sex, and age. To measure the entrance skin dose, we attached 24 calibrated thermoluminescent dosimeters to every patient. The calculation of the effective dose, which represents the risk of late radiation-induced effects, was based on measurements on an anthropomorphic phantom. Image quality of all three systems was evaluated by five experienced radiologists, using the European Quality Criteria for Chest Radiology. In addition, 33 a contrast-detail phantom study was set up to assess the low-contrast detection of all three systems. The amorphous silicon flat-panel detector radiography system allowed an important and significant reduction in both entrance skin dose and effective dose compared with the filmscreen radiography (× 2.7 decrease) or computed radiography (× 1.7 decrease) system. In addition, image quality produced by the amorphous silicon flat-panel detector radiography system was significantly better than the image quality produced by the film-screen or computed radiography systems, confirming that the dose reduction was not detrimental to image quality. The introduction of digital flat-panel radiography systems based on amorphous silicon and cesium iodide is an important step forward in chest imaging that offers improved image quality combined with a significant reduction in the patient radiation dose. Introduction Chest radiography is the most commonly applied diagnostic radiographic procedure [1]. Most chest radiographs are still acquired with conventional film-screen radiography systems that provide good image quality, high spatial resolution, and generally low costs [2,3]. However, typical disadvantages of film-screen radiography techniques are a limited exposure range, a rather high retake rate, and the inflexibility of image display and film management [2]. Because computer technology and storage capacity have developed rapidly during recent years, PACS (picture archiving and communication systems) have become more important, and the accurate implementation of a PACS depends on digital radiography techniques. Digital radiography systems offer an instant image display, a wide dynamic range, and a linear signal response [2]. Moreover, digital images allow flexibility in processing and archiving, thereby providing a solution to the major disadvantages of the film-screen radiography systems. The first step in digital chest radiography was the use of storage phosphor plates. Introduced some 20 years ago [4], these computed radiography systems are widely used because of their compatibility with existing radiography equipment. However, conflicting results have been reported concerning comparisons of the image quality and radiation dose delivered by computed radiography systems with those of conventional film-screen radiography combinations [2]. Recently, full-field digital amorphous silicon flat-panel X-ray detector radiography systems based on cesium iodide and amorphous silicon have become commercially available. These systems combine all advantages of digital radiography with a higher quantum detection efficiency than is attainable with film-screen or computed radiography systems [5]. Several 34 studies have confirmed the excellent image quality provided by the amorphous silicon flat-panel detector radiography system [2,5–7], but none has measured and calculated the patient radiation dose in correlation with the diagnostic performance of the detector. Hence, our aim was to compare the patient radiation dose delivered by a full-field digital amorphous silicon flat-panel detector radiography system with those of state-of-the-art conventional film-screen and storage phosphor-based computed radiography systems in clinical chest imaging. Image quality performance was evaluated to ensure that the potential dose reduction was not detrimental to quality. Subjects and Methods Patients Three hundred patients referred to our institution for routine posteroanterior and lateral chest radiography were randomly assigned to undergo imaging with one of three methods—film-screen radiography, computed radiography, or amorphous silicon flat-panel detector radiography. All three patient groups were matched for body mass index, sex, and age (Table 1). Table 1: Demographic data of three groups of 100 patients each imaged with three types of imaging systems Demographic Factor DFPD CR FSR Sex (male/female) 56 / 44 58 / 42 65 / 35 Mean 53.7 57.0 57.8 Range 21 – 83 20 – 83 20 – 84 Mean 24.1 25.3 24.4 p* Age (years) 0.337 BMI Range 0.418 17.5 – 33.8 17.5 – 48.8 16.6 – 42.2 Note – DFPD=digital flat-panel detector radiography, CR=computed radiography, FSR=film-screen radiography, BMI=body mass index. *Determined with Kruskal-Wallis test. Patients who had undergone pneumectomy, had extremely deformed spines, or were unable to raise their arms high enough to allow lateral imaging were excluded because the radiation exposure in these patients would have been higher than that in the average patient and could 35 therefore have artificially increased the mean measured radiation dose of the patient groups. Image Acquisition Standard posteroanterior and lateral chest radiographs were obtained with the patients standing at a focus-to-detector distance of 180 cm. All imaging was performed at 125 kVp using automatic exposure control. Exposure values (milliampere-seconds) for each acquisition were noted. Digital radiography The amorphous silicon flatpanel detector radiography system used in this study (Siemens, Erlangen, Germany; Trixell, Moirans, France) consisted of an X-ray tube (Optilix 150/30/50 HC-100, Siemens; focal spot size, 0.6 mm), a highvoltage generator (Polydoros LX 30 or 50 Lite, Siemens), and a motorized receptor wall stand with the flat-panel detector mounted behind a stationary antiscatter grid (80 lines per centimeter; ratio, 15:1). This amorphous silicon image detector is equipped with a 43×43 cm Xray sensing surface with a 3000×3000 matrix and a 143µm pixel size. The detector consists of a needle-structured thallium-doped cesium iodine scintillator layer and an amorphous silicon thin-film transistor array. The cesium iodine scintillator converts the X rays into the visible light that is deposited directly onto the amorphous silicon matrix and is subsequently converted by an amorphous silicon photodiode into an electrical charge, resulting in a 14-bit digital signal. The automatic exposure control of the flat-panel detector radiography system was adjusted to a 400-speed class. After acquisition, the digital data were sent to a PACS workstation (MagicView, Siemens) for assessment of image quality. Computed radiography The computed radiography system consisted of an X-ray tube (Opti 150/40/73 C, Siemens; focal spot size, 0.6 mm), a high-voltage generator (Polydoros 80S; Siemens), and a wall stand with an in-height adjustable cassette holder. A moving grid (40 lines per centimeter; ratio 12:1) was used to reduce scatter. Each of the storage phosphor cassettes used (GP DirectView cassettes, Eastman Kodak, Rochester, NY) has a 35×43 cm detection surface, with a 2048×2500 pixel matrix and a pixel size of 168µm. After the patients were irradiated, the storage screen data were reviewed on a dedicated unit (DirectView CR-900, Eastman Kodak) by scanning the screen line-by-line with a laser beam. The resulting digital images were sent to a PACS workstation (MagicView, Siemens). 36 Conventional film-screen radiography Conventional film-screen radio-graphy was performed using a dedicated automatic chest film changer (Thoramat, Siemens) combined with a 250speed wide-latitude asymmetric film-screen system optimized for thoracic imaging (InSight Thoracic imaging film and screen, Eastman Kodak). The X rays were generated using an X-ray tube (Optitop 150/40/80 HC-100, Siemens) with a focal spot size of 0.6 mm and a generator (Polydoros 80S, Siemens). A moving antiscatter grid was used (40 lines per centimeter; ratio, 12:1). Dose Measurements Entrance skin dose For the measurement of the entrance skin dose, 24 calibrated thermoluminescent dosimeters (Harshaw TLD-100, Thermo Electron, Solon, OH) were attached to each patient at six well-defined and reproducible locations. To ensure the reproducibility of the location of all thermoluminescent dosimeters, we used the square pattern in the light beam of the X-ray tube. For the posteroanterior acquisition, we spread 20 thermoluminescent dosimeters equally over five locations on the back of the patient: the center and all four corners of the light field. For the lateral radiographs, we attached four thermoluminescent dosimeters to the right side of the patient (toward the X-ray tube) in the center of the light pattern. Each set of 24 thermoluminescent dosimeters was used to measure radiation dose in 20 successive patients to obtain a high signalto-noise ratio. We analyzed the thermoluminescent dosimeters using a Harshaw 3500 reader (Thermo Electron). Effective dose The concept of the effective dose, introduced by the International Commission on Radiological Protection, is used to measure nonuniform radiation exposure [8]. The effective dose (E), representing the risk of late radiation-induced effects such as malignancies, is defined by the expression: E = ∑ wT H T T In this equation, HT is the equivalent radiation dose to tissue T and wT is the weighting factor representing the relative radiation sensitivity of the tissue T. An overview of the tissue-weighting factors is given in Table 2. 37 Table 2: Mean equivalent tissue doses for posteroanterior and lateral chest radiographs obtained on digital flat-panel detector, computed radiography, and film-screen radiography systems Equivalent Tissue Dose HT (µSv) Tissue wT TLD used for each tissue DFPD CR SFR PA LAT PA LAT PA LAT Gonads 0.20 4 0.3 0.8 0.6 1.2 0.4 1.9 Active bone marrow 0.12 20# 12.0 25.8 23.6 41.5 30.5 73.4 Lungs 0.12 42 30.5 98.6 60.1 158.4 70.0 260.9 Colon 0.12 18 4.9 6.2 9.7 10.0 10.9 17.4 Stomach 0.12 6 8.9 17.3 17.5 27.7 20.2 45.1 Liver 0.05 20 14.7 68.0 29.0 109.1 34.9 191.6 Thyroid 0.05 2 6.1 12.7 11.9 20.4 19.8 66.1 Esophagus 0.05 2 15.9 45.8 31.4 73.5 42.2 146.3 Breasts 0.05 4 5.4 10.5 10.7 16.8 17.2 42.9 Urinary bladder 0.05 3 0.2 0.6 0.4 1.0 0.3 1.3 Bone surface 0.01 20# 31.4 68.1 61.8 109.4 79.2 188.9 Skin 0.01 16 6.8 31.9 13.4 51.2 31.5 102.3 Remainder* 0.05 29 4.9 25.9 9.7 41.6 11.1 73.6 Note – DFPD=digital flat-panel detector radiography, CR=computed radiography, FSR=film-screen radiography, PA=posteroanterior, LAT=lateral, wT=tissue weighting factors [8] for determination of effective dose to phantom, TLD= thermoluminescent dosimeters. #Same thermoluminescent dosimeters were used for calculating radiation dose to both active bone marrow and bone surface. *Radiation dose of remainder was calculated as mass-averaged radiation dose to brain, spleen, pancreas, adrenal glands, kidneys, uterus, small intestine, and muscle tissues. Equivalent radiation doses to the tissues of the organs listed in Table 2 were determined by placing 166 calibrated thermoluminescent dosimeters on an anthropomorphic (representing an average-sized man) Rando phantom (The Phantom Laboratory, Salem, NY) on regions that represented these organs and tissues. We adopted the distribution of bone marrow over the body described by Christy [9]. The choice of the thermoluminescent dosimeter locations was based on a complete CT scan of the phantom. The distribution of the 166 thermoluminescent dosimeters over the phantom is also given in Table 2. Posteroanterior and lateral chest radiographs of the Rando phantom were obtained (125 kVp; focus-to-detector distance, 180 cm) on the digital flat-panel detector, computed, and filmscreen radiography systems. To obtain dose 38 measurements well above the detection limit of the thermoluminescent dosimeters, we set the exposure to 300 mAs. We used the data from the thermoluminescent dosimeters to calculate the mean equivalent organ doses per milliampere-second. Multiplying this result by the mean registered exposures of the three patient groups, we derived the mean equivalent organ doses (measured in microsieverts [µSv]) for the flat-panel detector, computed, and film-screen radiography systems (Table 2). We used equation 1 to calculate the effective dose (Table 3). Image Quality Scoring of patient images Five experienced chest radiologists assessed all chest radiographs and recorded the score of the image quality on a questionnaire. All images were interpreted independently. Each radiologist rated the visibility and radiographic quality of 12 anatomic regions as either clearly visible (scored as 1) or not (scored as 0). The choice of the anatomic structures used for image evaluation was based on the European Guidelines on Quality Criteria for Diagnostic Radiographic Images [10]. The evaluated regions are indicated in Table 4. The mean overall score was calculated using the individual scores of all evaluated regions. For the digital images (flat-panel detector radiography and computed radiography systems), the radiologists were allowed to adjust the image brightness and contrast as well as to magnify the images. All digital images were scored on a 21-inch (53 cm), high-contrast gray-scale monitor (SMM 21140P, Siemens) with a resolution of 1280×1600. Contrast-detail phantom study Because no reference images were available, the analysis of image quality based on patient data was subjective. Therefore, a contrast-detail phantom study (using Contrast-Detail Phantom for Digital and Conventional Radiography, version 2.0, University Hospital Nijmegen, St. Radboud, The Netherlands) was set up for a more objective analysis of the image quality produced by the three radiography systems. A detailed description of the CDRAD 2.0 phantom can be found elsewhere [2,7,11]. This phantom was used to assess the minimum contrast required to visualize objects of different sizes above the signal-to-noise threshold. The phantom was placed between two layers of 5-cm polymethylmethacrylate to simulate patient scatter. Three images of the phantom were acquired with all three radiography systems under the same 39 conditions as were used to image the patients. All images were scored independently by the five radiologists using the methodology described by the manufacturers of the phantom [11]. The reviewers were again allowed to adjust the image brightness and contrast of the digital images as well as to magnify the images. The results were presented in contrastdetail curves. Statistical Analysis The Kruskal-Wallis test was used to compare the age and body mass index of the three patient groups. Differences between two mean values were tested for significance using the two-tailed Mann-Whitney test (95% confidence level). For the comparison of the image quality (expressed as a percentage) between two imaging systems, chi-square statistics were used. To check the reliability of the image quality evaluation by the five independent radiologists, we assessed the interobserver correlation by calculating Spearman’s rank correlation for all image quality criteria for all observer combinations. A significant correlation indicated that the scores of the observers could be combined into a mean score for the image quality parameters [6]. All statistical calculations were performed using MedCalc software (MedCalc, Gent, Belgium). Results Demographic data of the three patient groups are summarized in Table 1. No statistical difference in age (p=0.337) or body mass index (p=0.418) was found among the patient groups. Figure 1 represents the frequency distributions of the recorded milliampere-second values for posteroanterior chest images acquired on the flat-panel detector, computed, and film-screen radiography systems for the patient groups. Mean equivalent radiation doses to all tissues for the posteroanterior and lateral radiographs are compared in Table 2. Table 3 summarizes the results of the exposure and dose measurements. For both the posteroanterior and lateral acquisitions, a highly significant reduction in exposure, entrance skin dose, and effective dose was found with the amorphous silicon digital flat-panel detector radiography system. For the posteroanterior image, the flat-panel detector radiography system showed a reduction (p<0.0001) in effective dose from 18.8µSv with the computed radiography system and with 23.2µSv for the film-screen radiography system to 9.6 µSv. For the lateral image, the effective dose decreased (p<0.0001) from 43.5µSv with the computed radiography 40 system and 77.0µSv with the film-screen radiography system to a value of 27.1µSv with the flat-panel detector radiography system. Figure 1: Bar graph shows frequency distribution of measured milliampere-seconds for posteroanterior chest images acquired on three imaging systems: amorphous silicon flatpanel detector radiography (A), computed radiography (B), and filmscreen radiography (C) systems 41 Table 3: Mean measured exposure, effective dose and entrance skin dose values for posteroanterior and lateral chest radiographs obtained on digital flat-panel detector radiography, computed radiography, and film-screen radiography systems Posteroanterior Lateral p* Factor DFPD CR FSR p* DFPD vs CR DFPD CR FSR FSR DFPD vs CR FSR Exposure (mAs) Mean 1.25 2.46 3.95 Range 0.752.74 1.526.63 2.0510.50 Mean 9.6 18.8 23.2 Range 5.721.0 11.650.8 12.162.1 Mean 66.8 164.9 199.0 Range 56.276.3 123.3- 149.8205.3 230.9 <0.0001 <0.0001 4.83 7.74 20.9 1.1117.98 2.8837.4 4.2792.2 27.1 43.5 77.0 6.2101.0 16.2210.2 19.8426.9 346.7 733.6 1286.2 283.6396.6 523.9- 1162.3823.3 1464.6 <0.0001 <0.0001 E (µSv) <0.0001 <0.0001 <0.0001 <0.0001 ESD (µGy) 0.0001 <0.0001 0.009 0.008 Note – DFPD=digital flat-panel detector radiography, CR=computed radiography, FSR=film-screen radiography, E=effective dose, ESD=entrance skin dose. *Determined using two-tailed Mann-Whitney test. Comparing the entrance skin doses allowed similar conclusions to be made. In the posteroanterior images, the mean entrance skin dose decreased from 164.9µGy with the computed radiography system (p=0.0001) and 199.0µGy with the film-screen radiography system (p<0.0001) to 66.8µGy with the flat-panel detector radiography system. For the lateral images, a decrease in the mean entrance skin dose was from 733.6µGy with the computed radiography system (p=0.009) and 1286.2µGy with the film-screen radiography system (p=0.008) to 346.7µGy with the flat-panel detector radiography system. For the image quality study, a significant interobserver agreement was found (p=0.034), indicating that the individual scores of all observers could be combined into an averaged value. Analysis of image quality showed a mean overall score (95%) for the amorphous silicon digital flatpanel detector radiography system that was significantly better than the mean overall score of the computed radiography system (85%, p=0.0339) or the film-screen radiography system (82%, p=0.0129). 42 Table 4: Mean image quality scores for three imaging systems derived from scores of five radiologists p* Regions DFPD CR FSR DFPD vs CR FSR Medial border of the scapulae 93 60 69 Rib 95 91 94 0.4057 1 Peripheral vessels 99 88 92 0.0041 0.0407 Trachea and proximal bronchi 98 85 86 0.0023 0.0041 Borders of the heart and aorta 99 94 96 0.1238 0.365 Diafragm and lateral costo-phrenic angles 99 96 98 0.3650 1 Retrocardiac lung and mediastinum 94 88 52 0.2167 <0.0001 Spine 84 57 28 0.0001 <0.0001 0.7mm high contrast 92 88 86 0.4795 0.2585 2mm low contrast 96 93 96 0.5350 1 0.3mm high contrast 93 92 88 1 0.3347 2mm low contrast 99 93 97 0.1724 0.6135 95 (4) 85 (13) 82 (22) 0.0339 0.0129 <0.0001 <0.001 Small round details in the lung and retrocardiac areas Linear and recticular details out to the lung periphery Mean overall score (standard deviation) Note – DFPD=digital flat-panel detector radiography, CR=computed radiography, FSR=film-screen radiography. *Determined with Chi-square test. No significant difference in mean overall quality was found (p=0.7032) between the computed radiography system and the filmscreen radiography system. In two regions (the retrocardiac lung and mediastinum and the spine), however, the computed radiography system performed statistically better than did the film-screen radiography system (p<0.0001). The contrast-detail phantom study showed the flat-panel detector radiography system had a significantly better low-contrast performance than the computed radiography (p=0.002) or film-screen radiography (p=0.001) systems. No significant differences in low-contrast detectability were found between the computed radiography and film-screen radiography systems (p=0.3). The contrast-detail curves are presented in Figure 2. 43 Figure 2: Graph shows average experimental contrast-detail curves for the film-screen radiography (◊), computed radiography (▲), and digital flat-panel detector radiography (●) systems. Data points were obtained by averaging response of five radiologists who reviewed images independently. Bars on either side of symbol indicate range. Discussion Because computer technology and storage capacity are developing rapidly, PACS-integrated hospital information systems will become more important in clinical practice in the near future. The different postprocessing tools, the possibility for multimodality image display, and the use of computer-aided diagnosis software are just some examples of the possibilities of digital image management with a PACS. The variable quality seen in conventional radiographs that is caused by the process of developing the X-ray films is eliminated with the use of digital radiography. In addition, radiologic reporting of images on the display screen eliminates the cost of film material and X-ray film archiving. Digital radiography will play an important role in this evolution because conventional radiographs are the most frequently obtained images in medical imaging. Furthermore, chest radiographs represent about 25% of all diagnostic radiography examinations [1] and are often obtained repeatedly for the followup of patients. Recently, amorphous silicon radiography systems with direct readout capabilities became commercially available. The diagnostic performance of this new amorphous silicon flat-panel detector radiography technology still requires evaluation, but it is expected to be at least as good as that of 44 conventional radiography. Previous experimental and clinical studies have shown that excellent image quality is achieved with the silicon flatpanel detector radiography system compared with the image quality produced by the conventional film-screen radiography and the computed radiography systems [2,3,6,7]. In a phantom study of Strotzer et al. [12], the depictions of inearstructured and micronodular-simulated lesions were significantly better on the amorphous silicon flat-panel detector radiography system than on conventional film-screen radiography. The two systems were equally capable of revealing pulmonary nodules and reticular patterns. In a CT-controlled clinical study, Garmer et al. [3] concluded that the diagnostic performance of the amorphous silicon flatpanel detector radiography system was equivalent or superior to that of the film-screen radiography system. Fink et al. [6] illustrated an improvement in the visibility of various anatomic structures on flat-panel detector chest radiographs compared with conventional film-screen radiographs. In our study, the quality of flat-panel detector chest radiographs was significantly superior in four anatomic regions (the medial border of the scapulae, the peripheral vessels, the trachea and proximal bronchi, and the spine) compared with that of computed radiographs and film-screen radiographs, findings consistent with those of the previously described studies. In addition, the visualization of the retrocardiac lung and mediastinum was significantly better with the amorphous silicon flat-panel detector radiography system compared with that of the film-screen radiography system. An important characteristic of the amorphous silicon flat-panel detector radiography system is that its quantum detection efficiency is higher than either computed radiography or film-screen radiography systems [3,5]. Quantum detection efficiency combines spatial resolution and image noise to provide a measure of the signal-to-noise ratio of all frequency components of the image [13]. Hence, a higher quantum detection efficiency provides improved capability to reveal an object in a noisy background [5], in addition to the possibility of reducing the patient radiation dose with no loss of diagnostic information. Previous studies have postulated that a dose reduction might be possible with the amorphous silicon flat-panel detector radiography system [3,5,6]. In our study, the flat-panel detector radiography system showed a strong and significant dose reduction compared with that possible with computed radiography or film-screen radiography systems. We measured and calculated the radiation dose for three comparable patient groups using the entrance skin dose and the effective dose. On the basis of the speed classes of the amorphous silicon flat-panel detector 45 radiography (400) and film-screen (250) radiography systems, a dose reduction of a factor of 1.6 could be expected. However, the high quantum detector efficiency of the flat-panel detector radiography system resulted in the dose being significantly lower than that delivered by the film-screen radiography system. For the combination of a posteroanterior and a lateral acquisition —the most common imaging combination in clinical chest radiography—the flat-panel detector radiography system showed a reduction in entrance skin dose of a factor of 3.6 compared with the film-screen radiography system and 2.2 compared with the computed radiography system. For the effective dose, the dose reduction factors were 2.7 and 1.7 compared with the film-screen radiography and the computed radiography systems, respectively. This finding corresponds with the assertion of Aufrichtig [7] that an amorphous silicon flat-panel detector radiography system only needs 30% of the exposure required by a film-screen radiography system to achieve the same contrast-detail detectability. The image quality assessment proved that despite the significant dose reduction, image quality had been not affected. The overall performance of the amorphous silicon flat-panel detector radiography system was significantly superior to the performances of the computed radiography and the film-screen radiography systems. Another important advantage of digital radiography systems is the wide dynamic range and histogram equalization [3]. Hence, low-contrast regions such as the mediastinum are better visualized, as was illustrated by the statistically better score of both digital systems compared with the score of the film-screen radiography system in imaging the mediastinal region (Table 4). The contrast-detail phantom study confirmed the superior low-contrast detection of the amorphous silicon flat-panel detector radiography system as compared with the low-contrast detection of the film-screen and computed radiography systems (Fig. 2). This result corresponds with the results reported by Garmer et al. [3], who found that mediastinal abnormalities were more clearly seen with the amorphous silicon flat-panel detector radiography system than with a conventional film-screen radiography technique, and by Schaefer et al. [14], who found that the computed radiography system was superior to the film-screen radiography system for revealing mediastinal lesions. Other studies have proven that limiting the spatial resolution of digital systems, in contrast to film-screen radiography systems, does not influence the diagnostic performance. MacMahon et al. [15] showed that a pixel size of 200µm is sufficient for the detection of necessary details using computed radiography systems. Aufrichtig [7] proved that an amorphous silicon flat-panel detector radiography system with a pixel 46 size of 200µm is superior to a dedicated film-screen radiography system in revealing small objects. Although the radiation dose is already low in acquisitions with amorphous silicon flatpanel detector radiography systems, additional dose reductions may be possible to achieve. In a phantom experiment, Strotzer et al. [12] did not find any significant difference in image quality of a flat-panel detector image obtained with a standard radiation dose and a flat-panel detector image obtained with a dose reduction of 50%. Therefore, the amorphous silicon flatpanel detector radiography systems would be appropriate for pediatric use, where it is crucial to keep the patient radiation dose as low as possible. Amorphous silicon flat-panel detector radiography systems are not exclusively designed for thoracic imaging; they have already proven their value in skeletal radiography [16], particularly in revealing cortical bone defects and fractures [17]. Flatpanel detector radiography has the additional advantage over computed radiography in that it offers an instant image display and the elimination of the need for cassettes. Therefore, amorphous silicon flat-panel detector radiography systems could be a cost-effective replacement for conventional radiography systems in the future. In conclusion, digital flat-panel detector radiography systems based on amorphous silicon and cesium iodide are an important step forward in chest imaging, offering improved image quality and a significant reduction in the radiation dose delivered to patients. Acknowledgment We thank the technical staff of the radiology department of the Ghent University Hospital for their cooperation in measuring patient radiation doses. References 1. United Nations Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation, vol 1. Sources, annex D. Medical radiation exposure. New York: United Nations, 2000, 355 2. Rong XJ, Shaw CC, Liu X, Lemacks MR, Thompson SK. Comparison of an amorphous silicon/cesium iodide flat-panel digital chest radiography system with screen/film and computed radiography systems: a contrast-detail phantom study. Med Phys 2000; 28: 2328–2335 47 3. Garmer M, Hennigs SP, Jäger HJ, et al. Digital radiography versus conventional radiography in chest imaging: diagnostic performance of a large-area flat-panel detector in a clinical CT-controlled study. AJR 2000; 174:75–80 4. Sonoda M, Takano M, Miyahara J, Kato H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology 1983; 148:833–838 5. Floyd CE, Warp RJ, Dobbins JT, et al. Imaging characteristics of an amorphous silicon flat-panel detector for digital chest radiography. Radiology 2001; 218:683–688 6. Fink C, Hallscheidt PJ, Noeldge G, et al. Clinical comparative study with a large-area amorphous silicon flat-panel detector: image quality and visibility of anatomic structures on chest radiography. AJR 2002; 178:481–486 7. Aufrichtig R. Comparison of low-contrast detectability between a digital amorphous silicon and a screen-film–based imaging system for thoracic radiography. Med Phys 1999; 26:1349–1358 8. International Commission on Radiological Protection. Recommendations of the International Commission on Radiological Protection. Oxford, UK: Pergamon, 1991. Publication no. 60 9. Christy M. Active bone marrow distribution as a function of age in humans. Phys Med Biol 1981; 26:389–400 10. Commission of the European Communities. European guidelines on quality criteria for diagnostic radiographic images. Brussels: Commission of the European Communities, 1996. Publication EUR-16260 11. Thijssen MAO, Bijkerk KR, van der Burght RJM. Manual contrast-detail phantom CDRAD type 2.0: project quality assurance in radiology. University Hospital Nijmegen: St. Radboud, The Netherlands, 1998 12. Strotzer M, Gmeinwieser J, Voelk M, Fruend R, Setz J, Feuerbach S. Detection of simulated chest lesions with normal and reduced radiation dose: comparison of conventional screen-film radiography and a flat-panel X-ray detector based on amorphous silicon. Invest Radiol 1998; 33:98–103 13. Chotas HG, Dobbins JT, Ravin CE. Principles of digital radiography with large-area electronically readable detectors: a review of the basics. Radiology 1999; 210:595–599 14. Schaefer CM, Greene R, Oestmann JW, et al.Digital storage phosphor imaging versus conventional film radiography in CT-documented chest disease. Radiology 1990; 174:207–210 15. MacMahon H, Vyborny CJ, Metz CE. Digital radiography of subtle pulmonary abnormalities: a ROC study of effect of pixel size on observer performance. Radiology 1986; 158:21–26 16. Strotzer M, Gmeinwieser J, Völk M, et al. Clinical application of a flat-panel X-ray detector based on amorphous silicon technology: image quality and potential for dose reduction in skeletal radiography. AJR 1998; 171:23–27 17. Strotzer M, Gmeinwieser J, Spahn M, et al. Amorphous silicon, flat-panel, X-ray detector versus screen-film radiography: effect of dose reduction on the detectability of cortical bone defects and fractures. Invest Radiol 1998; 33:33–38 48 3.2 Part II Image quality and radiation dose in digital chest imaging: comparison of an amorphous silicon and an amorphous selenium flat-panel system Klaus Bacher1 – Peter Smeets2 – Ludo Vereecken3 – An De Hauwere1 – Philippe Duyck2 – Robert De Man3 – Koenraad Verstraete2 – Hubert Thierens1 1Department of Medical Physics and Proeftuinstraat 86, Gent B-9000, Belgium. Radiation Protection, Ghent University, 2Department of Radiology, Ghent University Hospital, De Pintelaan 185, Gent B-9000, 3Department of Radiology, Heilig Hart Hospital, Wilgenstraat 2, B-8800 Roeselare, Belgium. Belgium. Accepted for publication in AJR Abstract The aim of this study was to compare the image quality and the radiation dose in chest imaging using an amorphous silicon and an amorphous selenium flat-panel detector system. In addition, the low contrast performance of both systems with standard and low radiation dose was compared. In two groups of 100 patients each, digital chest radiographs were acquired either with the amorphous silicon or the amorphous selenium flat-panel system. The effective dose of the examination was measured using thermoluminescent dosimeters placed in an anthropomorphic Rando phantom. The image quality of the digital chest radiographs was assessed by five experienced radiologists using the European Guidelines on Quality Criteria for Diagnostic Radiographic Images. In addition, a contrast-detail phantom study was set up to assess the low contrast performance of both systems at different radiation dose levels. Differences between two groups were tested for significance using the two-tailed Mann-Whitney test. The amorphous silicon flat-panel system allows an important and significant reduction in effective dose in comparison with the amorphous selenium flat-panel system (p<0.0001) for both the PA and lateral views. In addition, clinical image quality analysis showed that the dose reduction was not detrimental to image quality. Compared to the amorphous selenium flatpanel detector system, a significantly better low-contrast phantom performance of the amorphous silicon detector system was shown for phantom entrance dose values up to 135 µGy. Chest radiographs using the amorphous silicon flat-panel system can be acquired with a significantly lower patient dose compared to those made with the amorphous selenium system, thereby producing an image quality that is equal to or even superior to that of the amorphous selenium flat-panel detector system. 49 Introduction Since computer technology and storage capacity have developed rapidly during the last years, picture archiving and communication systems (PACS) are gaining more importance [1,2]. Digital radiography techniques will play an important role in this evolution because conventional radiographs are the most frequently obtained images in medical imaging [3]. In general, digital radiography systems offer an instant image display, a wide dynamic range and a linear signal response [2,4,5]. Moreover, digital images are very flexible in processing and archiving, thereby providing a solution to the major disadvantages of the screen-film systems. Storage phosphor plates, introduced some 20 years ago [6], were the first step towards full digitization of the radiology department. Nowadays, these computed radiography (CR) systems are widely used due to compatibility with existing radiographic equipment and offer an image quality that is comparable to conventional screen-film combinations [1,2]. Only recently, large-area full digital flat-panel radiography detectors based on active matrix thin-film transistor technology became commercially available. Depending on detector type, X-ray conversion into an electrical signal is done either directly or indirectly [1,4,5]. Direct X-ray conversion is performed in an amorphous selenium (a-Se) flatpanel system, whereas flat-panel detectors based on cesium iodide and amorphous silicon (a-Si) are used for indirect conversion of X-rays. Flatpanel systems combine all advantages of CR together with a higher detective quantum efficiency (DQE) than both screen-film and CR systems [1,4,5]. In addition, these detectors allow an optimized working procedure due to the instant image display and the elimination of the use of film cassettes. Only few studies discuss the comparison between a-Si and a-Se flat-panel systems in clinical settings. The published results are mainly based on the measurement of noise characteristics, modulation transfer function (MTF) and DQE and do not take into account patient acquisitions [7,8]. As chest radiography is the most commonly applied diagnostic X-ray procedure [9], the aim of the present study was to compare the image quality and the patient dose in clinical chest imaging using either an a-Si or an a-Se flat-panel detector. Furthermore, a detailed contrast-detail analysis was performed, to compare the low-contrast performance of both systems at different dose settings. 50 Materials and Methods Image acquisition and viewing The physical characteristics of the two active matrix flat panel systems used in this study are summarized in Table 1. Table 1: Overview of the technical characteristics of the a-Si and the a-Se system used in this study Active matrix flat panel system a-Si system a-Se system Manufacturer/Model Siemens Vertix FD Hologic EPEX Detector Trixell Pixium 4600 DirectRay DR 1000 X-ray detection indirect: CsI (Tl) direct: a-Se Imaging area (cm²) 43 × 43 35 × 43 Matrix size (pixels) 3001 × 3001 2560 × 3072 Pixel size (µm) 143 139 Image depth (bit) 14 14 Antiscatter grid stationary grid; ratio 15:1 moving grid; ratio 10:1 Note – a-Si=amorphous silicon, a-Se=amorphous selenium The amorphous silicon (a-Si) flat-panel detector system (Siemens, Erlangen, Germany and Trixell, Moirans, France) consisted of a ceiling mounted X-ray tube (Opti 150/30/50 HC, focal spot size 0.6 mm), a highvoltage generator and a motorized receptor wall stand with the flat panel detector mounted behind a stationary antiscatter grid. In the amorphous silicon flat panel detector, a needle-structured thallium-doped CsI scintillator was used to convert the X-rays into visible light, which was deposited directly on the 43x43 cm amorphous silicon matrix. Subsequently, an amorphous silicon photodiode converted the light into electrical charge, resulting in a 14-bit digital signal in a 3001x3001 pixel matrix. The amorphous selenium (a-Se) flat-panel detector (Hologic - Direct Radiography Corp., Newark, DE) was used in combination with a ceiling mounted X-ray tube (Varian A192, focal spot size 0.6 mm) and a highvoltage generator. The flat panel detector was mounted behind a moving antiscatter grid. A detailed description of the selenium-based flat-panel detector can be found elsewhere [1,4,5]. Briefly, free electrons are released by the interaction of X-rays with the amorphous selenium semiconductor layer. Due to the application of an electric field across the selenium layer, 51 the electric charges are drawn directly to the charge-collecting electrodes. The latter operation results in a 14-bit image, stored in a 2560x3072 pixel matrix. The automatic exposure control (AEC) of both flat-panel systems was used in the standard settings as advised by the manufacturers. After acquisition, the digital data were sent to a PACS workstation (MasterPage, Eastman Kodak, Rochester, NY), for image quality assessment. All digital images were scored on a 21-inch, high-contrast gray-scale monitor (MGD 2621P, Barco, Kortrijk, Belgium) with a resolution of 1280x1600 and a maximum luminance of 600 Cd/m². The DICOM header of all images was changed to remove system-specific information on the image display. In this way, no difference could be seen between the a-Se and a-Si soft copy images allowing a blind study. Patient study Patients Two hundred patients were referred to the radiology department for both routine posteroanterior (PA) and lateral chest radiography. At random, images were taken either with the a-Si or with the a-Se flat-panel detector system. Pneumonectomy patients, patients with extreme spinal deformity or those who were unable to raise their arms for the lateral view, were excluded. The exposure for such patients would be excessive and could therefore artificially increase the mean measured radiation dose of a patient group. The PA and lateral chest radiographs were obtained with the patients in an upright position at a focus-to-detector distance of 180 cm. All studies were performed at 125 kVp, using AEC. The displayed exposure values (mAs) after each acquisition were recorded. Scoring of patient images Five experienced chest radiologists assessed all soft-copy PA chest radiographs and scored the image quality using a methodology adopted from the European Guidelines on Quality Criteria for Diagnostic Radiographic Images [10]. In the latter document, the image quality criteria refer to characteristic features of imaged anatomical structures with a specific degree of visibility. In the Guidelines, three levels of visibility are defined: visualization (an anatomical feature is detectable but details are not fully reproduced), reproduction (details of anatomical structures are visible but not necessary clearly defined) and visually sharp reproduction (anatomical details are clearly defined) [10]. The images of 52 both systems were presented in a random order and all images were interpreted independently. The radiologists were allowed to adjust the image brightness and contrast as well as to magnify the images to full resolution. The five radiologists had to decide if a certain criterion (visualization, reproduction or visually sharp reproduction) was fulfilled (score 1) or not (score 0) in a total of seven anatomic structures and four image details (Table 3). For low contrast details (2mm), the visualisation of the normal bronchovascular structures up to the parietal pleura was considered. These peripheral structures are always smaller than the mentioned criteria (< 2mm), i.e. a score of “1” was given when visible. When these bronchovascular structures couldn't be followed all the way up to the visceral pleura the diameter of the smallest visible structure was measured. For high contrast details, small calcifications, surgical material or electrode leads, were measured systematically. For each region or detail, the scores of all 100 chest radiographs were summed. In this way, the percentage of images where the specific image criterion was fulfilled was calculated. An average percentage, using the scores from all five observers, was used in the data analysis. Dose measurements The effective dose (E), representative for the risk of late radiation induced effects as malignancies, is defined by the expression [11]: E = ∑ wT H T (1), T where HT is the equivalent dose to tissue T and wT is the weighting factor representing the relative radiation sensitivity of tissue T. The equivalent organ doses HT were determined by placing 166 calibrated thermoluminescent dosemeters (TLDs) in an anthropomorphic (average man) Rando phantom (The Phantom Laboratory, Salem, NY) in positions representative for these organs and tissues. Distribution of bone marrow over the body was adopted from Christy [12]. The choice of the TLD locations was based on a complete CT scan of the phantom. PA and lateral chest radiographs of the Rando phantom were obtained (125 kVp, 180 cm focus-to-detector distance) on both the a-Si and the a-Se system. To obtain dose measurements well above the detection limit of the TLDs, the exposure was set equal to 300 mAs. After the reading-out procedure of the TLDs, mean equivalent organ doses per mAs could be calculated. Multiplying these results with the mean registered mAs-values of the two patient groups, the mean 53 equivalent organ doses (unit: µSv) for both imaging systems were derived. By combining these results with the corresponding tissue weighting factors, wT, in expression (1), the effective dose (unit: µSv) could be calculated. Contrast detail study CDRAD phantom acquisition To achieve a more objective image quality measure, a CDRAD 2.0 (Artinis Medical Systems, Andelst, The Netherlands) contrast-detail phantom study was set up [13,14]. In this experiment, the CDRAD 2.0 phantom was exposed at different entrance dose values. The CDRAD 2.0 phantom consists of a Plexiglas plate (26.5 x 26.5 x 1 cm³) with a 15x15 array of 1.5 x 1.5 cm² cell regions in which circular holes are drilled. The latter holes are logarithmically sized from 0.3 to 8.0 mm in both diameter and depth (Figure 1). Figure 1: Sample image acquisition of the CDRAD 2.0 phantom. The phantom contains circular holes logarithmically sized from 0.3 to 8.0 mm in both diameter and depth. For 4 mm and smaller holes, an additional hole of matching diameter and depth, is located at one of the four corners. The presence of these additional holes allows a four-alternative forced-choice experiment in which the observer must select the location of the low-contrast objects among the four possible corners. For 4 mm and smaller objects, the phantom contains an additional hole of matching diameter and depth, placed at random in one of the four cell corners. The presence of these additional objects allows a four-alternative forced-choice experiment in which the observer must select the location of 54 the low-contrast objects among the four possible corners [15]. In this way, these extra holes help to minimize potential biases due to a priori knowledge of the presence of objects in every square region [16]. The phantom was used to assess the minimum contrast required to visualize objects of different sizes above the noise threshold. The phantom was placed between two layers of 5-cm polymethyl methacrylate (PMMA), to simulate patient scatter and to generate the same mean mAs value in AEC mode as obtained for the patient chest radiographs. For both radiography systems, three phantom images were acquired in the same conditions as used for patients, using AEC. In addition, phantom images were taken with varying mAs settings corresponding to equal phantom entrance doses on both systems (range: 22 µGy to 435 µGy). For each phantom exposure, entrance doses were measured with a R100 solid state detector (RTI, Sweden). For each imaging modality and exposure setting, a set of three images was available for scoring. Image quality scoring of phantom images As for the scoring of the patient images, the a-Si and a-Se images were displayed at random and analysed by five independent readers. The readers were allowed to adjust the image brightness and contrast as well as to magnify the images to full resolution. The observers had to identify, in every square cell region, the locations of the corner holes. The results were entered on a score sheet for each image reviewed. After comparing the score forms to a reference form containing the correct locations of all corner holes, a correction scheme was used taking into account the nearest neighbours in order to get more accurate results [13-16]. Finally, for each different diameter (Di) the threshold contrast value (Ci,th) was determined as the minimum depth in regions of valid detection [16]. The obtained threshold contrast value results were averaged over 15 observations (3 images x 5 readers) and then plotted as the function of the object diameter to form the contrast-detail curves (Figure 2). Holes above and to the right of this curve are visualised and holes below and to the left are not seen. The inverse image quality figure (IQFinv) was introduced for quantitative comparison of the CDRAD images [13]. The IQFinv is defined as: IQFinv = 100 15 ∑C i =1 55 i * Di ,th where Ci represents the object depth in the contrast-column i and Di,th denotes the corresponding smallest visible diameter (threshold diameter) in this column [13]. The higher the IQFinv, the better the low contrast visibility. The IQFinv was calculated for all analysed images, resulting in 15 IQFinv values for each entrance dose setting and each digital flat-panel system. Afterwards the IQFinv values were averaged and plotted in function of the entrance dose (Figure 3). Finally the IQFinv values of both systems are compared using non-parametric statistics. Statistical analysis Differences between two groups were tested for significance using the two-tailed Mann-Whitney test. For the comparison of the clinical image quality (expressed as a percentage) of the digital chest radiographs of the two imaging systems, Chi-square statistics were used. To check the reliability of the image quality evaluation by the five independent radiologists, we assessed the interobserver correlation by calculating Spearman’s rank correlation for all image quality criteria for all observer combinations. A significant correlation indicated that the scores of the observers could be combined into a mean score for the image quality parameters [2]. In all statistical calculations a confidence interval of 95% was applied. Calculations were performed by means of the MedCalc software (MedCalc Software, Gent, Belgium). Results For both the a-Si and a-Se system, one hundred PA and lateral chest radiographs were acquired. The a-Si patient population (54 men and 46 women) had a mean age of 58.8 years (range, 21-83 years). The mean age of the a-Se study group (56 men and 44 women) was 60.0 years (range, 1882). There was no statistical difference in BMI (p=0.984) between the patient groups, guaranteeing that no bias was introduced due to differences between groups. In Table 2, the results of the exposure and dose measurements are summarized. For the PA as well as for the lateral acquisition, a significantly lower exposure and effective dose could be demonstrated with the a-Si system when using the AEC settings of the manufacturers. For the PA view, the effective dose resulted in a value of 9.6 µSv and 22.6 µSv for the a-Si and a-Se system respectively. For the lateral view, effective dose values of 27.1 µSv (a-Si) and 79.2 µSv (a-Se) were obtained. 56 Table 2: Mean registered exposure and calculated effective dose for the two imaging modalities after a posteroanterior and a lateral chest acquisition Lateral Posteroanterior Factor a-Si a-Se p* a-Si a-Se p* Mean 1.25 3.07 <0.0001 4.83 17.5 <0.0001 Range 0.75-2.74 1.20-6.50 1.11-17.98 3.51-43.8 Mean 9.6 22.6 27.1 79.2 Range 5.7-21.0 8.9-47.9 6.2 -101.0 15.9-198.2 Exposure (mAs) Effective dose (µSv) <0.0001 <0.0001 Note – a-Si=amorphous silicon, a-Se=amorphous selenium. *Determined using two-tailed Mann-Whitney test. For the image quality study, a significant interobserver agreement was found (p ≤ 0.027), indicating that the individual scores of all observers could be combined in an averaged value. In Table 3, the mean scores for the seven anatomic regions and the four image details in both systems are indicated (maximum possible score: 100), together with the likelihood that both systems gave the same result. The image quality scores of Table 3 are based on PA chest radiographs acquired with AEC. In general, lowcontrast anatomical structures were better visualized with the a-Si flatpanel detector. This difference reached significance only for the thoracic spine (p=0.0022). Low contrast details were significantly better scored on the a-Si system (small round details, p=0.0425; linear and recticular details, p=0.0041), whereas small high contrast details were slightly better visualized on the a-Se system. However, the latter difference was not significant. 57 Table 3: Mean image quality scorings of posteroanterior chest radiographs of both flat-panel systems according to the European Guidelines on Quality Criteria for Diagnostic Radiographic Images. a-Si system a-Se system p* Visualization of the retrocardiac lung and the mediastinum 94 88 0.2167 Visualization of the spine through the heart shadow 84 64 0.0022 Reproduction of the whole rib cage above the diaphragm 95 97 0.7182 Visually sharp reproduction of the vascular pattern in the whole lung, particulary the peripheral vessels 99 93 0.0712 Visually sharp reproduction of the trachea and proximal bronchi 98 93 0.1724 Visually sharp reproduction of the borders of the heart and aorta 99 98 1.0000 Visually sharp reproduction of the diaphragm and lateral costophrenic angles 99 94 0.1238 0.7mm high contrast 92 98 0.1048 2mm low contrast 96 87 0.0425 0.3mm high contrast 93 99 0.0712 2mm low contrast 99 88 0.0041 Image quality criteria ANATOMIC REGIONS IMAGE DETAILS Small round details in the lung and retrocardiac areas Linear and recticular details out to the lung periphery Note – The scoring results indicate the percentage of images where the specific image criterion was fulfilled, averaged over all five observers. a-Si=amorphous silicon, a-Se=amorphous selenium. *Determined with Chi-square test. In Figure 2, average experimental contrast-detail curves of both flat panel detectors are presented. In Figure 2a, the CDRAD images were made with a phantom entrance dose of 54 µGy. Figure 2b and Figure 2c indicate the results obtained with AEC and with a phantom entrance dose of 135 µGy respectively. Figure 2a and Figure 2c clearly indicate a (significant) better low-contrast detectability of the a-Si system, whereas the detection of small high-contrast regions is comparable for both detectors. The CDRAD images taken with AEC (Figure 2b), reflect in very good agreement the results of the patient chest images (also taken with AEC), where only in low-contrast regions a difference between systems could be observed. 58 Figure 2: Average experimental contrast-detail curves. Data obtained from the a-Si and a-Se CDRAD images taken with a phantom entrance dose of 54 µGy (A), with AEC (B) or with a phantom entrance dose of 135 µGy (C). Data points were obtained by averaging responses of five radiologists who reviewed all images independently. Bars indicate standard deviation of 15 image scorings (5 readers × 3 images). Note – a-Si=amorphous silicon, a-Se=amorphous selenium, ED=phantom entrance dose. 59 Figure 3 presents the mean IQFinv values obtained from the CDRAD images as a function of the applied entrance dose. Overall, the contrastdetail study showed a significantly better low-contrast performance of the a-Si system compared to the a-Se detector system for phantom entrance doses up to 135 µGy (p<0.032). For entrance dose values of 218 µGy and higher, no significant difference in low-contrast detectability was found. Figure 3: Inverse image quality figure (IQFinv) of both the a-Si and the a-Se system, derived from the CDRAD images, as a function of the phantom entrance dose value. Bars indicate the standard deviation of 15 image scorings (5 readers x 3 images). Note – a-Si=amorphous silicon, a-Se=amorphous selenium. Discussion Conventional projection radiography is most frequently performed in diagnostic radiology. Therefore, digital radiography systems are playing an important role in the evolution towards a completely digital medical imaging environment. In particular, chest radiographs represent about 25 % of all diagnostic X-ray examinations [9]. Moreover, chest radiographs are often taken repeatedly for the follow up of patients. The majority of the chest acquisitions are still performed with conventional screen-film or CR systems. With CR, digital images are produced with an image quality that is comparable with conventional screen-film combinations [1,2]. However, no significant dose reductions could be obtained in these systems due to the limited DQE [1,5]. 60 A few years ago, direct-readout radiography systems, based on active matrix thin-film transistor technology became commercially available. These systems have a higher DQE compared to both CR and screen-film systems and have the additional advantage of an optimized working procedure due to the instant image display and the elimination of the use of cassettes [1,2,5]. Depending on detector type, digital signals are generated either directly, using amorphous selenium, or indirectly, using a scintillator and an amorphous silicon photodiode [1,4,5]. Previous experimental and clinical studies showed excellent results concerning image quality of direct readout detectors in thoracic radiography in comparison with conventional screen-film and computed radiography systems [2,3,24-28]. Most of them, however, were done with the a-Si flat-panel detector type [2,17-23] or refer to the selenium drum detector [24-27]. Only limited image quality information is found with respect to the a-Se flat-panel detector [3,28]. Moreover, only very few studies were performed to compare the diagnostic performance of direct (a-Se) and indirect (a-Si) flat-panel radiography units [7,8]. The latter comparisons are mainly based on phantom measurements to compare noise characteristics, MTF and DQE of both detector types. In our study, two commercially available flat-panel systems based on amorphous silicon or amorphous selenium are compared with respect to the image quality and radiation dose of digital chest acquisitions. Both systems were used as advised by the manufacturer, using a specific grid (Table 1) and the standard AEC settings. Analysis of all patient images showed that both flat-panel systems produced an excellent image quality using the AEC settings of the manufacturer. In general, low contrast details, such as the thoracic spine through the heart shadow and the normal bronchovascular structures up to the parietal pleura, were significantly better scored on the a-Si system, whereas small high contrast details were slightly better visualized on the a-Se system. The latter observation is in accordance with the higher MTF-value of the a-Se system at high frequencies [7,8]. Using the AEC, the chest radiographs of the a-Si flat-panel system could be acquired with a significantly lower patient effective dose compared to those taken with the a-Se detector. For the combination of a PA and a lateral acquisition – as is mostly the case in clinical chest radiography –, the a-Si system demonstrated a reduction in effective dose of about 60% compared to the direct flat-panel system when using the AEC settings of the manufacturers. Similar results were found by Fishbach et al who reported that low-dose a-Si phantom images scored better compared with a-Se drum images [29]. Samei et al and Borasi et al calculated DQE values 61 of both direct and indirect flat-panel types. In both studies, the DQE of the a-Si detector was significantly higher than the value for the a-Se detector, explaining the dose reduction obtained with the indirect flatpanel detector [7,8]. Previous measurements already showed the dosesaving effect of using a-Si flat-panel detectors in chest imaging, compared to CR and screen-film radiography [2,16,17,20,22,23]. However, no significant dose reductions were reported using an a-Se flat-panel detector. Contrast-detail studies are widely used for an objective analysis of the image quality performance of digital radiography systems [2,8,16,19,29]. The construction of the CDRAD 2.0 phantom allows a four-alternative forced-choice protocol for the analysis of contrast and detail perception [13,15]. The latter procedure is more reliable and accurate than the image quality analysis with other contrast-detail objects, without fouralternative forced-choice possibility [15,16]. The average experimental contrast-detail curves (Figure 2a and Figure 2c) of both flat-panel detectors clearly indicate a (significant) better lowcontrast detectability of the a-Si system, whereas the detection of small high-contrast regions is comparable for both detectors. This is in agreement with the contrast-detail curves calculated by Borasi et al, in which no significant difference was observed in the detection of small objects [8]. The CDRAD images taken with AEC (Figure 2b), reflect in very good agreement the results of the patient chest images where only in low-contrast regions a difference between both systems could be proven. The overall contrast-detail performance (expressed in the quantity IQFinv) as a function of the radiation dose level, showed a significantly better low-contrast performance of the a-Si system compared to the a-Se detector for clinical exposure settings (entrance dose values up to 135 µGy). For entrance dose values of 218 µGy and higher, no significant difference in low-contrast detectability was found. Figure 3 illustrates that the entrance dose value for the a-Se should be about 135 µGy (3.10 mAs) to obtain an equal contrast-detail performance of an a-Si flat-panel radiograph taken at an entrance dose of 54 µGy (1.25 mAs). The latter situation reflects the clinical practice in excellent agreement (Table 2). In conclusion, chest radiographs of a digital a-Si flat-panel system could be acquired with a significantly lower patient dose compared to chest radiographs taken with the a-Se system, thereby producing an image quality that is equal to or even superior to that of the a-Se flat-panel detector system. Further research should reveal whether both systems provide the same diagnostic accuracy. An excellent agreement was 62 obtained between the clinical image evaluation and the contrast-detail study, demonstrating the value of the CDRAD phantom as a tool for objective image quality analysis in digital radiography. References 1. Schaefer-Prokop C, Uffmann M, Eisenhuber E, Prokop M. Digital radiography of the chest: detector techniques and performance parameters. Journal of Thoracic Imaging 2003; 18:124-137 2. Bacher K, Smeets P, Bonnarens K, De Hauwere A, Verstraete K, Thierens H. Dose reduction in patients undergoing chest imaging: digital amorphous silicon flat-panel detector versus conventional film-screen radiography and phosphor-based computed radiography. AJR 2003; 181:923-929 3. Goo JM, Im JG, Kim JH, et al. Digital chest radiography with a selenium-based flatpanel detector versus a storage phosphor system: comparison of soft-copy images. AJR 2000; 175:1013-1018 4. Chotas HG, Dobbins JT, Ravin CE. Principles of digital radiography with large-area electronically readable detectors: a review of the basics. Radiology 1999; 210:595-599 5. Kotter E, Langer M. Digital radiography with large-area flat-panel detectors. Eur Radiology 2002; 12:2562-2570 6. Sonoda M, Takano M, Miyahara J, Kato H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology 1983; 148:833-838 7. Samei E, Flynn MJ. An experimental comparison of detector performance for direct and indirect digital radiography systems. Med Phys 2003; 30: 608-622 8. Borasi G, Nitrosi A, Ferrari P, Tassoni D. On site evaluation of three flat panel detectors for digital radiography. Med Phys 2003; 30: 1719-1731 9. UNSCEAR, 2000. Sources and effects of ionizing radiation – Vol I: Sources. Report to the General Assembly, United Nations, New York. 10. Commission of the European Communities. European guidelines on quality criteria for diagnostic radiographic images. (EUR 16260). Brussels: CEC, 1996 11. ICRP, Publication 60, Recommendations of the International Commission on Radiological Protection, Pergamon Press. Oxford, 1991 12. Christy M. Active bone marrow distribution as a function of age in humans. Phys Med Biol 1981; 26: 389-400 13. Thijssen MAO, Thijssen HOM, Merx JL, Lindeijer JM, Bijkerk KR. A definition of image quality: the image quality figure. In: Moores BM, Wall BF, Eriskat H, Schibilla H, editors. BIR Report 20: Optimization of image quality and patient exposure in diagnostic radiology. London: The British Institute of Radiology; 1989. p. 29-34. 14. Thijssen MAO, Bijkerk KR, van der Burght RJM. Manual Contrast-Detail Phantom CDRAD type 2.0. Project Quality Assurance in Radiology, Department of Radiology, University Hospital Nijmegen, St. Radboud, The Netherlands, 1998 15. Aufrichtig R, Xue P. Dose efficiency and low-contrast detectability of an amorphous silicon x-ray detector for digital radiography. Phys Med Biol 2000; 45:2653-2669 63 16. Rong XJ, Shaw CC, Liu X, Lemacks MR, Thompson SK. Comparison of an amorphous silicon/cesium iodide flat-panel digital chest radiography system with screen/film and computed radiography systems – A contrast-detail phantom study. Med Phys 2000; 28: 2328-2335 17. Strotzer M, Gmeinwieser J, Voelk M, Fruend R, Setz J, Feuerbach S. Detection of simulated chest lesions with normal and reduced radiation dose: comparison of conventional screen-film radiography and a flat-panel X-ray detector based on amorphous silicon. Invest Radiol 1998; 33:98-103 18. Garmer M, Hennings SP, Jäger HJ, et al. Digital radiography versus conventional radiography in chest imaging: diagnostic performance of a large-area flat-panel detector in a clinical CT-controlled study. AJR 2000; 174: 75-80 19. Chotas HG, Ravin CE. Digital chest radiography with a solid-state flat-panel X-ray detector: contrast-detail evaluation with processed images printed on film hard copy. Radiology 2001; 218:679-682 20. Fink C, Hallscheidt PJ, Noeldge G, et al. Clinical comparative study with a large-area amorphous silicon flat-panel detector: image quality and visibility of anatomic structures on chest radiography. AJR 2002; 178: 481-486 21. Goo JM, Im JG, Lee HJ, et al. Detection of simulated chest lesions by using soft-copy reading: comparison of an amorphous silicon flat-panel-detector system and a storagephosphor system. Radiology 2002; 224:242-246 22. Rapp-Bernhardt U, Roehl FW, Gibbs RC, Schmidl H, Krause UW, Bernhardt TM. Flatpanel X-ray detector based on amorphous silicon versus asymmetric screen-film system: phantom study of dose reduction and depiction of simulated findings. Radiology 2003; 227:484-492 23. Ganten M, Radeleff B, Kampschulte A, Daniels MD, Kauffmann GW, Hansmann J. Comparing image quality of flat-panel chest radiography with storage phosphor radiography and film-screen radiography. AJR 2003; 181:171-176 24. van Heesewijk HPM, van der Graaf Y, de Valois JC, Vos JA, Feldberg MAM. Chest imaging with a selenium detector versus conventional film radiography: a CTcontrolled study. Radiology 1996; 200:687-690 25. Schaefer-Prokop CM, Prokop M, Schmidt A, Neitzel U, Galanski M. Selenium radiography versus storage phosphor and conventional radiography in the detection of simulated chest lesions. Radiology 1996; 201:45-50 26. Bernhardt TM, Otto D, Reichel G, et al. Detection of simulated interstitial lung disease and catheters with selenium, storage phosphor, and film-based radiography. Radiology 1999; 213:445-454 27. Awai K, Komi M, Hori S. Selenium-based digital radiography versus high-resolution storage phosphor radiography in the detection of solitary pulmonary nodules without calcification: receiver operating characteristic curve analysis. AJR 2001; 177:1141-1144 28. Kim TS, Im JG, Goo JM, at al. Detection of pulmonary edema in pigs: storage phosphor versus amorpous selenium-based flat-panel-detector radiography. Radiology 2002; 223:695-701 29. Fischbach F, Freund T, Pech M, et al. Comparison of indirect CsI/a:Si and direct a:Se digital radiography. Acta Radiologica 2003; 44: 616-621 64 3.3 Part III Analysis of image quality in digital chest imaging An De Hauwere1 – Klaus Bacher1 – Peter Smeets2 – Koenraad Verstraete2 – Hubert Thierens1 1Department of Medical Physics and Proeftuinstraat 86, Gent B-9000, Belgium. 2Department Belgium. Radiation Protection, Ghent University, of Radiology, Ghent University Hospital, De Pintelaan 185, Gent B-9000, Reprint from Rad Prot Dosim 2005; 117: 174-177 Abstract An evaluation of the image quality of an amorphous silicon flat-panel detector system and a computed radiology system compared with a screen-film system was performed by means of contrast-detail phantom images. Hard and soft copy images were evaluated. Although patient dose at clinical settings was strongly decreased with the amorphous silicon system, the lowcontrast visibility with this system was still significantly better than with the screen-film system. For the computed radiology system, low-contrast visibility was comparable to the screen-film system. Best results were obtained by soft copy reading at full resolution with adaptation of contrast and brightness. Changing tube voltage (102–133 kV), or additional filtration, did not significantly affect image quality. However, low-contrast visibility improved significantly with increasing exposure. It was clearly demonstrated that, in chest imaging, the amorphous silicon system has superior imaging characteristics compared to the screen-film and the computed radiology system. Introduction The first digital radiographs were introduced about 20 y ago with the development of computed radiology systems based on phosphor plate detectors. Full field, full digital, flat-panel detector systems have been commercially available for a few years. Previous studies [1–4] showed the potential of a strong dose reduction without substantial loss of image quality in chest radiography performed with digital flat-panel detector systems compared to conventional screen-film systems. At Ghent University Hospital, a dosimetry study [5] had shown that dose reductions of ~35 and 60% could be achieved in clinical settings with a storage phosphor plate detector (computed radiology) and an amorphous silicon flat-panel detector, respectively, compared to the 250 speed screenfilm system conventionally used for thoracic imaging. A subjective 65 clinical study [5] had shown that images from the amorphous silicon detector system were of significantly better quality than images from the screen-film and the computed radiology system. No significant difference in image quality was found between the computed radiology system and the screen-film system. In this study, a more objective and thorough evaluation of the image quality of the digital systems compared to the conventional screen-film system was performed. As the detection of noncalcified small nodules is considered a critical issue for chest radiography, low-contrast resolution was assessed using a contrast-detail phantom. Materials and Methods Imaging systems The amorphous silicon flat panel detector system (FP) used in this study (Vertix FD; Siemens, Germany) consists of an X-ray tube (Optilix 150/30/50 HC-100; Siemens, focal spot size, 0.6/1.0 mm), a high-voltage generator (Polydoros LX-30/50 Lite; Siemens), and a motorized receptor wall stand with the flat panel detector mounted behind a stationary antiscatter grid (80 lines cm-1, ratio, 15:1). The 43x43 cm amorphous silicon image detector (Trixell pixium 4600, France) has a 3000x3000 pixel matrix, corresponding to a 143 µm pixel size. When images are sent to a workstation or printer the 14 bit digital signal is reduced to 12 bit by applying a sigmoidal lookup table (LUT)[6]. The automatic exposure control of the flat panel detector system is adjusted to a 400 speed class. The computed radiography system (CR) used in the study consists of an X-ray tube (Optitop 150/40/73 C, Siemens; focal spot size, 0.6/1.0 mm), a high-voltage generator (Polydoros 80S; Siemens), and a wall stand with an in-height adjustable cassette holder and a moving anti-scatter grid (40 lines cm-1; ratio 12:1). Each of the storage phosphor plates used (GP DirectView CR cassettes, Eastman Kodak, USA) has a 35x43 cm detection surface. After acquisition, the storage phosphor plates are placed into a read-out unit (DirectView CR 900; Eastman Kodak), resulting in a pixel size of 168 µm and a 2048x2500 pixel matrix. The resulting 16 bit digital signal is logarithmically converted into a 12 bit pixel-value [7]. Conventional screen-film radiography (SF) was performed using a dedicated automatic chest film changer (Thoramat; Siemens) combined with a 250 speed wide latitude asymmetric screen-film system optimised for thoracic imaging (InSight Thoracic imaging film and screen, Eastman Kodak). The X-rays are generated using an X-ray tube (Optitop 66 150/40/80 HC-100, Siemens; focal spot size, 0.6/1.0 mm) and a generator (Polydoros 80S, Siemens). A moving anti-scatter grid was used (40 lines cm-1; ratio, 12:1). Contrast-detail phantom For evaluation of the image quality taking into account both spatial and contrast resolution, the CDRAD 2.0 contrast-detail phantom (University Hospital Nijmegen - St. Radboud, The Netherlands)[8] was used. The phantom is a plate of PMMA (265x265x10 mm³ polymethyl methacrylate) containing a grid of 15x15 cells. The cells contain cylindrical holes with depths and diameters varying exponentially from 0.3 mm to 8.0 mm. Within each column the depth remains constant and within each row the diameter is constant. For the largest three diameters one hole is situated in the centre of the cell. In all other cells two identical holes are present, one in the centre and one in a random corner. As objects get smaller and lower in contrast, their signal-to-noise ratio is reduced and they become harder to see on the image. With this type of phantom an observer has to decide not only which holes are visible in the phantom image, but he/she must also indicate in which of the four corners of the cell the holes are situated. Because this is a four-alternative forced choice experiment the chance of a false positive is limited to 25% and accuracy is improved in comparison with a traditional contrast-detail phantom study. After comparing the indicated positions of the eccentric holes with the true hole positions in the phantom, a correction scheme was used taking into account the nearest neighbours in order to get more accurate results [8]. For each row i (detail Di) the smallest visible depth (Ci,th, threshold contrast) was determined, and for each column j (contrast Cj) the smallest visible diameter (Dj,th, threshold detail). By plotting Ci,th versus Di, for all rows i, a contrast-detail curve is obtained. Objects above and to the right of this curve are visualised and objects below and to the left are not seen. A so called inversed image quality figure (IQFinv) is determined by the following formula: IQFinv = 100 15 ∑C i =1 i * Di ,th The higher the IQFinv, the better the low contrast visibility. Image acquisition For all imaging modalities the source image distance was 180 cm, the field of view 40x40 cm and the focal spot size 0.6 mm. In a clinical setting the 67 automatic exposure control is used at a tube voltage of 125kV without additional cupper filtration. To simulate attenuation and scatter by the patient chest, the CDRAD 2.0 phantom was placed between two slabs of 5 cm PMMA, resulting in a total phantom thickness of 11 cm. This thickness was chosen to mimic a standard PA-exposure, since the obtained mAsvalues corresponded well with the mean mAs-values of 100 patients for the PA-projection in clinical settings [5]. Phantom images were made with the different imaging systems for a standard PA-exposure. Furthermore the phantom thickness was varied, as well as the depth position of the low contrast objects within the phantom. The effect of tube voltage, additional cupper filtration and exposure was examined. For each setting two or three images were made in order to take into account the effect of statistical fluctuations in the number of photons emitted by the X-ray tube. Image display After acquisition the digital CR and FP images were sent to a PACS workstation (SIENET MagicView 1000; Siemens) and to a laserprinter (DryView 8500 Laser Imager-Dryview Laser Imaging Film, Kodak; spot spacing 78 µm, resolution 325 dpi, grey value 12 bit). The images were printed with a resolution of 75%. At the workstation soft copy (SC) CRand FP-images were evaluated on a high contrast greyscale monitor (SMM 21140P, Siemens; 21 inch CRT, resolution 1280x1600, luminance ~800 Cd/m²). The soft copy images were scored in two ways. First the images were evaluated with reduced resolution (42%) and postprocessing typical for chest imaging (SC(-)). Then the same images were evaluated at full resolution and with adaptation of contrast and brightness (SC(+)). The SF images as well as the hard copy (HC) CR and FP images were evaluated on a light box. Image reading For each setting the phantom images were distributed at random among eight readers. Radiologists as well as non-radiologists were included in the reading group since there would not be a significant difference in performance [9]. During the evaluation, the ambient light level remained low. No restrictions were made about the viewing distance and the duration of the reading sessions. One image had to be scored at a time, so mutual comparison was impossible. 68 Statistical analysis For all displaying modalities (HC, SC(-) and SC(+)) the FP-system and the CR-system were compared to the SF-system and within a displaying modality the FP-system was compared to the CR-system. For each digital system comparisons were made between image quality of the three displaying modalities. For each setting results were averaged over the different readings. Resulting contrast-detail curves were compared by means of the two-tailed Wilcoxon test (95% confidence level). The two tailed Mann-Whitney U test (95% confidence level) or the Kruskal-Wallis test was used to compare two or more IQFinv. The statistical analysis was performed using SPSS software (version 10.0.5.) for Windows. Results and discussion For a standard PA-exposure contrast-detail curves of the FP-system were significantly better than contrast-detail curves of the SF-system for all displaying modalities (Figure 1). Figure 1: Contrast-detail curves for the different imaging systems in clinical settings (HCdisplay) 69 The FP-system performed also significantly better than the CR-system. No statistical difference was found between the contrast-detail curves of the CR-system and the SF-system. These results are consistent with the results of a subjective clinical image quality study on the same systems, based on SC(+) readings [5]. It is noted that the FP-system operated at about 40% of the dose of the SF-system, and the CR-system at about 65% of that dose [5]. For both digital systems SC(+) readings lead to significantly better contrast-detail curves than both SC(-) and HC readings, this is illustrated in Figure 2 for the FP-system. Figure 2: Contrast-detail curves for the FP-system in clinical settings (different displaying modalities) No significant difference was found between SC(-) and HC readings. Analysis of the IQFinv lead to similar results, although 95% statistical significance was not reached for the difference between FP-SC(-) and CRSC(-) and between FP-SC(+) and FP-HC. As expected the image quality decreased significantly with the phantom thickness (7-20 mm PMMA) for all imaging systems. The depth position of the low contrast objects in the phantom did not affect the image quality significantly, neither did the tube voltage (102-133 kV) or additional filtration (0.0-0.3 mm Cu). However low contrast visibility significantly improved with increasing 70 exposure for the digital systems. Figure 3 clearly indicates that for a comparable exposure the FP-system provides superior image quality than the SF- and the CR-system. This implies that strong dose reductions with the FP-system are possible without loss of image quality. The difference between the CR- and the SF-system for a comparable exposure is not convincing. Figure 3: Inversed Image Quality Figure as a function of exposure for the different imaging systems and the different displaying modalities These results are consistent with literature where for the same exposure low-contrast performance of amorphous silicon FP-systems showed to be superior compared to CR-systems [1] and SF-systems [1,2]. For the same exposure there was no significant difference between contrast-detail curves of the CR- and the SF-systems [1,9]. Aufrichtig et al. [2] attributed the better performance of the FP-system to the higher detective quantum efficiency, and estimated that a dose reduction up to 70% could be achieved in comparison with a SF-system. Clinical studies and anthropomorphic chest phantom studies also support previous findings [3,4]. All these studies were based on hard copy display. Figure 3 however demonstrates that when applying digital systems best results are 71 obtained with soft copy reading at full resolution with adaptation of contrast and brightness. It is noted that because of the high dynamic range of digital detectors and the increase in low contrast visibility with exposure, care must be taken that the dose levels of the automatic exposure control are well calibrated and a good compromise is made between low dose and acceptable image quality, keeping in mind the ALARA-principle. Conclusion Full Field Digital Radiography systems, based on amorphous silicon flat panel detector technology, are a breakthrough in chest imaging. These systems show an improved image quality in combination with a strong patient dose reduction. References 1. Rong, X.J., Shaw, C.C., Liu, X., Lemacks, M.R. and Thompson, S.K. Comparison of an amorphous silicon/cesium iodide flat-panel digital chest radiography system with screen/film and computed radiography systems – A contrast-detail phantom study. Med. Phys. 28, 2328-2335 (2001) 2. Aufrichtig, R. Comparison of low contrast detectability between a digital amorphous silicon and a screen-film based imaging system for thoracic radiography. Med. Phys. 26, 1349-1358 (1999) 3. Fink, C., Hallscheidt, P., Noeldge, G., Kampschulte, A., Radeleff, B., Hosch, W.P., Kauffmann, G.W. and Hansmann, J. Clinical comparative study with a large-area amorphous silicon flat-panel detector: image quality and visibility of anatomic structures on chest radiography. AJR 178, 481-486 (2002) 4. Strotzer, M., Gmeinwieser, J., Völk, M., Fründ, R., Seitz, J. and Feuerbach, S. Detection of simulated chest lesions with normal and reduced radiation dose, comparison of conventional screen-film radiography and a flat-panel X-ray detector based on amorphous silicon. Invest. Radiol. 33, 98-103 (1998) 5. Bacher, K., Smeets, P., Bonnarens, K., De Hauwere, A., Verstraete, K. and Thierens, H. Dose reduction in patients undergoing chest imaging: digital amorphous silicon flatpanel detector radiography versus conventional film-screen radiography and phosphorbased computed radiography. AJR 181, 923-929 (2003). 6. Herrmann, K.A., Stäbler, A., Bonél, H., Kulinna, C., Holzknecht, N., Geiger, B., Böhm, S., Maschke, M., Reiser, M.F. Initial experiences in clinical application of the THORAXFD: flat-panel detector radiography in thoracic diagnosis. Electromedia 68, 25-30 (2000) 7. Guidelines for Acceptance Testing and Quality Control: Kodak Direct View CR 800 System and Kodak DirectView CR 900 System. Kodak Technical and Scientific Bulletin, 1-25 72 8. Thijssen, M.A.O., Bijkerk, K.R. and van der Burght, R.J.M. Manual contrast-detail phantom CDRAD type 2.0. Project Quality Assurance in Radiology, Section Clinical Physics, Department of Radiology, University Hospital Nijmegen, St. Radboud (1998) 9. Cook, L.T., Insana, M.F., McFadden, M.A., Hall, T.J. and Cox, G.G. Comparison of the low-contrast detectability of a screen-film system and third generation computed radiography. Med. Phys. 21, 691-695 (1994) 73 3.4 Part IV Image quality performance of liquid crystal display systems: influence of display resolution, magnification and window settings on contrast-detail detection Klaus Bacher1 – Peter Smeets2 – An De Hauwere1 – Tony Voet2 – Philippe Duyck2 – Koenraad Verstraete2 – Hubert Thierens1 1Department of Medical Physics and Proeftuinstraat 86, Gent B-9000, Belgium. 2Department Belgium. Radiation Protection, Ghent University, of Radiology, Ghent University Hospital, De Pintelaan 185, Gent B-9000, Accepted for publication in Eur J Radiol (in press) Abstract The aim of this study was to investigate the combined effects of liquid crystal display (LCD) resolution, image magnification and window/level adjustment on the low contrast performance in soft-copy image interpretation in digital radiography and digital mammography. In addition, the effect of a new LCD noise reduction mechanism on the low-contrast detectability was studied. Digital radiographs and mammograms of two dedicated contrast-detail phantoms (CDRAD 2.0 and CDMAM 3.4) were scored on five LCD devices with varying resolutions (1-, 2- , 3- and 5megapixel) and one dedicated 5-megapixel cathode ray tube monitor. Two 5-megapixel LCDs were included. The first one was a standard 5-megapixel LCD, the second had a new (Per Pixel Uniformity) noise reduction mechanism. A multivariate analysis of variance revealed a significant influence of LCD resolution, image magnification and window/level adjustment on the image quality performance assessed with both the CDRAD 2.0 and the CDMAM 3.4 phantoms. The interactive adjustment of brightness and contrast of digital images did not affect the reading time, whereas magnification to full resolution resulted in a significantly slower softcopy interpretation. For digital radiography applications, a 3-megapixel LCD is comparable with a 5-megapixel CRT monitor in terms of low-contrast performance as well as in reading time. The use of a 2megapixel LCD is only warranted when radiographs are analysed in full resolution and when using the interactive window/level adjustment. In digital mammography, a 5-megapixel monitor should be the first choice. In addition, the new PPU noise reduction system in the 5-megapixel LCD devices provides significantly better results for mammography reading as compared to a standard 5-megapixel LCD or CRT. If a 3megapixel LCD is used in mammography setting, a very time-consuming magnification of the digital mammograms would be necessary. 75 Introduction Since the introduction of picture archiving and communication systems (PACS) in radiology, computer-based image interpretation is rapidly replacing the film-based evaluation [1-3]. High-resolution monochrome cathode ray tube (CRT) monitors are currently the golden standard in soft-copy reading and are considered to be at least as efficient and accurate as conventional film-based reading [2-8]. However, CRT monitors have some disadvantages, including a non-isotropic modulation transfer function (MTF) and a large vulnerability to reflections and ambient lighting conditions [4,7-9]. In addition, due to their curved faceplate surface, CRT displays can cause peripheral distortion artefacts [4,7-9]. Furthermore, CRT displays are heavy and bulky and have high levels of power consumption [2,4]. A few years ago, high quality medical grade active matrix Liquid Crystal Display (LCD) devices became commercially available. LCDs have a nearly perfect MTF behaviour, have higher luminance and are less susceptible to light reflections compared to CRT monitors [1,4,7-9]. Moreover, LCDs are compact and energy-efficient display systems [1,2]. Today, a large variety of LCD devices are available for medical use. Their resolutions vary from 1-megapixel up to 5-megapixel for monochrome displays and even 9-megapixels for colour LCDs. However, up to now, the knowledge about the image quality performance of LCD devices is limited. Most studies focus only on the comparison between a 3megapixel LCD device and a 5-megapixel CRT display with respect to their diagnostic performance in digital radiography [1,2,4,7,10-12] or on the angular luminance and contrast dependence of both monitor types [13]. Only few initial results are available with respect to the image quality performance of 5-megapixel LCDs [8,9]. Furthermore, the influence of image magnification and interactive adjustment of the brightness and contrast levels on LCDs of different matrix sizes remains unclear. Therefore, the objective of this study was to examine the combined effects of LCD resolution, image magnification and window adjustment on the low-contrast performance, using dedicated contrast-detail phantoms for radiography and mammography. In addition, the effect of a new LCD noise reduction mechanism (Per Pixel Uniformity) on the low-contrast detectability was studied. 76 Materials and Methods Contrast-detail phantoms The image quality of different soft-copy display systems was analyzed in terms of the contrast-detail performance of the monitors, using both a CDRAD 2.0 and a CDMAM 3.4 contrast-detail phantom (Artinis Medical Systems, Andelst, The Netherlands). The CDRAD 2.0 phantom consists of a Plexiglas plate (26.5 x 26.5 x 1 cm³) with a 15x15 array of 225 square cell regions in which circular holes are drilled. The latter holes are logarithmically sized from 0.3 to 8.0 mm in both diameter and depth (Figure 1). For 4 mm and smaller objects, the phantom contains an additional hole of matching diameter and depth, placed at random in one of the four cell corners. Figure 1: Digital acquisition of the CDRAD 2.0 phantom. The CDMAM 3.4 phantom, designed for image quality evaluation in mammography, consists of an aluminium base with gold disks of various thicknesses and diameters, attached to a Plexiglas cover (18 x 24 x 0.3 cm³) (Figure 2). The discs are arranged in a 16x16 matrix divided into 205 squares cell regions. Within a row, the disc thickness logarithmically increases from 0.03 to 2 µm, while the diameter is constant; within a column, the disc diameter logarithmically increases from 0.06 to 2.00 mm, while the thickness is constant. Each of the 205 cell regions of the phantom contains one gold disk in the centre and another one at a randomly chosen corner. 77 Figure 2: Sample image of the CDMAM 3.4 phantom. The presence of these additional corner objects in both the CDRAD 2.0 and the CDMAM 3.4 contrast-detail phantoms allows a four-alternative forced-choice experiment in which the observer must select the location of the low-contrast objects among the four possible corners [14]. In this way, these extra holes (disks) help to minimize potential biases due to a priori knowledge of the presence of objects in every square region [15]. The phantoms are used to assess the minimum contrast required to visualize objects of different sizes above the noise threshold in digital radiography (CDRAD 2.0) and digital mammography (CDMAM 3.4). Contrast-detail phantoms acquisitions The CDRAD 2.0 phantom was placed between two layers of 5 cm polymethyl methacrylate (PMMA) to simulate patient scatter. Afterwards, three digital radiographs were taken of the phantom set-up using an amorphous silicon flat-panel detector system (Siemens, Erlangen, Germany and Trixell, Moirans, France). In this detector, a needle-structured thallium-doped CsI scintillator was used to convert the X-rays into visible light, which was deposited directly on the 43 x 43 cm amorphous silicon matrix with a 143 µm pixel pitch. Subsequently, an amorphous silicon photodiode converted the light into electrical charge, resulting in a 14-bit digital signal in a 3001 x 3001 pixel matrix. All images were acquired at a focus-to-detector distance of 180 cm using the same exposure parameters (125 kVp, 1.5 mAs). The CDMAM 3.4 phantom was placed between two layers of 2 cm PMMA to simulate the absorption of a standard breast. Three digital 78 CDMAM images were acquired on a full-field digital mammography system, using a 24 x 29 cm amorphous selenium flat-panel detector with a pixel pitch of 70 µm (Lorad Selenia, Lorad/Hologic, Danbury, CT). In this detector system, free electrons are released by the interaction of X-rays with the amorphous selenium semiconductor layer. Due to the application of an electric field across the selenium layer, the electric charges are drawn directly to the charge-collecting electrodes. The latter operation results in a 14-bit image, stored in a 3328 x 4096 pixel matrix. The imaging conditions were selected as 30 kVp, 55 mAs, with a Mo/Mo anode-filter combination. After acquisition, both the digital CDRAD 2.0 and the CDMAM 3.4 images were transferred to a workstation, for image quality assessment. Soft-copy display systems The contrast-detail performance of five LCDs with varying resolution was compared to that of a dedicated 5-megapixel CRT monitor (MGD 521, BARCO, Kortrijk, Belgium). The LCDs had a matrix resolution of 1-, 2-, 3or 5-megapixel. Two 5-megapixel LCDs were included (MFGD 5421 and MFGD 5621 HD, BARCO, Kortrijk, Belgium). The first one was a standard 5-megapixel LCD, the second had a Per Pixel Uniformity (PPU) functionality, designed for real-time elimination of screen noise, thereby making each individual pixel DICOM-compliant [16]. Monitors were installed using their corresponding 10-bit display controller card. The technical characteristics of all displays used are summarized in Table 1. To assure consistency in image appearance with the monitors included in this study, all displays were calibrated to comply with the DICOM Part 3.14 Greyscale Standard Display Function (GSDF), using calibration software provided by the manufacturer (MediCal Pro, BARCO, Kortrijk, Belgium) [17]. The maximum luminance of all monitors was adjusted to 400 Cd/m²; the minimum luminance was set to 0.4 Cd/m². Before each reading session, compliance to the DICOM GSDF was measured with a telescopic luminance meter (LS-100, Minolta, Osaka, Japan), using the methodology as proposed by the American Association of Physicists in Medicine (AAPM), Task Group 18 [18]. 79 Table 1: Summary of the monitor specifications. Monitor Type BARCO BARCO BARCO BARCO BARCO BARCO MFGD 1318 MFGD 2320 MFGD 3420 MFGD 5421 MFGD 5621HD MGD 521 1-MP 2-MP 3-MP 5-MP 5-MP 5-MP TFT AMLCD TFT AMLCD TFT AMLCD TFT AMLCD TFT AMLCD, PPU CRT, P45 phosphor 459.7 510.0 528.0 540.9 540.9 486.6 Pixel Number 1024 × 1280 1200 × 1600 1536 × 2048 2048 × 2560 2048 × 2560 2048 × 2560 Pixel Pitch (mm) 0.2805 0.255 0.207 0.165 0.165 N.A. 600 600 700 700 >800 600 Factor Display Type Viewable size (diameter, mm) Maximum Luminance (Cd/m²) Note – MP=megapixel, N.A.=not applicable, TFT AMLCD=Thin Film Transistor Active Matrix Liquid Crystal Display, PPU=Per Pixel Uniformity functionality. Soft-copy image evaluation Contrast-detail phantom evaluation For both the CDRAD 2.0 as for the CDMAM 3.4 phantom images, the observers had to identify, in every square cell region, the locations of the corner holes (disks). The results were entered on a score sheet for each image reviewed. After comparing the score forms to a reference form containing the correct locations of all corner holes (disks), a correction scheme was used taking into account the nearest neighbours in order to get more accurate results [19-21]. Finally, for each different diameter (Di) the threshold contrast value (Ci,th) was determined as the minimum depth (thickness) in regions of valid detection. The inverse image quality figure (IQFinv) was introduced for quantitative comparison of the contrastdetail images [19]. The IQFinv is defined as: IQFinv = 100 15 ∑C i =1 i * Di ,th where Ci represents the object depth (thickness) in the contrast-column i and Di,th denotes the corresponding smallest visible diameter (threshold diameter) in this column. The parameter n represents the number of rows/columns in the contrast-detail phantom (n=15 for the CDRAD 2.0; 80 n=16 for the CDMAM 3.4). The higher the IQFinv, the better the low contrast visibility. Monitor low-contrast quality analysis All image reading sessions were conducted in the same evaluation room under subdued lighting conditions. Before each reading session, room illuminance was measured with an illuminance meter (L100, RTI, Mölndal, Sweden) to ensure an illuminance level lower than 10 lux at the position of the monitor. Three CDRAD 2.0 and three CDMAM 3.4 contrast-detail images were analysed in four different interpretation sessions. In the first session, the images were visualized using the standard representation on the display (zoom to fit display surface, automatic contrast and brightness selection). Secondly, the observers were forced to use a pc-mouse driven interactive adjustment of the window (contrast) and level (brightness) settings. In the next session, the images were presented at full resolution, but window and level adjustment were not allowed. Finally, observers scored the images at full resolution in combination with the interactive window and level adjustment. To avoid learning effects, reading sessions were organized with an interval of at least two weeks. A total of 15 observers contributed in this study. All of them were familiar with analyzing CDRAD 2.0 and CDMAM 3.4 contrast-detail images. Because monitor types could be easily distinguished based on their physical appearance, a possible bias could be introduced by this a priori knowledge of the observers. Therefore only three of them analysed the contrast-detail images on all displays. The twelve remaining observers were proportionally subdivided over the six monitor types. Hence 15 CDRAD 2.0 and 15 CDMAM 3.4 evaluations were made for all displays in each of the four different scenarios. The IQFinv-values were calculated for the analysed images and mean values were computed for each display type. Reading times were registered for all contrast-detail image interpretations. Statistical analysis A three-way analysis of variance (ANOVA) with two repeated factors (magnification and adjustment of window/level settings) and one between-subject parameter (monitor type) was conducted to analyse the possible effects of the monitor type, magnification and window/level setting adjustment on the observer performance for low-contrast details 81 in general. Significance of differences among interpreting times was evaluated using the same three-way ANOVA technique. To test whether the different LCD monitor types performed significantly better (worse) in comparison with the ‘golden-standard’ 5 MP CRT monitor, post-hoc comparisons were made by means of Dunnett’s t-test. The latter test treats one group as a control (i.e. CRT display) and compares all other groups against it. To analyse the influence of image magnification (to full resolution) and window level adjustments on each monitor type separately, a two-way repeated measures ANOVA was applied. A p-value < 0.05 was considered as significant. All calculations were performed by means of the SPSS 12.0 software (SPSS, Chicago, Ill). Results Mean IQFinv For each monitor type, a set of 60 IQFinv-values were calculated from the CDRAD 2.0 as well as from the CDMAM 3.4 images (5 observers x 3 images x 4 scenarios). During the study, the measured greyscale display function of all monitors did not deviate more than 10% from the GSDF as is required for primary class displays [17,18]. standard view 4 3.5 3 2.5 2 1.5 1 0.5 0 window adjustment magnification magnification + window adjustment 1MP LCD 2MP LCD 3MP LCD 5MP LCD 5MP 5 MP LCD CRT PPU Figure 3: Mean calculated IQFinv-values for all monitor types in the different reading scenarios based on the CDRAD 2.0 image analysis. Bars indicate standard deviation. 82 The mean calculated IQFinv-values for all monitor types in the different reading scenarios are summarized in Figure 3 and Figure 4 for the CDRAD 2.0 and the CDMAM 3.4 images respectively. standard view 100 window adjustment Mean IQFinv 120 80 magnification 60 magnification + window adjustment 40 20 0 1MP LCD 2MP LCD 3MP LCD 5MP LCD 5MP LCD PPU 5 MP CRT Figure 4: Mean calculated IQFinv-values for all monitor types in the different reading scenarios based on the CDMAM 3.4 image analysis. Bars indicate standard deviation. Influence of monitor type on contrast-detail performance In Table 2 the p-values from the three-way ANOVA analysis of the IQFinv-values are presented. The latter test showed a significant influence of the monitor type on the contrast-detail performance for both the CDRAD 2.0 and the CDMAM 3.4 phantoms (p=0.023 and p=0.009 respectively). Table 2: P-values for assessment of effects of monitor type, image magnification and window/level adjustment on the contrast-detail performance (IQFinv). Results of the CDRAD 2.0 and the CDMAM 3.4 analysis are indicated separately. Factors CDRAD 2.0 CDMAM 3.4 Monitor type 0.023 0.009 Magnification 0.002 0.001 Window/Level adjustment 0.001 0.001 Magnification*Monitor type (1) 0.029 0.011 Magnification*Window/Level adjustment (1) 0.364 0.289 Window/Level adjustment*Monitor type (1) 0.465 0.512 Magnification*Window/Level adjustment*Monitor type (1) 0.315 0.425 Note – P-values were estimated with three-way ANOVA (1): Factors represent multi-variate interactions 83 The results of the post-hoc comparison of all LCDs with the 5-megapixel CRT display control are indicated on Table 3. Dunnett’s t-test revealed a significant inferior contrast-detail performance of the 1-megapixel LCD for the digital radiography experimental setting (CDRAD 2.0). In the digital mammography setting, the 1-megapixel as well as the 2-megapixel LCDs scored significantly lower in comparison with the CRT control (p=0.001 and p=0.016 respectively). The 5-megapixel LCD with PPU outperformed the standard 5-megapixel CRT display. The latter finding was significant for the CDMAM 3.4 image analysis (p=0.039), but not for the CDRAD 2.0 data (p= 0.386). Table 3: P-values of post-hoc comparison of the IQFinv-values of different LCD types with CRT control group. Results of the CDRAD 2.0 and the CDMAM 3.4 analysis are indicated separately. 2-sided comparisons CDRAD 2.0 CDMAM 3.4 1-MP LCD vs 5-MP CRT 0.014 0.001 2-MP LCD vs 5-MP CRT 0.103 0.016 3-MP LCD vs 5-MP CRT 0.426 0.161 5-MP LCD vs 5-MP CRT 0.899 0.632 5-MP LCD, PPU vs 5-MP CRT 0.386 0.039 Note – MP=megapixel. P-values were estimated by means of Dunnett’s t-test. Influence of monitor type on reading time Table 4 summarizes the three-way ANOVA with respect to the reading time needed to analyse the contrast-detail phantom images. Again, the monitor type influence was significant (p=0.033 for CDRAD 2.0 and p=0.019 for CDMAM 3.4 reading). Table 4: P-values for assessment of effects of monitor type, image magnification and window/level adjustment on reading times. Results of the CDRAD 2.0 and the CDMAM 3.4 analysis are indicated separately. Factors CDRAD 2.0 CDMAM 3.4 Monitor type 0.033 0.019 Magnification 0.012 0.007 Window/Level adjustment 0.298 0.101 Magnification*Monitor type (1) 0.039 0.021 Magnification*Window/Level adjustment (1) 0.354 0.389 Window/Level adjustment*Monitor type (1) 0.565 0.613 Magnification*Window/Level adjustment*Monitor type (1) 0.415 0.435 Note – P-values were estimated with three-way ANOVA (1) Factors represent multi-variate interactions 84 Post-hoc test results of the reading times are given in Table 5. For both the 1-megapixel as well as the 2-megapixel monitors observers needed significantly more time compared to the CRT-reference (Table 5). This was also the case for the 3-megapixel LCD, but only when analysing the CDMAM 3.4 images (p=0.028). For the 5-megapixel LCD devices, similar interpretation times were needed as compared to the 5-megapixel CRT monitor. Table 5: P-values of post-hoc comparison of the phantom reading times using different LCD types compared with the CRT control group. Results of the CDRAD 2.0 and the CDMAM 3.4 analysis are indicated separately. 2-sided comparisons CDRAD 2.0 CDMAM 3.4 1-MP LCD vs 5-MP CRT 0.011 0.003 2-MP LCD vs 5-MP CRT 0.037 0.012 3-MP LCD vs 5-MP CRT 0.560 0.028 5-MP LCD vs 5-MP CRT 0.859 0.762 5-MP LCD, PPU vs 5-MP CRT 0.866 0.674 Note – MP=megapixel. P-values were estimated by means of Dunnett’s t-test. Impact of magnification and interactive window/level adjustment on the contrast-detail performance According to the three-way ANOVA, both magnification to full resolution and window/level adjustment significantly influenced the contrast-detail performance (Table 2). No interactions were found between the window/level adjustment and the monitor type (p=0.465 and p=0.512 for the CDRAD 2.0 and the CDMAM 3.4 analysis respectively), showing that interactively changing the window/level setting significantly improved the low-contrast performance of all monitors in both the radiography and the mammography settings. However, the mean IQFinv-value increase was higher for the CDMAM 3.4 images compared to the CDRAD 2.0 images (Table 6). With respect to magnification, significance was reached only for the 1and 2-megapixel LCDs in the CDRAD 2.0 analysis (p=0.009 and p=0.012, respectively) and for the 1-, 2- and 3-megapixel LCDs in the CDMAM 3.4 experiment (p=0.001, p=0.007 and p=0.011, respectively). This is supported by a significant interaction between the monitor type and the use of magnification (p=0.029 and p=0.011 for the CDRAD 2.0 and the CDMAM 3.4 analysis respectively). There were no significant interactions between magnification and window/level adjustment, indicating that the effect of window/level adjustment on the IQFinv-value was not affected 85 by the effect of magnification to full resolution (p=0.364 and p=0.289 for the CDRAD 2.0 and the CDMAM 3.4 analysis respectively). Table 6: Mean IQFinv-value increase by using interactive window/level adjustment. Monitor types % IQFinv increase in CDRAD 2.0 reading % IQFinv increase in CDMAM 3.4 reading 1-megapixel LCD 5.6 (1.8) 12.4 (1.9) 2-megapixel LCD 7.3 (1.9) 13.5 (2.4) 3-megapixel LCD 6.4 (2.0) 10.7 (2.4) 5-megapixel LCD 5.5 (1.0) 12.1 (1.5) 5-megapixel LCD, PPU 3.7 (2.1) 8.8 (3.7) 5-megapixel CRT 7.1 (1.3) 9.7 (2.9) 5.9 11.2 Mean Note – Values are mean percentages. Number in parentheses indicate standard deviation. MP=megapixel. Impact of magnification and interactive window/level adjustment on the reading time In present phantom study, magnification of the digital images to full resolution significantly influenced the reading time of the digital images (p=0.012 and p=0.007 for the CDRAD 2.0 and the CDMAM 3.4 analysis respectively) (Table 4). However, there was a significant interaction with the monitor type (p=0.039 and p=0.021, respectively). For the digital radiographs of the CDRAD 2.0 phantom, the significant difference was only true in the case of the 1 and 2-megapixel LCDs (p=0.009 and p=0.032). In the digital mammography setting, the analysis with the 3megapixel LCD also resulted in a significant longer evaluation time (p=0.035). The interactive adjustment of brightness and contrast did not affect the reading time (p=0.298 and p=0.101 for the CDRAD 2.0 and the CDMAM 3.4 analysis respectively). Discussion The last years, digital radiography and mammography systems are rapidly replacing the screen-film based imaging. As a result, radiology departments are now evolving towards a completely digital medical imaging environment. PACS integrated in a hospital information systems are playing an important role in this evolution. The different post86 processing tools, the possibility for multi-modality image display and the use of computer aided detection software are just some examples of the possibilities of a digital image management in a PACS [22]. Accurate image interpretation with the use of soft-copy viewing stations is one of the major requirements for implementing a PACS [3]. Highresolution grey-scale CRT monitors are now widely accepted for primary image interpretation [2-8]. A few years ago, high-resolution active matrix LCDs became available for soft-copy medical image reading. The latter display devices provide excellent MTF characteristics, higher luminance and, in comparison with the traditional curved-surface CRT monitors, a virtual elimination of veiling glare and peripheral distortions [1,2,4-11,13]. In addition, LCDs have some ergonomic advantages as well, related to their compact construction and the elimination of display flicker [1,2]. Up to now, the knowledge about the image quality performance of LCD devices for primary image analysis is limited. In contrast to the large variety of available matrix resolutions in LCD technology, most studies discuss the diagnostic performance of a 3-megapixel LCD device in comparison with a 5-megapixel CRT monitor [1,2,4,7,10-12]. Only recently, Krupinski et al presented the first results with respect to the image quality performance of a 5-megapixel LCD [8,9]. In present study, the image quality performance of a series of LCD devices was compared with the quality that can be obtained with a 5megapixel reference CRT monitor. The included LCDs had a matrix resolution of 1-, 2-, 3- or 5-megapixel. The image quality was assessed in terms of the ability to detect low-contrast details in the background noise of digital radiography and mammography acquisitions. Therefore, a contrast-detail phantom analysis was set up. For image quality analysis in the digital radiography setting, a CDRAD 2.0 phantom was used. The subtle details and contrasts typical for digital mammography, were simulated using the CDMAM 3.4 phantom. Both contrast-detail phantoms are widely used for an objective analysis of the image quality performance of digital radiography and mammography systems and provide an objective quantitative value for the low-contrast performance (IQFinv) [14,15,22,23]. Our results showed that the matrix resolution of the LCD devices had a significant influence on the contrast-detail performance. Even when using magnification, a 1-megapixel LCD could not reach the quality of a 5megapixel CRT in the digital radiography setting. Similar results were 87 found by Peer et al, where a contrast-detail analysis showed a significantly lower performance of a 1-megapixel CRT monitor in comparison with a 5-megapixel CRT [24]. The CDMAM 3.4 results indicate that the quality of 1- and 2-megapixel monitors is not sufficient for digital mammography viewing. The CDRAD 2.0 analysis showed that a 3-megapixel LCD performed as well as a dedicated 5-megapixel CRT monitor, with respect to the image quality and the efficiency. These conclusions are confirmed by different clinical comparative studies [1,2,4,7,10-12]. For the scoring of digital mammograms, only the 5-megapixel LCD could obtain a similar quality and efficiency compared to the 5-megapixel CRT monitor. The 5megapixel LCD equipped with the recently developed PPU function, significantly outperformed the 5-megapixel CRT display. The latter function was designed for real-time elimination of the typical LCD screen noise, thereby making each individual pixel DICOM-compliant [16]. The improvement in contrast-detail performance by using PPU was, however, only significant when analysing CDMAM 3.4 images, indicating that the PPU functionality eliminates very subtle noise patterns which are only relevant for mammography applications. Clinical comparative studies should be performed to confirm the latter finding. Magnification to full resolution significantly improves the image quality when using lower resolution LCD devices. As a result, a 2-megapixel monitor could be used in digital radiography when the radiographs are evaluated under full resolution. Similarly, a 3-megapixel monitor can approach the quality of a 5-megapixel monitor in digital mammography when the magnification function is used. However, using magnification is very time-consuming and will therefore reduce the diagnostic throughput in the radiology department. The latter time considerations are especially relevant in applications for screening purposes. Moreover, with magnification one may lose the overview of the total digital image and this could make diagnosis more difficult. In general, the interactive adjustment of the brightness and contrast results in a significantly better contrast-detail performance. The ANOVA analysis showed no significant interaction between the window/level adjustment and the monitor type, indicating that changing the window/level settings provided a similar image quality improvement for all monitor types in both the digital radiography as well as in the digital mammography setting. In the study of Fuchsjäger et al, adjustment of the window/level setting had a significant influence on the detectability of catheters in chest radiographies [5]. Our results indicate that the image quality improvement by using window/level adjustment is much more 88 pronounced in digital mammography compared to digital radiography (Table 6). Hence, the change of brightness and contrast during the evaluation of digital mammograms should be strongly recommended. Despite the excellent quality of high-resolution LCDs, the angular dependence of luminance and contrast is the major disadvantage [8,13]. Currently, a new generation of LCDs is in development taking into account this problem. However, today, off-axis viewing of radiographs should be avoided [8]. Conclusion Medical grade high-resolution LCD devices provide excellent contrastdetail detectability. As the interactive adjustment of brightness and contrast of digital images significantly improved the image quality of all included displays without affecting the throughput, the use of this functionality should be strongly recommended, especially in digital mammography. For digital radiography applications, a 3-megapixel LCD is comparable with a 5-megapixel CRT monitor in terms of low-contrast performance as well as in reading time. The use of a 2-megapixel LCD is only warranted when radiographs are analysed in full resolution and when using the interactive window/level adjustment. In digital mammography, a 5megapixel monitor should be the first choice. In addition, the new PPU noise reduction system in the 5-megapixel LCD devices provides excellent results for mammography reading. If a 3-megapixel LCD is used in mammography setting, a very time-consuming magnification of the digital mammograms would be necessary. Acknowledgements The authors thank the staff of the Department of Radiology of the Ghent University Hospital for their cooperation in this study. We also thank MSc Geert Carrein, MSc Luc Colle and BSc Tom Martens of BARCO Medical Imaging Systems (Kortrijk, Belgium) for their technical support. 89 References 1. Balassy C, Prokop M, Weber M, Sailer J, Herold CJ, SchaeferProkop C. Flat-panel display (LCD) versus high-resolution gray-scale display (CRT) for chest radiography: an observer preference study. Am J Roentgenol 2005; 184:752-756. 2. Hwang SA, Seo JB, Choi BK, et al. Liquid-crystal display monitors and cathode-ray tube monitors: a comparison of observer performance in the detection of small solitary pulmonary nodules. Korean J Radiol 2003; 4:153-156. 3. Goo JM, Choi JY, Im JG, et al. Effect of monitor luminance and ambient light on observer performance in soft-copy reading of digital chest radiographs. Radiology 2004; 232: 762-766. 4. Scharitzer M, Prokop M, Weber M, Fuchsjäger M, Oschatz E, Schaefer-Prokop C. Detectability of catheters on bedside chest radiographs: comparison between liquid crystal display and high-resolution cathode-ray tube monitors. Radiology 2005; 234:611616. 5. Fuchsjäger MH, Schaefer-Prokop CM, Eisenhuber E, et al. Impact of ambient light and window settings on the detectability of catheters on soft-copy display of chest radiographs at bedside. Am J Roentgenol 2003; 181:1415-1421. 6. Ishihara S, Shimamoto K, Ikeda M, et al. CRT diagnosis of pulmonary disease: influence of monitor brightness and room illuminance on observer performance. Comput Med Imag Graph 2002; 26: 181-185. 7. Oschatz E, Prokop M, Scharitzer M, Weber M, Balassy C, Schaefer-Prokop C. Comparison of liquid crystal versus cathode ray tube display for the detection of simulated chest lesions. Eur Radiol 2005; 15:1472-1476 8. Krupinski EA, Johnson J, Roehrig H, Nafziger J, Lubin J. On-axis and off-axis viewing of images on CRT displays and LCDs: Observer performance and vision model predictions. Acad Radiol 2005; 12:957-964. 9. Krupinski AE, Johnson J, Roehrig H, Nafziger J, Fan J, Lubin J. Use of a human visual system model to predict observer performance with CRT vs LCD display of images. J Digit Imaging 2004; 17: 258-263. 10. Langer S, Bartholmai B, Fetterly K, et al. SCAR R&D symposium 2003: Comparig the efficacy of 5-MP CRT versus 3-MP LCD in the evaluation of interstitial lung disease. J Digit Imaging 2004; 17:149-157. 11. Kotter E, Bley TA, Saueressig U, et al. Comparison of the detectability of high- and lowcontrast details on a TFT screen and a CRT screen designed for radiologic diagnosis. Invest Radiol 2003; 38: 719-724. 12. Averbukh AN, Channin DS, Homhual P. Comparison of human observer performance of contrast-detail detection across multiple liquid crystal displays. J Digit Imaging 2005; 18: 66-77. 13. Bandado A, Flynn MJ, Martin S, Kanicki J. Angular dependence of the luminance and contrast in medical monochrome liquid crystal displays. Med Phys 2003; 30: 2602-2613. 14. Aufrichtig R, Xue P. Dose efficiency and low-contrast detectability of an amorphous silicon x-ray detector for digital radiography. Phys Med Biol 2000; 45:2653-2669 15. Rong XJ, Shaw CC, Liu X, Lemacks MR, Thompson SK. Comparison of an amorphous silicon/cesium iodide flat-panel digital chest radiography system with screen/film and computed radiography systems – A contrast-detail phantom study. Med Phys 2000; 28: 2328-2335 90 16. Kimpe T, Xthona A, Matthijs P, De Paepe L. Solution for nonuniformities and spatial noise in medical LCD displays by using pixel-based correction. J Digit Imaging 2005; 18: 209-218. 17. National Electrical Manufacturers Association (NEMA). Digital Imaging and Communications in Medicine (DICOM), Part 3.14: Grayscale Standard Display Function. Technical report. Virginia: ACR/NEMA, 1998. 18. Samei E, Badano A, Chakraborty D, et al. Assessment of display performance for medical imaging systems: Executive summary of AAPM TG18 report. Medical Physics 2005; 32(4):1205-1225. 19. Thijssen MAO, Thijssen HOM, Merx JL, Lindeijer JM, Bijkerk KR. A definition of image quality: the image quality figure. In: Moores BM, Wall BF, Eriskat H, Schibilla H, editors. BIR Report 20: Optimization of image quality and patient exposure in diagnostic radiology. London: The British Institute of Radiology; 1989: 29-34. 20. Thijssen MAO, Bijkerk KR, van der Burght RJM. Manual Contrast-Detail Phantom CDRAD type 2.0. Project Quality Assurance in Radiology, Department of Radiology, University Hospital Nijmegen, St. Radboud, 1998. 21. Bijkerk KR, Thijssen MAO, Arnoldussen TJM. Manual Contrast-Detail Phantom CDMAM type 3.4. Nijmegen: University Medical Centre Nijmegen, St. Radboud, 2000. 22. Bacher K, Smeets P, Bonnarens K, De Hauwere A, Verstraete K, Thierens H. Dose reduction in patients undergoing chest imaging: digital amorphous silicon flat-panel detector versus conventional film-screen radiography and phosphor-based computed radiography. AJR 2003; 181:923-929. 23. Bonardi R, Ambrogetti D, Ciatto S, et al. Conventional versus digital mammography in the analysis of screen-detected lesions with low positive predictive value. Eur J Radiol 2005; 55: 258-263. 24. Peer S, Giacomuzzi SM, Peer R, Gassner E, Steingruber I, Jaschke W. Resolution requirements for monitor viewing of digital flat-panel detector radiographs: a contrastdetail analysis. Eur Radiol 2003; 13:413-417. 91 Chapter 4 General discussion and conclusions 4.1 Digital x-ray acquisition The last years, radiology departments are increasingly moving towards an integrated digital environment where digital image archiving systems (PACS) are coupled to a patient management database (RIS/HIS) [1-3]. A significant increase in workflow is expected, when moving to digital radiology [3-5]. In fact, after acquisition, images are immediately available on the PACS for interpretation by the radiologists. After the report has been sent to the RIS/HIS, images as well as the corresponding reports are directly distributed within the entire hospital [3,5]. Moreover, in a digital setting, post-processing tools, the multi-modality image display and the use of CAD software may facilitate the image interpretation and diagnosis. It is obvious that digital imaging techniques are an important requirement for making these systems work accurately and effectively. The transition from screen-film x-ray projection techniques (including mammography) to digital acquisition was therefore a very important step, as conventional radiographs are the most frequently obtained images in medical imaging [1,6,7]. As the conventional screen-film technique is principally incompatible with a digital environment, it was the last modality to make the transition to digital acquisition [8]. In fact, up to now, screen-film systems are still in use because of their good image quality, high spatial resolution and general low costs [8-10]. Major disadvantages of screen-film techniques, however, are the limited exposure range, a rather high retake rate and the inflexibility of image display and archiving [8,9]. 93 Computed radiography A first step to overcome screen-film radiography was made in 1982 by introducing storage phosphor-based imaging cassettes [7,11]. These CR systems are typically characterized by a wide dynamic range and a linear signal response [9,10]. The digital images are generated in a dedicated laser reader. As a result, the well-known image quality variability due to the developing procedure of X-ray films can be avoided and significant reductions of retake rates were reported with CR [3,12]. CR technology can easily be implemented in existing radiographic equipment, resulting in a relative low installation cost [2,3]. In addition, CR detector plates are portable and therefore a digital alternative for bedside radiography [2]. On the other hand, due to the cassette handling and transport to a cassette reader, only a marginal workflow improvement over a conventional screen-film unit can be achieved [2-4,7,8]. Different studies reported equivalent overall image quality performance of storage phosphor plates in chest radiography compared to conventional screen-film combinations [13-18]. However, the wide dynamic range of the CR imaging plates significantly improves the contrast resolution features of these systems [18]. This enables a simultaneous adequate representation of tissues with substantially different x-ray absorption characteristics. As a result CR systems are superior to the screen-film systems in depicting low-contrast regions such as mediastinal structures and lesions [16,18-19]. The latter results are confirmed by our investigations [20,21]. The limiting spatial resolution of CR systems, in contrast to screen-film radiography systems, does not influence the diagnostic performance. MacMahon et al. [22] showed that a pixel size of 200µm is sufficient for the detection of necessary details using computed radiography systems. Other studies compared the performance of a 2K versus 4K matrix size CR system [23,24]. No significant difference could be proven. With respect to the radiation dose needed for accurate CR imaging, conflicting results were published. Some studies reported that a higher radiation dose is needed to achieve a similar contrast resolution in CR chest imaging as in screen-film radiography [25,26], whereas others do not find such a dose increase [16,18,27-29]. Our studies support the latter group, as we found a slight but not significant dose reduction in CR chest imaging, compared to the state-of-the-art screen-film technique [20,21]. In general skeletal radiography, equivalent image quality can be obtained with the same radiation dose as used in conventional radiography [30-32]. However, for the specific detection of small bone lesions or subtle rib 94 fractures, a conventional screen-film system outperforms CR using a similar dose level [33,34]. Selenium drum radiography In 1992, the first direct-readout digital detector specifically designed for chest radiography became available (Thoravision, Philips Medical Systems) [16,35]. The latter detector used an amorphous selenium-coated drum as a radiographic detector which directly converts x-rays into a charge pattern [2]. Hence no light conversion is needed to achieve an image. The detector system was characterized by a high DQE as compared to conventional screen-film and CR systems [2,35,36]. Different studies showed an excellent image quality in chest imaging. The amorphous selenium drum detector outperformed both screen-film and CR systems with respect to visualization of anatomic structures [37-39], detection of simulated lesions in an anthropomorphic phantom [16,35,40] and detection of lung nodules in patients [41,42]. Moreover, significant dose reductions of 50% were reported compared to screen-film and CR acquisitions without being detrimental for quality [16]. Despite the fact that a selenium drum detector was a dedicated chest unit, studies were performed dealing with the detection of subtle bone lesions and fissures [30]. The digital selenium detector performed equally well compared to screen-film and CR systems, while being operated at half the detector dose [30]. As flat-panel detectors were commercially introduced, selenium drum systems were no longer constructed. CCD-based radiography A few years after the introduction of selenium drum systems, large-area detectors using CCDs became available. As CCDs are physically small, CCD-based radiographic systems must include some means of optical coupling to reduce the size of the projected visible light image and transfer the image to the face of one or more CCDs [2,7,8,43]. Lens optical coupling in CCD radiography substantially reduces the number of photons that reach the CCD, resulting in only modest DQE-values [2,44,45] compared to the newest flat-panel detectors. In clinical practice, lens-coupled CCD detectors show better chest lesion detection performance at lower patient dose as compared to CR systems [35]. The last years, digital CCD detectors are gaining more importance due to the introduction of slot-scan designs [35,45-47]. In the slot-scan CCD 95 systems, the rather low DQE performance of the CCD is compensated by a significant reduction of scatter radiation within the system [35]. Hence the effective DQE of the complete system results in high scores [35,45-47]. In chest imaging, slot-scan CCD devices perform at least as well as stateof-the-art conventional screen-film systems [45,46]. In recent studies, significantly better performance of a slot-scan CCD system was shown in chest radiography compared to CR [35,48] and lens-coupled CCDs [35]. However, the latter operated at a slightly lower dose. TFT-based flat-panel radiography A few years ago, direct-readout radiography systems, based on active matrix thin-film transistor technology became commercially available. These systems have a higher DQE compared to both CR and screen-film systems [2,8,43]. As other direct-readout detectors, they have the additional advantage of an optimized working procedure due to the instant image display and the elimination of the use of image cassettes [2,4,8,43,49]. Unfortunately, the latter detectors are rather expensive. Hence, a high patient volume is needed to be cost effective over CR [49]. Depending on the detector type, digital signals are generated either directly, using amorphous selenium, or indirectly, using a scintillator (unstructured Gd2O2S or needle-structured CsI) and an amorphous silicon photodiode [2,8,43]. A large amount of data is available with respect to the dose and image quality performance of these flat-panel detectors. Most of them, however, are performed with a CsI/a-Si detector type and focus on comparisons with conventional screen-film systems and CR. In thoracic radiography, we found a significantly superior quality of flatpanel detector radiographs in four anatomic regions (the medial border of the scapulae, the peripheral vessels, the trachea and proximal bronchi, and the spine) compared with that of computed radiographs and filmscreen radiographs [20]. In addition, the visualization of the retrocardiac lung and mediastinum was significantly better with the flat-panel detector system compared with that of the film-screen system [20]. For the effective dose, we found dose reduction factors of 2.7 and 1.7 when comparing flat-panel chest acquisitions with film-screen radiography and CR respectively [20]. Comparable results were obtained in other studies. In a CT-controlled clinical study, Garmer et al. concluded that the diagnostic performance of the a-Si flat-panel detector radiography system was equivalent or superior to that of the film-screen radiography system [10] . In a phantom 96 study of Strotzer et al., micronodular-simulated chest lesions were significantly better visualized on the a-Si flat-panel detector radiography system than on conventional film-screen radiography, despite the low dose used for the a-Si flat-panel acquisitions [50]. Fink et al. illustrated an improvement in the visibility of various anatomic structures on flat-panel detector chest radiographs compared with conventional film-screen radiographs, even though a dose reduction of 50% was applied using the a-Si detector [51]. In a subjective clinical study of Ganten et al., a-Si chest radiographs were better rated in comparison with screen-film or CR chest images [18]. Goo et al. found that the a-Si system was superior to a CR system for the detection of pulmonary nodules [52]. The excellent performance of CsI/a-Si flat-panel detectors can be attributed to their high DQE [53-58]. Hence a superior low-contrast behavior of the CsI/a-Si detector can be expected [21,53,57]. These findings are consistent with the CDRAD 2.0 studies performed in this work, where a significantly better contrast-detail detectability was proven in CsI/a-Si radiographs compared to that of conventional screen-film radiographs and CR images, despite the low acquisition dose used for the a-Si system [20,21]. Similar results were found in other contrast detail studies [9,53,57,59,60-63]. Based on these phantom studies, some authors claim that additional dose reductions would be possible when using a CsI/a-Si detector [53,57,61-63]. Clinical studies confirmed the latter finding: an extra dose reduction of 50% and more did not affect diagnostic value of the chest images [64-67]. Therefore, CsI/a-Si flat-panel detector systems are particularly interesting in pediatric radiographic imaging as children are more sensitive to radiation in comparison to an adult population [68]. Amorphous silicon flat-panel detector systems have also proven their value in skeletal and abdominal radiography. In observer preference studies, the a-Si flat-panel detector performed at least as good as compared to conventional screen-film and computed radiography for the visualization of anatomic skeletal regions [69-71], abdominal regions [71] and for the detection of subtle wrist fractures [72]. Different studies assessed the potential for dose reduction when using a CsI/a-Si detector in skeletal radiography. In a ROC analysis, Ludwig et al. showed that dose reductions up to 50% relative to a high-speed screen-film and phosphor-based radiography, are not detrimental for an accurate detection of low-contrast bone lesions [33] or subtle rib fractures [34]. Similar findings were reported by Strotzer et al. for the detection of simulated arthritic lesions [73]. A few studies reported even dose reductions up to 75%, without significant detriment for the image quality 97 [74-76]. The latter, is of particular importance for applications in children. Ludwig et al proved in a pediatric model that a 75% dose reduction is feasible for lumbar spine radiography [31]. With respect to direct-conversion flat-panel detectors based on amorphous selenium, only few data are available. In studies of Goo et al. and Kim et al., chest radiographs obtained with an a-Se flat-panel detector were rated equally compared to storage phosphor-based radiography [1,77]. However no dose reductions were reported using this flat-panel detector. In contrast to a-Si flat-panel detector systems, significant memory artifacts were reported in a-Se flat panel radiography [55,78]. In Figure 4.1, a typical memory artifact is illustrated. Hereby, part of a previously acquired lateral chest radiograph (left) is clearly depicted on the posteroanterior acquisition of the next patient (right), taken approximately 1 minute after the lateral view. Figure 4.1: Memory artefact in a-Se flat-panel radiography [78] Only few studies were performed to compare the image quality generated by different types of digital direct-readout detector. We compared a CsI/a-Si and an a-Se flat panel detector in chest radiography. Both systems provided an excellent image quality as showed in the depiction of anatomic structures on patient chest images. However, the aSe flat-panel detector needed a significantly higher dose level compared to the CsI/a-Si system [79]. The corresponding contrast-detail study revealed similar conclusions. For lower radiation dose settings, the CsI/aSi detector significantly outperformed the a-Se system with respect to low-contrast detectability, whereas for higher doses no differences could be shown [79]. The latter results can be compared with those from the study of Fischbach et al. [80] who found a better low-contrast performance in an a-Si system compared to an a-Se drum system for low dose settings. For higher doses no differences were found. 98 These results can be related to the higher DQE at lower spatial frequencies (< 2.5 mm-1) of a CsI/a-Si system compared to an a-Se detector [81,82], which is further linked with the absorption efficiency of the different detector materials. Two recent studies, taking into account different direct-readout detector systems, further confirm our results [35,48]. In the latter experiments, the CsI/a-Si systems provided the best performance at the lowest dose, whereas direct-conversion systems must be used at higher radiation dose levels to obtain the same image quality. 4.2 Image quality analysis in digital radiography When new technology is introduced in clinical practice, care has to be taken that these systems provide at least a similar image quality compared to conventional radiography systems and that images can be acquired at an acceptable dose level to the patient [83,84]. Moreover, it is important to compare these new digital detector systems as their performance may considerably differ [35,48,79,81,82]. Unfortunately, image quality analysis is not as straightforward as is the case with dose measurements. In order to achieve an acceptable image quality level, local signal-to-noise ratios should be sufficiently high in order to be able to detect image details within the background noise [8]. Because the latter SNR is proportional to the square root of the amount of X-ray quanta reaching the digital detector, the radiation dose will play an important role in the discussion of the image quality. In addition, the SNR will be lower in highly attenuating anatomic regions. Hence, the SNR values in the latter regions will be determinative for the minimum dose that can be applied in a specific radiography examination. Normally, when new detector systems are introduced, basic objective image quality characteristics (such as MTF, NPS, DQE) are measured. These parameters provide information about the fundamentals of the detector technology. However, they are not always simple to measure in clinical practice and a direct link with diagnostic image quality is rather difficult to make [81,83]. With respect to image quality, MTF is regarded as a rather inaccurate parameter as MTF values are largely dependent on post-processing parameters [43,85]. Moreover, MTF does not take into account the signalto-noise ratio. As a result, the detection of small low-contrast objects is determined by the limitations of the DQE, rather than by the MTF 99 characteristics [53]. Therefore, DQE can be considered as the best overall performance parameter of the acquisition system [43]. The DQE combines spatial resolution and image noise to provide a measure of the signal-to-noise ratio of all frequency components of the image [43]. Hence, a higher quantum detection efficiency provides improved capability to reveal an object in a noisy background [55], in addition to the possibility of reducing the patient radiation dose with no loss of diagnostic information. Measurement of DQE is difficult in clinical practice, as (unprocessed) raw data are not always available [81,82]. Moreover, Neitzel et al. showed that depending on the measurement methodology used, DQE-values could differ up to 15% [86]. The latter differences were attributed to inaccuracies in the MTF calculation [86]. Recently an IEC standard (IEC 62220-1) was published with respect to the measurement of the DQE [87]. The latter standard will possibly contribute to an objective measure of DQE-values of digital x-ray detector systems[88]. However, problems may occur when using high purity aluminum for the determination of the DQE, as imposed by the IEC standard [89]. In general, a large variety in measurement methodologies exists in literature for the calculation of MTF, NPS and DQE. Due to the sensitivity of the latter parameters on the applied methodology, a direct comparison of the published results of different research groups is difficult [82]. Moreover, the actual clinical performance of the detector depends on many other facts other than DQE, including the operating exposure ranges for the acquisition of clinical images, detector sensitivity to scattered radiation, the use of antiscattergrids, and image processing [82]. In addition, previous discussed parameters do not include the observer in the process of medical image quality analysis notwithstanding the important influence of psychophysical factors related with the observer in the imaging chain [61,90]. With the use of a contrast-detail phantom, the just-visible contrasts and details in the phantom image is measured in a semi objective way [59,9193]. This is particularly relevant as the detection of subtle low-contrast details is considered as one of the most important aspects in medical imaging [92]. To avoid a potential bias in the phantom image reading due to a priori knowledge of the presence of a low-contrast object [9], the CDRAD 2.0 and CDMAM 3.4 phantoms were developed. In the latter phantoms a four-alternative forced-choice experiment is set up, resulting in a more objective image quality measure compared to other contrastdetail phantoms [59,91,92]. 100 The contrast-detail methodology allows an image quality assessment of the complete digital imaging chain, including the image display and the observer [59,92]. Differences in image quality between imaging systems or between different acquisition settings can be visualized easily, even when the differences are small [59]. As observers are included in the analysis, multiple readers are required to obtain reliable results. However, rather large inter-observer variations of reading results are reported in contrast-detail analysis [9,59]. The latter variations may complicate the use of a contrast-detail analysis within a routine constancy check of a quality control program. On the other hand, despite possible large inter-observer variations, evident trends in quantified contrastdetail performance were observed in our study when changing basic exposure parameters (kV, filtration, mass) [21]. Different groups studied the feasibility of automatic computer-based assessment of low-contrast details in phantoms [81,94,95]. Preliminary results for the CDRAD 2.0 phantom show that computer reading can reject the inter-observer variability normally seen in manual contrastdetail analysis, resulting in the possibility of performing an accurate and objective image quality quantification [94]. The computer programs can be adjusted to produce results comparable to those achieved by human observers [94]. Moreover, computerized contrast-detail reading significantly reduces the workload needed for a complete analysis. In fact, manual contrast-detail reading can be very time-consuming and it is therefore not feasible to implement manual reading in routine quality control. On the other hand, a computer analysis does not allow for the quality of the image presentation (quality of hard-copies, quality of the monitor system used). Consistent results were obtained when comparing a contrast-detail analysis with MTF, DQE and NPS measurements [81]. In our study, clinical image quality data comparing a-Si and a-Se chest radiographs, corresponded very well with the extensive CDRAD 2.0 contrast-detail study that was performed on both flat-panel systems [79]. The latter findings indicate that the use of contrast-detail phantoms is warranted for accurate image quality analysis [79,83]. A general point of concern with respect to phantom image quality analysis is related to the image processing of the digital images. In fact, most manufacturers are using different types of (post)processing algorithms. The latter software tools are especially designed for patient image processing and not for (contrast-detail) phantom images. Therefore, comparisons of different digital systems could be performed on raw data. Unfortunately, raw data is not always easily accessible. 101 Moreover, there is no general standard with respect to the definition of raw data. Each manufacturer will apply own device-specific ‘corrections’ to obtain a raw image data file (image conditioning) [96]. However, it is not always clear which/whether specific processing algorithms are included in the creation of these raw datasets. As a result, some systems could provide better image quality scorings only related to the fact that more ‘corrections’ were applied on the raw data. In our studies, the standard processing for chest imaging was used as advised by each of the manufacturers. No additional post-processing (e.g. extra edge enhancement,…) was performed. We preferred to use the standard processed digital data in our studies for several reasons. First of all, when comparing a screen-film system with a digital acquisition system, one has to take into account that the film images are completely processed, i.e. the phantom images are processed/presented in the same way as for patient radiographs. Our initial experiments with digital CDRAD-data showed that the image quality scores based on raw data resulted in lower scorings when compared with processed data. Therefore, it would not be ‘fair’ to compare only raw datasets with the processed film images. Secondly, as mentioned earlier, well-defined raw datasets were not always available. In addition, it is worthwhile to mention that in a lot of the published contrast-detail studies processed images were used [9,28,53,57,60,61]. Most of the other studies do not mention which type of data set they were using. Despite the value of phantom images, the most realistic approach to clinical image quality, however, is the analysis of patient images. In our studies (as in most other image quality studies) we opt for quality analysis on chest radiographs [20,79]. First of all, chest radiographs are the most frequently obtained images in diagnostic radiology and are often obtained repeatedly for the follow-up of patients [10,101]. Moreover, the chest radiography technique is highly demanding because a wide variety of tissue densities must be taken into account [10,46]. Therefore, this technique is particularly relevant for testing the image quality capability of radiography systems. Most of the published studies are based on visual grading techniques, in which the observer must compare an image with a reference acquisition. However, this methodology requires two ore more exposures of the same patient when comparing two or more acquisition systems. Similarly, in ROC patient studies a reference dataset is needed to obtain the knowledge of the true presence and locations of lesions [8]. As the benefit of extra exposures is only marginal for the patient, the latter approach is not compatible with good clinical practice [97]. 102 To facilitate visual grading and ROC studies and to avoid unnecessary extra patient exposures, anthropomorphic phantoms are used. In fact, these phantoms mimic the human anatomy very accurately and different structures (low and high-contrast objects) can be superimposed over the phantom on well-defined positions, simulating different pathologies [45,50,65,66]. In our studies, clinical image quality was based on the predefined image quality criteria of the Commission of the European Communities [20,79,98]. In these guidelines, the visibility of different characteristic features of imaged anatomical structures must be scored subjectively. Compared to ROC studies, studies based on image quality criteria are much easier to set up and are less time-consuming. Previous studies showed that the latter criteria were accurate for image quality assessments [99]. Moreover, no additional exposures are needed as comparisons are made on populations, rather than on the individual. Some of the quality criteria, however, could be revised in the future. In fact, different parameters are only related to patient positioning and are therefore not reflecting the quality of the (digital) acquisition system. Some other criteria associated with image details of a specific predefined size, are very difficult to assess objectively. Specific requirements for digital radiographic procedures are currently under development [96,100]. General image quality criteria are easy to use and require only a limited amount of time. However, objective quantification of the image quality is not possible as the clinical evaluations are performed on a subjective basis. Therefore, (objective) phantom images should be preferred in quality audits. 4.3 Patient dosimetry and dose optimization Today, patient dosimetry is an integral part of a quality assurance program [102-105]. The determination of reference levels for a radiological examination is regarded as an important tool for dose minimization and procedure optimization and is imposed by the European Union council directive 97/43/Euratom [96,102,104-106]. The latter investigations are particularly relevant in the digital setting as an overexposure can occur without an adverse effect on the image quality, in contrast with the conventional screen-film systems [96,105,107]. Hence inadequate technique can result in significant increase of dose when using digital detectors [96,105,107]. Moreover, different studies report an 103 increase in number of acquired images because of the relative ease of obtaining and archiving the images with digital systems [5,96,108]. Unfortunately, digital images can be easily deleted before being archived in the PACS, making accurate audits of exact numbers of retakes rather difficult [96]. Previous arguments stress the need for an accurate dose management in digital radiography. At commissioning of new digital systems, the imaging capabilities of the system should be assessed and compared with the radiation dose needed to obtain a specific image quality level [84,96]. In fact, by applying the same exposure settings as in a previous conventional system, dose levels would not be optimized and are, as a result, in most cases too high. Test objects, such as contrast-detail phantoms are particularly relevant for the latter task [96]. Recent studies revealed that the techniques that are conventionally assumed to be optimum, might be revised for digital radiography [100]. For chest imaging, for example, some authors suggest to use lower kVp-values (90 kVp) while maintaining the patient dose level constant [109,111]. However, other groups suggest to use a highly filtered (0.2mm Cu) x-ray beam at the usual kVp-values (120 kVp), while reducing the patient radiation exposure with 25% [112]. Digital radiography systems, especially flat-panel detectors based on CsI/a-Si, have a large potential for dose reduction in clinical practice. The latter dose reduction should be in agreement with an acceptable image quality needed for the specific clinical application, rather than aiming the best possible image quality [96]. In a study of Perslinden et al., a doseimage quality analysis was performed for pelvic examinations using a CsI/a-Si detector [76]. Radiologists analyzed the noise level that was acceptable for accurate diagnosis. After the latter optimization procedure, the effective dose for pelvic examinations could be reduced with 50% (from 0.12 mSv to 0.06 mSv) without being detrimental for image quality (Figure 4.2) [76]. 104 A B C Figure 4.2: Digital x-ray radiographs of a phantom pelvis acquired with a standard entrance surface dose of 0.48 mGy (A), an optimized entrance surface dose of 0.24 mGy (B) and lowdose setting of 0.05 mGy. Radiographs (A) and (B) were rated as equivalent. Acquisition (C) was insufficient for diagnosis due to the higher noise level [76]. In Figure 4.3 a detail of a DR chest radiograph is presented, demonstrating a large opacification and a linear atelectatic area. After a dose reduction up to 75%, the discrimination of structures remains perfectly possible [2]. The latter dose reduction in flat-panel chest radiography is in agreement with our contrast-detail study where similar dose reductions on a CsI/a-Si flat-panel system resulted in an equivalent image quality as compared to a state-of-the-art screen-film system [21]. Figure 4.3: Detail of a flat-panel chest radiograph illustrating a large opacification and a linear atelectatic area. Image were acquired with a dose comparable to a film speed of 200 (A), 400 (B) and 800 (C). Even with the highest dose reduction, structures are still well visible [2]. Image quality must always be taken into account when trying to achieve dose reductions. In Figure 4.4, a dose reduction of 50% in a CR chest radiograph resulted in a significantly inferior detection of catheters [2]. 105 Therefore, the latter dose reduction should not be implemented in clinical practice. Figure 4.4: Zoomed view of the upper mediastinum of a storage phosphor image obtained with a standard dose (A) and with a half-dose protocol (B). The central venous catheter (arrow) is no longer visible in B due to increased image noise [2]. Recently, attempts are being made for the integration of central dose management systems into the PACS/RIS/HIS environment [105,113,114]. In the latter systems, dose indicators should be presented by default on the soft-copy image to give the observer the ability to assess the patient dose that was used for the acquisition [115]. The dose information that is included within these systems will help the medical physics expert to facilitate the optimization process in the radiology department [113]. In direct digital radiography applications, for example, dose surveys can be easily performed as most relevant parameters for dose calculations (tube current, exposure time, tube voltage, source-detector distance, dose area product,…) are available in the DICOM header of the image [113,114]. As a result, local diagnostic reference levels can directly be calculated using data on the PACS/RIS/HIS. Unfortunately, the latter information is mostly not available in computed radiography acquisitions as the image cassette normally does not have a connection with the x-ray generator. Nevertheless, exposure indicators measuring the detector dose (e.g. “exposure index”, “sensitivity index”,…) may be useful in computed radiography for optimization purposes [116] despite they are not directly related to patient entrance doses [105]. 4.4 Digital image display As image acquisition and image presentation are separated in digital radiography applications, both parameters (acquisition and display) can be optimized independently [2,3]. When making hard-copy prints of digital images for conventional analysis on lightboxes, most advantages 106 of the digital environment are lost [8]. In fact, soft-copy reading improves the efficiency in a medical imaging department using a PACS [2,4]. Moreover, soft-copy reading on monitor systems permit to zoom in on specific structures of interest and to interactively adjust contrast and brightness levels [2,3,8]. With this functionality the observer is using optimally the wide dynamic range of the digital acquisition systems. The latter has been shown in our study, where soft-copy contrast-detail images (assessed at full resolution) were significantly better rated compared to the hard-copy versions when level and window setting were adjusted by the observer [21]. Similar results were obtained in a clinical study of Kim et al. where soft-copy reading improved the diagnostic accuracy for the detection of urinary calculi compared to hard-copy interpretation [117]. On the other hand, the 75% hard-copy prints of our study were equally rated in comparison with soft-copy readings without any manipulation. It should be noted that in the latter case, the soft-copy images were presented at about 70% and 77% of the original size for the FD and CR images respectively. In soft-copy reading, the interactive adjustment of the brightness and contrast should be recommended as this resulted in a significantly better contrast-detail performance [118]. This was confirmed by the study of Fuchsjäger et al, where adjustment of the window/level setting had a significant influence on the detectability of catheters in chest radiographies [119]. In our study we demonstrated that the latter interactive adjustments did not affect the throughput [118]. High-resolution grey-scale CRT monitors are widely accepted for primary image interpretation [2,119-125]. Due to their typical disadvantages such as a non-isotropic MTF, a large vulnerability to reflections and ambient light and peripheral distortion artifacts, CRT monitors are currently being replaced by LCD devices [119-126]. The latter monitor type is much more compact and is characterized by an excellent MTF, higher luminance and a nearly elimination of veiling glare and peripheral distortions [119124,126,127]. As a large variety of resolutions are commercially available in LCD devices, we assessed the influence of the LCD matrix resolution on the contrast-detail performance in comparison with a state-of-the art 5megapixel greyscale CRT monitor [118]. Subtle low-contrast details were achieved with a CDRAD 2.0 and a CDMAM 3.4 phantom, simulating digital radiography and digital mammography acquisitions respectively. In general, our study showed a significant influence of the LCD matrix resolution on the contrast-detail detectability. Magnification to full resolution significantly improved the image quality, but only for the 107 lower resolution LCD devices [118]. Depending on the application (digital radiography or digital mammography), different conclusions were obtained with respect to the LCD matrix resolution needed. For digital mammography, 1- and 2-megapixel LCDs scored significantly inferior compared to a 5-megapixel CRT even when using the magnification function [118]. After magnification, the CDMAM 3.4 contrast-detail scores of a 3-megapixel LCD device were equivalent to those of a 5-megapixel CRT monitor. However, the latter operation is unlikely to be applicable into clinical practice as the magnification operation significantly affects the throughput [118]. Up to date, no clinical data are available to confirm these findings. In the digital radiography setting, a 1-megapixel LCD performed significantly worse compared to a 5-megapixel CRT, despite the use of magnification [118]. The latter is in agreement with Peer et al, where a contrast-detail analysis showed a significantly lower performance of a 1megapixel CRT monitor in comparison with a 5-megapixel CRT [92]. Otto et al. reported a significantly better diagnostic performance on a 5megapixel CRT, compared to a 1-megapixel CRT monitor [128]. In a ROCstudy for detection of pulmonary nodules, Graf et al. found an overall better performance of a 5-megapixel CRT monitor in comparison with a 1megapixel CRT device where magnification was used [129]. In digital radiography, the use of a 2-megapixel LCD is warranted when images are scored with the magnification function [118]. However, the latter significantly affects the throughput [118]. Our contrast-detail study also confirms the previously obtained clinical results where the diagnostic equivalence of a 3-megapixel LCD device and a 5-megapixel CRT monitor was shown [120,121,123,127,130,131]. Similarly, Averbukh et al. demonstrated the equivalence between a 3- and 5-megapixel LCD device [132]. However, LCD devices have also some disadvantages. First of all, LCDs have a rather high spatial noise caused by LCD pixel luminance differences [133,134]. This may cause interference with the detection of fine details and subtle abnormalities in clinical images such as chest nodules, hairline fractures in bones or micro-calcifications in mammograms [133]. In present work a new LCD noise reduction function was evaluated (PPU - Per Pixel Uniformity [134]). Our phantom study showed an improved contrast-detail detectability when using PPU [118]. However, this was only significant in the mammography image analysis. Clinical comparative studies should be performed to confirm the latter finding. Another problem with LCD devices is the strong angular dependence of luminance characteristics [122,135]. As a result, a 108 significant reduction in image contrast can be observed depending on the direction wherein the images are viewed. Therefore, off-axis viewing of radiographs should be avoided when using an LCD [122]. In soft-copy reading, special attention has to be paid on the accurate calibration of the display devices. In fact, displays should be calibrated using the DICOM greyscale display function (DGDF) Part 3.14 [136,137] to obtain a reproducible contrast impression, independent of the monitor where the digital images are viewed on. The maximum luminance level should be high enough, otherwise a significant reduction in low-contrast detectability is observed [124,125,138]. As luminance levels are subject to change in function of time in both LCD and CRT devices, they should be carefully and strictly monitored and re-calibrated when necessary [137,139]. Accurate procedures proposed by the American Association of Medical Physicists (AAPM) exist for this purpose [137]. Finally, in soft-copy reading, good ambient conditions are essential for accurate quality. In fact, low-contrast performance of display devices is significantly affected by the ambient illuminance and light reflections. The influence of ambient light has been proven for the detection of catheters in chest radiographs [119,120] and in subjective observer studies in digital chest radiography [124,125,140]. Therefore, soft-copy reading rooms should be properly designed in order to avoid such problems. 4.5 Future Prospects 4.5.1 Evolution of digital technology Digital X-ray projection imaging systems are continuously evolving and new technologies are being introduced with respect to detector technology, image processing, image storage and image display. Some authors claim that CR technology will be replaced by direct-readout systems within the next 10 years [3]. However, recently introduced innovations have improved the performance of CR systems significantly, rendering it competitive with direct-readout digital radiography [141,142]. The latter improvements are achieved by the use of new storage phosphors and/or by applying more efficient high quality readout mechanisms. With respect to phosphor plate technology, more efficient phosphors such as BaFI:Eu [143] and CsBr:Eu [144] are being used. The latter phosphor 109 has the additional advantage that it can be produced in a needlestructure, resulting in a dose-image quality performance that is comparable with that of a flat-panel detector [144,145]. Some CR cassettes use a multiple-screen assembly in which two or more CR screens are combined in the cassette and are exposed together and separately read out. Afterwards both images are recombined into a single digital image with an improved SNR [2,141,146]. CR readout technology is also being optimized. In dual-side readout systems both sides of the photostimulable phosphor screen are read out simultaneously by incorporating two light-guide assemblies in the reader [141]. As a result, a greater fraction of the light signal emitted by the phosphor will be captured. First clinical studies document an excellent image quality with dose reductions up to 50% [147,148]. Some readout systems use a line scanning mechanism in which a row of multiple laser diodes is coupled with high-capacity CCDs to capture whole lines of light emission data from the phosphor screen instead of scanning the plate point-by-point [2,141,149]. Other systems are integrating the readout in a cassette unit, thereby removing the manual handling of imaging plates. As a result of the previous mentioned innovations, CR will continue to play an important role in projection radiography and will probably coexist for many years with direct-readout radiography systems [2,141,142]. Whereas CR technology is evolving to a performance of a direct-readout radiography system, the latter technology is now available in a portable format [150]. In fact, one of the disadvantages for direct-readout systems was the lack of portability. First evaluations of portable flat-panel detectors based on amorphous silicon indicated an improved image quality at lower dose levels [151,152]. Hence, the latter systems are particularly relevant for radiography of neonates and children [151,152]. Unfortunately, flat-panel detectors are rather fragile as TFT arrays are implemented on a glass substrate. New more flexible substrates are under development and should overcome this problem [153]. With respect to direct x-ray conversion flat-panel technology, other materials such as HgI2, CdTe and PbI2 are being studied for using in flatpanel detectors [8]. Finally, experience from experimental high-energy physics has prompted the consideration of silicon strip detectors and new types of detectors based on gas amplification in x-ray imaging applications [153]. The latter detectors, both photon counters, show promising results with respect to dose reduction in combination with high spatial resolution [153]. First applications of both conversion 110 technologies become mammography. now available for application in digital As illustrated above, new technologies are emerging for digital image acquisition. Information on system performance with respect to image quality and patient radiation dose of these systems is very limited up to date and these characteristics should therefore be thoroughly investigated before being widely implemented in clinical practice. Other evolutions, such as the use of CAD software to assist the radiologist in making a (better) diagnosis, are now under investigation. In addition, digital flatpanel systems are now increasingly implemented in other medical fields using x-ray projection imaging such as interventional cardiology and radiology. Studies should investigate the effect of these evolutions on patient care in general and more specifically on the patient dose. 4.5.2 Challenges for the medical physics expert As imposed by the European Union council directive 97/43/Euratom, the medical physics expert should play an important role in a department of medical imaging [96,102,104-106]. His main tasks are related to the assessment of the X-ray system quality (and constancy), the audit and calculation of patient doses and the procedure optimization. Especially in a digital imaging environment, optimization with respect to both image quality and patient radiation dose is particularly relevant [96,105,107]. Therefore, a routine image quality assessment should be implemented in quality audits. A CDRAD contrast-detail study is a valuable tool for the latter image quality analysis in (digital) radiography. However, an automated image scoring is an absolute requirement to manage the inter-observer variability and to reduce the workload. Software tools are currently being investigated for this purpose [154]. However, up to now, it is not clear whether these tools can provide accurate and reproducible results. Moreover, the correlation between phantom analysis and clinical information should be taken into account. Contrast-detail studies are not only restricted to applications in radiography. Similar phantoms exist for image quality management in mammography (e.g. CDMAM), for testing image intensifiers (e.g. CDDISC) and CT systems (e.g. Catphan phantom series). Recently, a dedicated contrast-detail phantom became available for digital intra-oral radiography (CDDENT). 111 If the contrast-detail methodology would be applied in routine practice, reference contrast-detail curves should be available. The latter reference curves can be derived from a multi-centre overview of the contrast-detail performance of a large number of radiography systems throughout Belgium. Recently, this project has been started at the Ghent University. Similar work has been done for the image quality of mammography installations, using a CDMAM phantom (Euref-project). It is worthwhile to mention that reference contrast-detail curves could also be a tool for an overall test of the monitor performance, including the reading conditions (ambient light,…). Due to the recent standardization in the measurement and calculation of the DQE, the latter quantity could provide an alternative approach for image quality analysis [87]. However, DQE normally does not include other parameters, such as the use of an anti-scatter grid. Therefore, a ‘system DQE’ could be used, taking into account these factors. Future experimental studies should reveal whether this methodology can be used in practice. The digital image processing has an important influence on the image quality. However, up to now, no methodologies are available for an objective check of the influence of the latter software tools. Further research is needed to investigate the importance of (post)processing within a contrast-detail set-up. It is obvious that in the digital setting the tasks of a medical physics expert will be much more complex, especially by introducing the image quality concept into routine quality audits. Different questions remain to be answered. However, this is an interesting challenge and the results will be beneficial for the patients. 4.6 Conclusions Present work has shown an excellent image quality for current digital chest radiography systems. However, not all digital detector types provide the possibility for significant patient dose reduction, without being detrimental for image quality. With an amorphous selenium flatpanel detector, for example, similar dose levels as measured in state-ofthe-art screen-film chest radiography must be applied. Similar conclusions were found for storage phosphor plate chest imaging. On the other hand, when using a flat-panel detector based on cesium iodide and amorphous silicon, dose reductions of more than 60% are feasible. 112 Image quality analysis based on patient images correlated very well with the more objective and less time-consuming contrast-detail analysis. Hence the latter methodology may be an interesting tool that can be implemented in a quality assurance program in digital radiography, especially when an automated phantom analysis can be performed. Moreover, contrast-detail phantoms can be used for the image quality assessment and optimization of the whole (digital) imaging chain, including image acquisition and display. With respect to digital image display, it was shown that soft-copy reading can significantly improve the contrast-detail detectability compared to hard-copy presentation. However, when using monitors with a lower matrix resolution (e.g. 2 megapixel), digital images should be magnified to full resolution. As the latter adjustment will cause a significant reduction of the throughput, monitors with a resolution of 3 megapixel or higher are recommended for the application in digital chest radiography. The contrast-detail analysis with the dedicated CDMAM 3.4 phantom revealed that for digital mammography applications, a 5-megapixel monitor should be the first choice. Interactive adjustment of brightness and contrast of digital images significantly improved the image quality in soft-copy reading without affecting the throughput. Therefore, the use of this functionality should be strongly recommended, especially for applications were subtle lowcontrast details must be visualized. Finally, medical grade high-resolution LCD devices provide excellent contrast-detail detectability, at least comparable with state-of-the-art greyscale CRT monitors. 4.7 References 1. Goo JM, Im JG, Kim JH, et al. Digital chest radiography with a selenium-based flat-panel detector versus a storage phosphor system: comparison of soft-copy images. AJR 2000; 175:1013-1018 2. Schaefer-Prokop C, Uffmann M, Eisenhuber E, Prokop M. Digital radiography of the chest: detector techniques and performance parameters. Journal of Thoracic Imaging 2003; 18:124-137 3. Verschakelen J, Bellon E, Deprez T. Digital chest radiography: quality assurance. Journal of Thoracic Imaging 2003; 18:169-177 113 4. May GA, Deer DD, Dackiewietz D et al. Impact of digital radiography on clinical workflow. J Digit Imaging 2000;13 (suppl):76-78 5. Reiner BI, Siegel EL, Flagle C, Hooper FJ, Cox RE, Scanlon M. Effect of filmless imaging on the utilization of radiologic services. Radiology 2000; 215:163-167 6. United Nations Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation, vol 1. Sources, annex D. Medical radiation exposure. New York: United Nations, 2000, 355 7. Reiff KJ. Flat panel detectors – closing the (digital) gap in chest and skeletal radiology. Eur J Radiol 1999; 31:125-131 8. Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. The essential physics of medical imaging, 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkens, 2002:145-173 9. Rong XJ, Shaw CC, Liu X, Lemacks MR, Thompson SK. Comparison of an amorphous silicon/cesium iodide flat-panel digital chest radiography system with screen/film and computed radiography systems – A contrast-detail phantom study. Med Phys 2000; 28: 2328-2335 10. Garmer M, Hennings SP, Jäger HJ, et al. Digital radiography versus conventional radiography in chest imaging: diagnostic performance of a large-area flat-panel detector in a clinical CTcontrolled study. AJR 2000; 174: 75-80 11. Sonoda M, Takano M, Miyahara J, Kato H. Computed radiography utilizing scanning laser stimulated luminescence. Radiology 1983; 148:833–838 12. Polumin N, Lim TA, Tan KP. Reduction in ratake rates and radiation dosage through computed radiography. Ann Acad Med Singapore 1998; 27:805-807 13. Busch HP, Lehmann KJ, Drescher P, Georgi M. New chest imaging techniques: a comparison of five analogue and digital methods. Eur Radiol 1992; 2:335-341 14. Slone RM, van Metter R, Senol E, Muka E, Pilgram TK. Effect of exposure variation on the clinical utility of chest radiographs. Radiology 1996; 199:497-504 114 15. Ishigaki T, Endo T, Ikeda M, et al. Subtle pulmonary disease: detection with computed radiography versus conventional chest radiography. Radiology 1996; 201:51-60 16. Bernhardt TM, Otto D, Reichel G, et al. Detection of simulated interstitial lung disease and catheters with selenium, storage phosphor, and film-based radiography. Radiology 1999; 213:445454 17. Niklason LT, Chan HP, Cacade PN, Chang CL, Chee PW, Mathews JF. Protable chest imaging: comparison of storage phosphor digital, asymmetric screen-film, and conventional screen-film systems. Radiology 1993; 186:387-393 18. Ganten M, Radeleff B, Kampschulte A, Daniels MD, Kauffmann GW, Hansmann J. Comparing image quality of flat-panel chest radiography with storage phosphor radiography and film-screen radiography. AJR 2003; 181:171-176 19. Schaefer CM, Greene R, Oestmann JW, et al.Digital storage phosphor imaging versus conventional film radiography in CTdocumented chest disease. Radiology 1990; 174:207–210 20. Bacher K, Smeets P, Bonnarens K, De Hauwere A, Verstraete K, Thierens H. Dose reduction in patients undergoing chest imaging: digital amorphous silicon flat-panel detector versus conventional film-screen radiography and phosphor-based computed radiography. AJR 2003; 181:923-929 21. De Hauwere A, Bacher K, Smeets P, Verstraete K, Thierens H. Analysis of image quality in digital chest imaging. Rad Prot Dosim, in press 22. MacMahon H, Vyborny CJ, Metz CE. Digital radiography of subtle pulmonary abnormalities: a ROC study of effect of pixel size on observer performance. Radiology 1986; 158:21–26 23. Miro SPM, Leung AN, Rubin GD, et al. Digital storage phosphor chest radiography: an ROC study of the effect of 2k versus 4k matrix size on observer performance. Radiology 2001; 218:527-532 24. Flynn MJ, Samei E. Experimental comparison of noise and resolution for 2k and 4k storage phosphor radiography system. Med Phys 1999; 26:1612-1623 115 25. Tylén U. Stimulable phosphor plates in chest radiology. Eur Radiol 1997; 73(Suppl): S83-S86 26. Dobbins JT, Rice JJ, Beam CA. Threshold perception performance with computed and screen-film radiography: implications for chest radiography. Radiology 1992; 183:179-187 27. Cook LT, Insana MF, McFadden MA, Hall TJ, Coc GG. Comparison of the low-contrast detectability of a screen-film screen system and third generation computed radiography. Med Phys 1994; 21:691-695 28. Launders JH, Cowen AR. A comparison of the threshold detail detectability of a screen-film combination and computed radiography under conditions relevant to high-kVp chest radiography. Phys Med Biol 1995; 40:1393-1398 29. Neitzel U, Bohm A, Maack I. Comparison of low-contrast detail detectability with five different conventional and digital radiographic imaging systems. Proc SPIE 2000; 3981:216-223 30. Zähringer M, Krug B, Kamm KF, et al. Detection of porcine bone lesions and fissures: comparing digital selenium, digital luminescence, and analog film-screen radiography. AJR 2001; 177:1397-1403 31. Ludwig K, Ahlers K, Wormanns, et al. Lumbar spine radiography: digital flat-panel detector versus screen-film and storage-phosphor systems in monkeys as a pediatric model. Radiology 2003; 229:140144 32. Jonsson A, Herrlin K, Jonsson K, Lundin B, Sanfridsson J, Pettersson H. Radiation dose reduction in computed skeletal radiography – effect on image quality. Acta Radiologica 1996; 37:128-133 33. Ludwig K, Lenzen H, Kamm, KF, et al. Performance of a flat-panel detector in detecting artificial bone lesions: comparison with conventional screen-film and storage-phosphor radiography. Radiology 2002; 222:453-459 34. Ludwig K, Schülke C, Diederich S, et al. Detection of subtle undisplaced rib fractures in a porcine model: radiation dose requirement – digital flat-panel versus screen-film and storagephosphor systems. Radiology 2003; 227:163-168 116 35. Kroft LJM, Veldkamp WJH, Mertens BJA, Boot MV, Geleijns J. Comparison of eight different digital radiography systems: variation in detection of simulated chest disease. AJR 2005; 185:339346 36. Neitzel U. Grids or air gaps for scatter reduction in digital radiography: a model calculation. Med Phys 1992; 19:475-481 37. Flyod CE, Baker JA, Chotas HG, Delong DM, Ravin CE. Seleniumbesed digital radiography of the chest: radiologists’ preference compared with film-screen radiographs. AJR 1995; 165:1353-1358 38. Biemans JMA, van Heesewijk JPM, van der Graaf Y. Selenium radiography versus storage phosphor imaging: comparison of visualization of specific anatomic regions of the chest. Invest Radiol 2002; 37:47-51 39. van Heesewijk HP, Neizel U, van der Graaf Y, de Valois JC, Feldberg MA. Digital chest imaging with a selenium detector: comparison with conventional radiography for visualization of specific anatomic regions in the chest. AJR 1995; 165:535-540 40. Schaefer-Prokop CM, Prokop M, Schmidt A, Neitzel U, Galanski M. Selenium radiography versus storage phosphor and conventional radiography in the detection of simulated chest lesions. Radiology 1996; 201:45-50 41. Awai K, Komi M, Hori S. Selenium-based digital radiography versus high-resolution storage phosphor radiography in the detection of solitary pulmonary nodules without calcification: receiver operating characteristic curve analysis. AJR 2001; 177:11411144 42. van Heesewijk HPM, van der Graaf Y, de Valois JC, Vos JA, Feldberg MAM. Chest imaging with a selenium detector versus conventional film radiography: a CT-controlled study. Radiology 1996; 200:687-690 43. Chotas HG, Dobbins JT, Ravin CE. Principles of digital radiography with large-area electronically readable detectors: a review of the basics. Radiology 1999; 210:595-599 44. Bath M, Sund P, Mansson LG. Evaluation of the imaging properties of two generations of a CCD-based system for digital radiography. Med Phys 2002; 29:2286-2297 117 45. Kroft LJM, Geleijns J, Mertens BJA, Veldkamp WJH, Zonderland HM, de Roos A. Digital slot-scan charge-coupled device radiography versus AMBER and screen-film radiography for detection of simulated nodules and interstitial disease in a chest phantom. Radiology 2004; 231:156-163 46. Veldkamp WJH, Kroft LJM, Mertens BJA, Geleijns J. Digital slotscan charge-coupled device radiography versus AMBER and bucky screen-film radiography: comparison of image quality in a phantom study. Radiology 2005; 235:857-866 47. Samei E, Saunders RS, Lo JY, et al. Fundamental imaging characteristics of a slot-scan digital chest radiographic system. Med Phys 2004; 31: 2687-2698 48. Pascoal A, Lawinski CP, Mackenzie A, Tabakov S, Lewis CA. Chest radiography: a comparison of image quality and effective dose using four digital systems. Rad Prot Dosim 2005; 114:273-277 49. Andriole KP, Luth DM, Gould RG. Workflow assessment of digital versus computed radiography and screen-film in the outpatient environment. J Digit Imaging 2002; 15:124-126 50. Strotzer M, Gmeinwieser J, Voelk M, Fruend R, Setz J, Feuerbach S. Detection of simulated chest lesions with normal and reduced radiation dose: comparison of conventional screen-film radiography and a flat-panel X-ray detector based on amorphous silicon. Invest Radiol 1998; 33:98–103 51. Fink C, Hallscheidt PJ, Noeldge G, et al. Clinical comparative study with a large-area amorphous silicon flat-panel detector: image quality and visibility of anatomic structures on chest radiography. AJR 2002; 178:481–486 52. Goo JM, Im JG, Lee HJ, et al. Detection of simulated chest lesions by using soft-copy reading: comparison of an amorphous silicon flatpanel-detector system and a storage-phosphor system. Radiology 2002; 224:242-246 53. Aufrichtig R, Xue P. Dose efficiency and low-contrast detectability of an amorphous silicon x-ray detector for digital radiography. Phys Med Biol 2000; 45:2653-2669 118 54. Spahn M, Strotzer M, Völk M, et al. Digital radiography with a large-area, amorphous-silicon, flat-panel x-ray detector system. Invest Radiol 2000; 35:260-266 55. Floyd CE, Warp RJ, Dobbins JT, et al. Imaging characteristics of an amorphous silicon flat-panel detector for digital chest radiography. Radiology 2001; 218:683–688 56. Granfors PR, Aufrichtig R. Performance of a 41x41-cm² amorphous silicon flat panel x-ray detector for radiographic imaging applications. Med Phys 2000; 27:1324-1331 57. Aufrichtig R. Comparison of low-contrast detectability between a digital amorphous silicon and a screen-film–based imaging system for thoracic radiography. Med Phys 1999; 26:1349–1358 58. Liu X, Shaw CC. A-Si:H/CsI(Tl) flat-panel versus computed radiography for chest imaging applications: image quality metrics measurement. Med Phys 2004; 31:98-110 59. Geijer H, Beckman KW, Andersson T, Perslinden J. Image quality vs radiation dose for a flat-panel amorphous silicon detector: a phantom study. Eur Radiol 2001; 11:1704-1709 60. Chotas HG, Ravin CE. Digital chest radiography with a solid-state flat-panel X-ray detector: contrast-detail evaluation with processed images printed on film hard copy. Radiology 2001; 218:679-682 61. Peer S, Neitzel U, Giacomuzzi SM, et al. Comparison of lowcontrast detail perception on storage phosphor radiographs and digital flat panel detector images. IEEE Trans Med Imag 2001; 20:239-242 62. Hamer OW, Völk M, Zorger N, Feuerbach S, Strotzer M. Amorphous silicon, flat-panel, x-ray detector versus storage phosphor-based computed radiography: contrast-detail phantom study at different tube voltages and detector entrance doses. Invest Radiol 2003; 38:212-220 63. Fischbach F, Ricke J, Freund T, et al. Flat panel digital radiography compared with storage phosphor computed radiography: assessment of dose versus image quality in phantom studies. Invest Radiol 2002; 37:609-614 119 64. Strotzer M, Völk M, Fründ R, et al. Routine chest radiography using a flat-panel detector: image quality at standard detector dose and 33% dose reduction. AJR 2002; 178:169-171 65. Rapp-Bernhardt U, Roehl FW, Gibbs RC, Schmidl H, Krause UW, Bernhardt TM. Flat-panel X-ray detector based on amorphous silicon versus asymmetric screen-film system: phantom study of dose reduction and depiction of simulated findings. Radiology 2003; 227:484-492 66. Metz S, Damoser P, Hollweck R, et al. Chest radiography with a digital flat-panel detector: experimental receiver operating characteristic analysis. Radiology 2005; 234:776-784 67. Herrmann KA, Bonel H, Stäbler A, et al. Chest imaging with flatpanel detector at low and standard doses: comparison with storage phosphor technology in normal patients. Eur Radiol 2002; 12:385390 68. ICRP, Publication 60, Recommendations of the International Commission on Radiological Protection, Pergamon Press. Oxford, 1991 69. Strotzer M, Gmeinwieser J, Völk M, et al. Clinical application of a flat-panel X-ray detector based on amorphous silicon technology: image quality and potential for dose reduction in skeletal radiography. AJR 1998; 171:23–27 70. Hamers S, Freyschmidt J, Neitzel U. Digital radiography with a large-scale electronic flat-panel detector vs screen-film radiography: observer preference in clinical skeletal diagnostics. Eur Radiol 2001; 11:1753-1759 71. Okamura T, Tanaka S, Koyama K, et al. Clinical evaluation of digital radiography based on a large-area cesium iodideamorphous silicon flat-panel detector compared with screen-film radiography for skeletal system and abdomen. Eur Radiol 2002; 12:1741-1747 72. Peer S, Neitzel U, Giacomuzzi SM, et al. Direct digital radiography versus strorage phosphor radiography in the detection of wrist fractures. Clin Radiology 2002; 57: 258-262 120 73. Strotzer M, Völk M, Wild T, von Landenberg P, Feuerbach S. Simulated bone erosions in a hand phantom: detection with conventional screen-film technology versus cesium iodideamorphous silicon flat-panel detector. Radiology 2000; 215: 512-515 74. Strotzer M, Gmeinwieser J, Spahn M, et al. Amorphous silicon, flatpanel, X-ray detector versus screen-film radiography: effect of dose reduction on the detectability of cortical bone defects and fractures. Invest Radiol 1998; 33:33–38 75. Völk M, Strotzer M, Holzknecht N, et al. Digital radiography of the skeleton using a large-area detector based on amorphous silicon technology: image quality and potiential for dose reduction in comparison with screen-film radiography. Clin Radiology 2000; 55: 615-621 76. Perslinden J, Beckman KW, Geijer H, Andersson T. Dose-uimage optimisation in digital radiology with a direct digital detector: an example applied to pelvic examinations. Eur Radiol 2002; 12: 15841588 77. Kim TS, Im JG, Goo JM, at al. Detection of pulmonary edema in pigs: storage phosphor versus amorpous selenium-based flatpanel-detector radiography. Radiology 2002; 223:695-701 78. Chotas HG, Floyd CE, Ravin CE. Memory artifact related to selenium-based digital radiography systems. Radiology 1997; 203:881-883 79. Bacher K, Smeets P, Vereecken L, De Hauwere A, Duyck P, De Man R, Verstraete K, Thierens H. Image quality and radiation dose in digital chest imaging: comparison of an amorphous silicon and an amorphous selenium flat-panel system. AJR, in press 80. Fischbach F, Freund T, Pech M, et al. Comparison of indirect CsI/a:Si and direct a:Se digital radiography. Acta Radiologica 2003; 44: 616-621 81. Borasi G, Nitrosi A, Ferrari P, Tassoni D. On site evaluation of three flat panel detectors for digital radiography. Med Phys 2003; 30: 1719-1731 82. Samei E, Flynn MJ. An experimental comparison of detector performance for direct and indirect digital radiography systems. Med Phys 2003; 30: 608-622 121 83. Martin CJ, Sharp PF, Sutton DG. Measurement of image quality in diagnostic radiology. Appl Radiat Isot 1999; 50:21-38 84. Martin CJ, Sutton DG, Sharp PF. Balancing patient dose and image quality. Appl Radiat Isot 1999; 50:1-19 85. Moy JP. Signal–to-noise ratio and spatial resolution in x-ray electronic imagers: is the MTF a relevant parameter? Med Phys 2000; 27:86-93 86. Neitzel U, Günther-Kohfahl S, Borasi G, Samei E. Determination of the detective quantum efficiency of a digital x-ray detector: comparison of three evaluations using a common image data set. Med Phys 2004; 31:2205-2211 87. IEC 62220-1. Medical electrical equipment – characteristics of digital x-ray imaging devices – part 1: determination of the detective quantum efficiency. International Electrotechnical Commission, Geneva, Switzerland, 2003 88. Illers H, Buhr E, Hoeschen C. Measurement of the detective quantum efficiency (DQE) of digital x-ray detectors according to the novel standard IEC 62220-1. Rad Prot Dosim 2005; 114:39-44 89. Ranger NT, Samei E, Dobbins JT, Ravin CE. Measurement of the detective quantum efficiency in digital detectors consistent with the IEC 62220-1 standard: practical considerations regarding the choice of filter material. Med Phys 2005; 32:2305-2311 90. Burgess AE. Comparison of receiver operating characteristic and forced choice observer performance measurement methods. Med Phys 1995;22:643-655 91. Thijssen MAO, Thijssen HOM, Merx JL, Lindeijer JM, Bijkerk KR. A definition of image quality: the image quality figure. In: Moores BM, Wall BF, Eriskat H, Schibilla H, editors. BIR Report 20: Optimization of image quality and patient exposure in diagnostic radiology. London: The British Institute of Radiology; 1989. p. 29-34 92. Peer S, Giacomuzzi SM, Peer R, Gassner E, Steingruber I, Jaschke W. Resolution requirements for monitor viewing of digital flatpanel detector radiographs: a contrast-detail analysis. Eur Radiol 2003; 13:413-417 122 93. Uffmann M, Schaefer-Prokop C, Neitzel U, Weber M, Herold CJ, Prokop M. Skeletal applications for flat-panel versus storagephosphor radiography: effect of exposure on detection of lowcontrast details. Radiology 2004; 231:506-514 94. Norman E, Gardestig M, Perslinden J, Geijer H. A clinical evaluation of the image quality computer program, CoCIQ. J Digit Imaging 2005; 18:138-144 95. Kwan ALC, Filipow LJ, Le LH. Automatic low contrast analysis of digital chest phantom radiographs. Med Phys 2003; 30:312-320 96. ICRP, Publication 93, Managing patient dose in digital radiology, Elsevier. Oxford, 2004 97. ICRP, Publication 62, Radiological protection in biomedical research, Pergamon Press. Oxford, 1992 98. Commission of the European Communities. European guidelines on quality criteria for diagnostic radiographic images. (EUR 16260). Brussels: CEC, 1996 99. Vaño E, Guibelalde E, Morillo A, Salvarez-Pedrosa CS, Fernandez JM. Evaluation of the European image quality criteria for chest examinations. Br J Radiol 1995; 68:1349-1355 100. Vetter S, Strecker EP. Clinical aspects of quality criteria in digital radiography. Rad Prot Dosim 2001 ; 94 :33-36 101. UNSCEAR, 2000. Sources and effects of ionizing radiation – Vol I: Sources. Report to the General Assembly, United Nations, New York 102. Faulkner K, Broadheas DA, Harrison RM. Patient dosimetry measurement methods. Appl Radiat Isot 1999; 50:113-123 103. National Radiological Protection Board. Patient dose reduction in diagnostic radiology. Documents of the NRPB, 1990: Vol 1-3 104. ICRP, Publication 73, Radiological protection and safety in medicine, Elsevier. Oxford, 1997 105. Vaño E, Fernandez JM, Ten JI, Guibelalde E, Gonzalez L, Pedrosa SCA. Real-time measurement and audit of radiation dose to patients undergoing computed radiography. Radiology 2002; 225:283-288 123 106. The Council of the European Union. Council Directive 97/43/Euratom. Official Journal of the European Communities 1997;L (180):22-27 107. Busch HP, Jaschke W. Adaption of the quality criteria concept to digital radiology. Rad Prot Dosim 1998; 80:61-63 108. Axelsson B, Bodén K, Fransson SG, Hansson IB, Perslinden J, Witt HH. A comparison of analogue and digital techniques in upper gastrointestinal examinations: absorbed dose and diagnostic quality of the images. Eur Radiol 2000; 10:1351-1354 109. Honey ID, MacKenzie A, Evans DS. Investigation of optimum energies for chest imaging using film-screen and computed radiography. Br J Radiol 2005; 78:422-427 110. Tingberg A, Sjöström D. Optimisation of image plate radiography with respect to tube voltage. Rad Prot Dosim 2005; 114:286-293 111. Uffmann M, Neitzel U, Prokop M, et al. Flat-panel-detector chest radiography: effect of tube voltage on image qualiy. Radiology 2005; 235:642-650 112. Dobbins JT, Samei E, Chotas HG, et al. Chest radioghraphy: optimisation of the x-ray spectrum for cesium iodide-amorphous silicon flat-panel detector. Radiology; 2003; 226:221-230 113. Ward M, Hudhes D, Connolly P, Moores BM. Central dose date management and analysis in IT-driven radiation protection strategies. Rad Prot Dosim 2005; 114:135-142 114. Schuncke A, Neitzel U. Retrospective patient dose analysis of a digital radiography system in routine clinical use. Rad Prot Dosim 2005; 114:131-134 115. Weatherburn GC, Davies JG. Comparison of film, hard copy computed radiography (CR) and soft copy picture archiving and communication (PACS) systems using a contrast detail test object. Br J Radiol 1999; 72:856-863 116. Peters SE, Brennan PC. Digital radiography: are the manufacturers’ settings too high? Optimisation of the Kodak digital radiography system with aid of the computed radiography dose index. Eur Radiol 2002; 12:2381-2387 124 117. Kim AY, Cho KS, Song KS, Kim JH, Kim JG, Ha HK. Urinary calculi on computed radiography: comparison of observer performance with hard-copy versus soft-copy images on different viewer systems. AJR 2001; 177:331-335 118. Bacher K, Smeets P, De Hauwere A, et al. Image quality performance of liquid crystal display systems: influence of display resolution, magnification and window settings on contrast-detail detection. Eur J Radiol, in press 119. Fuchsjäger MH, Schaefer-Prokop CM, Eisenhuber E, et al. Impact of ambient light and window settings on the detectability of catheters on soft-copy display of chest radiographs at bedside. Am J Roentgenol 2003; 181:1415-1421 120. Scharitzer M, Prokop M, Weber M, Fuchsjäger M, Oschatz E, Schaefer-Prokop C. Detectability of catheters on bedside chest radiographs: comparison between liquid crystal display and highresolution cathode-ray tube monitors. Radiology 2005; 234:611-616 121. Oschatz E, Prokop M, Scharitzer M, Weber M, Balassy C, SchaeferProkop C. Comparison of liquid crystal versus cathode ray tube display for the detection of simulated chest lesions. Eur Radiol 2005; 15:1472-1476 122. Krupinski EA, Johnson J, Roehrig H, Nafziger J, Lubin J. On-axis and off-axis viewing of images on CRT displays and LCDs: Observer performance and vision model predictions. Acad Radiol 2005; 12:957-964. 123. Hwang SA, Seo JB, Choi BK, et al. Liquid-crystal display monitors and cathode-ray tube monitors: a comparison of observer performance in the detection of small solitary pulmonary nodules. Korean J Radiol 2003; 4:153-156 124. Ishihara S, Shimamoto K, Ikeda M, et al. CRT diagnosis of pulmonary disease: influence of monitor brightness and room illuminance on observer performance. Comput Med Imag Graph 2002; 26: 181-185 125. Goo JM, Choi JY, Im JG, et al. Effect of monitor luminance and ambient light on observer performance in soft-copy reading of digital chest radiographs. Radiology 2004; 232: 762-766 125 126. Krupinski AE, Johnson J, Roehrig H, Nafziger J, Fan J, Lubin J. Use of a human visual system model to predict observer performance with CRT vs LCD display of images. J Digit Imaging 2004; 17: 258263 127. Balassy C, Prokop M, Weber M, Sailer J, Herold CJ, SchaeferProkop C. Flat-panel display (LCD) versus high-resolution gray-scale display (CRT) for chest radiography: an observer preference study. Am J Roentgenol 2005; 184:752-756 128. Otto D, Bernhardt TM, Rapp-Bernhardt U, et al. Subtle pulmonary abnormalities: detection on monitors with varying spatial resolutions and maximum luminance levels compared with detection on storage phosphor radiographic hard copies. Radiology 1998; 207:237-242 129. Graf B, Simon U, Eickmeyer F, Fiedler V. Bushberg JT, Seibert JA, 1K versus 2K monitor: a clinical alternative free-response receiver operating characteristic study of observer performance using pulmonary nodules. AJR 2000;174:1067-1074 130. Langer S, Bartholmai B, Fetterly K, et al. SCAR R&D symposium 2003: Comparig the efficacy of 5-MP CRT versus 3-MP LCD in the evaluation of interstitial lung disease. J Digit Imaging 2004; 17:149157 131. Kotter E, Bley TA, Saueressig U, et al. Comparison of the detectability of high- and low-contrast details on a TFT screen and a CRT screen designed for radiologic diagnosis. Invest Radiol 2003; 38: 719-724 132. Averbukh AN, Channin DS, Homhual P. Comparison of human observer performance of contrast-detail detection across multiple liquid crystal displays. J Digit Imaging 2005; 18: 66-77 133. Fan J, Roehrig H, Sundareshan MK, Krupinski E, Dallas Wj, Gandhi K. Evaluation of and compensation for spatial noise of LCDs in medical applications. Med Phys 2005; 32:578-587 134. Kimpe T, Xthona A, Matthijs P, De Paepe L. Solution for nonuniformities and spatial noise in medical LCD displays by using pixel-based correction. J Digit Imaging 2005; 18: 209-218 126 135. Bandado A, Flynn MJ, Martin S, Kanicki J. Angular dependence of the luminance and contrast in medical monochrome liquid crystal displays. Med Phys 2003; 30: 2602-2613 136. National Electrical Manufacturers Association (NEMA). Digital Imaging and Communications in Medicine (DICOM), Part 3.14: Grayscale Standard Display Function. Technical report. Virginia: ACR/NEMA, 1998. 137. Samei E, Badano A, Chakraborty D, et al. Assessment of display performance for medical imaging systems: Executive summary of AAPM TG18 report. Medical Physics 2005; 32(4):1205-1225 138. Ikeda M, Ishigaki T, Shimamoto K, et al. Influence of monitor luminance change on observer performance for detection of abnormalities depicted on chest radiographs. Invest Radiol 2003; 38:57-63 139. Seto E, Ursani A, Cafazzo JA, Rossos PG, Easty AC. Image quality assurance of soft-copy display systems. J Digit Imaging 2005; 18:280-286 140. Uffmann M, Prokop M, Kupper W, Mang T, Fiedler V, SchaeferProkop C. Soft-copy reading of digital chest radiographs: effect of ambient light and automatic optimization of monitor luminance. Invest Radiol 2005;40:180-185 141. Rowlands JA. The physics of computed radiography. Phys Med Biol 2002; 47:R123-R166 142. Neitzel U. Status and prospects of digital detector technology for CR and DR. Rad Prot Dosim 2005; 114:32-38 143. Nakano Y, Gido T, Honda S, Maezawa A, Wakamatsu H, Yanagita T. Improved computed radiography image quality from a BaFI:Eu photostimulable plate. Med Phys 2002; 29:592-597 144. Leblans P, Struye L, Willems P. New needle-crystalline CR detector. Proc SPIE 2001; 4320:59-67 145. Korner M, Wirth S, Treitl M, Reiser M, Pfeifer KL. Initial clinical results with a new needle screen storage phosphor system in chest radiograms. Rofo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der Beldgebenden Verfahren 2005; 177:1491-1496 127 146. Liu X, Shaw CC. Regional improvement of signal-to-noise and contrast-to-noise ratios in dual-screen CR chest imaging: a phantom study. Med Phys 2001;28:1293-1302 147. Fetterly KA, Schueler BA. Performance valuation of a "dual-side read" dedicated mammography computed radiography system. Med Phys 2003;30:1843-1854 148. Uffmann M, Prokop M, Eisenhuber E, Fuchsjager M, Weber M, Schaefer-Prokop C. Computed radiography and direct radiography: influence of acquisition dose on detection of simulated lung lesions. Invest Radiol 2005;40:249-256 149. Schaetzing R, Fasbender R, Kersten P. New high-speed scanning technique for computed radiography. Proc SPIE 2002;4682:511-520 150. Watanabe M, Mochizuki, Kameshima T, et al. Development and evaluantion of a portable amorphous silicon flat panel x-ray detector. Proc SPIE 2001; 4320:103-114 151. Samei E, Hill JG, Frey GD, Southgate WM, Mah E, Delong D. Evaluation of a flat panel digital radiographic system for low-dose portable imaging of neonates. Med Phys 2003; 30:601-607 152. Rapp-Bernhardt U, Bernhardt TM, Lenzen H, et al. Experimental evaluation of a portable indirect flat-panel detector for the pediatric chest: comparison with storage phosphor radiography at different exposures by using a chest phantom. Radiology 2005; 237:485-491 153. Orava R. New detectors for radiology. Phys Medica 1999; 15:295300 154. Pascoal A, Lawinski CP, Honey I, Blake P. Evaluation of a software package for automated quality assessment of contrast detail images--comparison with subjective visual assessment. Phys Med Biol 2005; 50: 5743-57 128 Curriculum Vitae Personal BACHER Klaus Born on the 4th of October 1977, Dendermonde Private address: Prinsdaal 57/4 9420 Bambrugge Working address: Ghent University Department of Medical Physics Proeftuinstraat 86 9000 Gent tel: 09/264 66 56 fax: 09/264 66 96 email : [email protected] Education • Burgerlijk natuurkundig ingenieur (UGent, 2000) Thesis: Dosimetrie bij 131I-Lipiodol therapie Promotor: Prof.dr. H. Thierens • Post Academische Vorming Multimedia ICT (UGent, 2002) • Gediplomeerde in de Gespecialiseerde Studies van Biomedical and Clinical Engineering – Radiation Physics (UGent, 2002) Thesis: Kwantificatie van 131I en 188Re activiteit op scintigrafische beelden: een vergelijkende studie Promotor: Prof. Dr. H. Thierens • Deskundige in de medische Geneeskunde/Radiologie) 129 stralingsfysica (Nucleaire Awards • Ingenieursprijs Koninklijke Vlaamse Ingenieursvereniging (Antwerpen, 2000) • Best Oral Presentation Award (Belgian Society for Nuclear Medicine Symposium, Knokke, 2001) Membership • Koninklijke Vlaamse Ingenieursvereniging • European Society for Engineering and Medicine • Belgische Vereniging voor Stralingsbescherming • Belgian Nuclear Society • Belgian Society for Nuclear Medicine • Working Group “Therapy” • Working Group “Radiological Protection” • Belgische Vereniging van Ziekenhuis Fysici • Working Group “Nuclear Medicine” • Working Group “Radiology” • The International Society for Optical Engineering Publications A1 Publications [1] Bacher K, Brans B, Monsieurs M, De Winter F, Dierckx RA, Thierens H. Thyroid uptake and radiation dose after 131I-lipiodol treatment: is thyroid blocking by potassiumiodide necessary?Eur J Nucl Med 2002; 29: 1311-1316 [2] Monsieurs M, Brans B, Bacher K, Dierckx RA, Thierens H. Patient dosimetry for 131I-MIBG therapy for neuroendocrine tumours based on 123I-MIBG scans. Eur J Nucl Med 2002; 29: 1581-1587 [3] Brans B, Bacher K, Vandevyver V, Vanlangenhove P, Smeets P, Thierens H, Dierckx RA, Defreyne L. Intra-arterial radionuclide therapy of liver tumors: effect of selectivity of catheterisation and I131-Iodized Lipiodol delivery on tumor uptake and response. Nucl Med Commun 2003; 24: 391-396 130 [4] Monsieurs M, Bacher K, Brans B, Vral A, de Ridder L, Dierckx RA, Thierens H. Patient dosimetry for 131I-Lipiodol therapy. Eur J Nucl Med 2003; 30: 554-561 [5] Lahorte CMM, Van de Wiele C, Bacher K, Van den Bossche B, Thierens H, Slegers G, Dierckx RA. Biodistribution and dosimetry of 123I-rh-Annexin V in Mice and Humans. Nucl Med Commun 2003; 24: 871-880 [6] Vandenbulcke K, De Vos F, Offner F, Philippé J, Apostolides C, Molinet R, Nikula TJ, Bacher K, de Gelder V, Vral A, Lahorte C, Thierens H, Dierckx RA, Slegers G. In vitro evaluation of 213BiRituximab versus external gamma irradiation for the treatment of BCLL patients: relative biological efficacy with respect to apoptosis induction and chromosomal damage. Eur J Nucl Med 2003; 30: 13571364 [7] Bacher K, Smeets P, Bonnarens K, De Hauwere A, Verstraete K, Thierens H. Dose reduction in patients undergoing chest imaging: digital amorphous silicon flat panel detector versus conventional screen-film and phosphor-based computed radiography systems. AJR 2003; 181: 923-929 [8] De Ruyck K, Lambert B, Bacher K, Gemmel F, De Vos F, Vral A, de Ridder L, Dierckx RA, Thierens H. Biologic dosimetry of Re-188HDD/lipiodol versus I-131-lipiodol therapy in patients with hepatocellular carcinoma. J Nucl Med 2004; 45: 612-618 [9] Lahorte CM, Bacher K, Burvenich I, Coene ED, Cuvelier C, De Potter C, Thierens H, Van de Wiele C, Dierckx RA, Slegers G. Radiolabeling, biodistribution and dosimetry of 123I-MAb 14C5: A new monoclonal antibody for radioimmunodetection of tumour growth and metastasis in vivo. J Nucl Med 2004; 45: 1065-1073 [10] Thierens H, Reynaert N, Bacher K, van Eijkeren M, Taeymans Y. Patient doses in gamma-intracoronary radiotherapy: The radiation burden assessment study. Int J Radiation Oncology Biol Phys 2004; 60: 678-685 [11] Vandenbulcke K, Thierens H, Offner F, Janssens A, de Gelder V, Bacher K, Philippé J, Devos F, Dierckx R, Apostolidis C, Morgenstern A, Slegers G. Importance of receptor density in alpharadioimmunotherapy in B cell malignancies: an in-vitro study. Nucl Med Commun 2004; 25: 1131-1136 131 [12] Van Den Bossche B , D’Haeninck E, Bacher K, Thierens H, Van Belle S, Dierckx RA, Van de Wiele C. Biodistribution and dosimetry of (99mTc)depreotide (P829) in patients suffering from breast carcinoma. Cancer Biother Radiopharm 2004; 19: 776-783 [13] Jacobs F, Thierens H, Piepsz A, Bacher K, Van de Wiele C, Ham H, Dierckx RA. Optimized tracer-dependent dosage cards to obtain weight-independent effective doses. Eur J Nucl Med 2005; 32: 581588 [14] Lambert B, Bacher K, Defreyne L, Gemmel F, Van Vlierberghe H, Jeong JM, Dierckx RA, Van de Wiele C, Thierens H, De Vos F. 188ReHDD/Lipiodol Therapy for Hepatocellular Carcinoma: A Phase I Clinical Trial. J Nucl Med 2005; 46: 60-66 [15] Bacher K, Bogaert E, Lapere R, De Wolf D, Thierens H. Patientspecific dose and radiation risk estimation in pediatric cardiac catheterisation. Circulation 2005; 111: 83-89 [16] Bacher K and Thierens H. Accurate dosimetry: an essential step towards good clinical practice in nuclear medicine. Nucl Med Commun 2005; 25: 581-586 [17] Thierens H, Monsieurs M, Bacher K. Patient dosimetry in radionuclide therapy: the whys and the wherefores. Nucl Med Commun 2005; 26: 593-599 [18] Lambert B, Bacher K, De Keukeleire K, Smeets P, Colle I, Jeong JM, Thierens H, Troisi R, De VosF, Van de Wiele C. 188Re-HDD/Lipiodol for Treatment of Hepatocellular Carcinoma: A Feasibility Study in Patients with Advanced Cirrhosis. J Nucl Med 2005; 46: 1326-1332 [19] De Hauwere A, Bacher K, Smeets P, Verstraete K, Thierens H. Analysis of image quality in digital chest imaging. Rad Prot Dosim 2005; 117: 174-177 [20] Lambert B, Bacher K, Defreyne L, Van Vlierberghe H, Jeong JM, Wang RF, van Meerbeeck J, Smeets P, Troisi R, Thierens H, De Vos F, Van de Wiele C. 188Re-HDD/Lipiodol Therapy for Hepatocellular Carcinoma: An Activity Escalation Study. Eur J Nucl Med Mol Imaging 2006; 33: 344-352 [21] Kersemans V, Cornelissen B, Bacher K, Kersemans K, Thierens H, Dierckx RA, De Spiegeleer B, Slegers G, Mertens J. In vivo evaluation and dosimetry of [123I]-2-iodo-D-phenylalanine, a new potential tumor specific tracer for SPECT, in an R1M rhabdomyosarcoma athymic mice model. J Nucl Med 2005; 46: 2104-2111 132 [22] Bacher K, Smeets P, Vereecken L, De Hauwere A, Duyck P, De Man R, Verstraete K, Thierens H. Image quality and radiation dose in chest imaging using digital flat-panel detectors: comparison of an amorphous silicon and an amorphous selenium flat-panel system. AJR 2006, in press [23] Bacher K, Smeets P, De Hauwere A, Voet T, Duyck P, Verstraete K, Thierens H. Image quality performance of liquid crystal display systems: influence of display resolution, magnification and window settings on contrast-detail detection. Eur J Radiol 2006, in press [24] De Ruyck K, Van Eijkeren M, Claes K, Bacher K, Vral A, De Neve W, Thierens H. TGFβ polymorphisms and late clinical radiosensitivity in patients treated for gynecologic tumors. Int J Radiation Oncology Biol Phys 2006, in press A3 Publications [1] Brans B, Monsieurs M, Bacher K, De Vos F, Thierens H, Slegers G, Dierckx RA. Toepassingen van de radionuclidentherapie. Radiofarmaca, werkingsmechanismen en stralingsbescherming. Tijdschrift voor Geneeskunde 2002; 58: 1090-1097 [2] De Winter F, Brans B, Lambert B, Gemmel F, Van Laere K, Defreyne L, Van Vlierberghe H, De Hemptinne B, Bacher K, Dierckx RA. Locoregionale 131I-lipiodoltherapie bij de behandeling van het hepatocellulair carcinoom. Tijdschrift voor Geneeskunde 2003; 59: 532-541 A4 Publications [1] Bacher K. Stralingsbelasting bij de behandeling van levertumoren. Het Ingenieursblad 2001; 5: 46-52 [2] Bacher K, Monsieurs M, Lambert B, Dierckx R, Thierens H. Towards individual Patient dosimetry in metabolic radiotherapy. Annales de l’Association belge de Radioprotection 2002; 27 (4): 183-189 [3] Bacher K, De Hauwere A, Smeets P, Verstraete K, Thierens H. Image quality in correlation with patient dose in radiology. Annales de l’Association belge de Radioprotection 2002; 27 (4): 215-222 [4] Bacher K, Vandenbulcke K, De Vos F, Phillipé J, Slegers G, Offner F, Dierckx RA, Thierens H. Application of alpha-emitters in nuclear medicine: facts and fiction. Annales de l’Association belge de Radioprotection 2003; 28 (1): 1-7 133 International communications [1] European Association of Nuclear Medicine Congress 2000. Paris, 2-6 IX 2000. Bacher K, Thierens H, Monsieurs M, Brans B, De Winter F, Defreyne L, Dierckx RA. Evaluation of radiation exposure during the administration of I131-Lipiodol therapy. Eur J Nucl Med 2000; 27 (8): 358 [2] European Association of Nuclear Medicine Congress 2001 Naples, 25-19 VIII 2001 Bacher K, Brans B, Troisi R, Monsieurs M, Defreyne L, Vanlangenhove P, Colle I, de Hemptinne B, Thierens H, Dierckx RA. 131I-Lipiodol therapy: influence of potassiumiodide on thyroidal uptake and dose. Eur J Nucl Med 2001; 28: 1093 [3] European Association of Nuclear Medicine Congress 2001 Naples, 25-29 VIII 2001 Bacher K, Thierens H, Van de Putte S, Brans B, Monsieurs M, Dierckx RA. 188-Re-Lipiodol: prediction of the patient dose based on 131-Lipiodol bi-planar scanning and monte-carlo simulation. Eur J Nucl Med 2001; 28: 1196 [4] American Roentgen Ray Society 2002 Annual Meeting Atlanta, 28 IV – 3 V 2002 Smeets P, Van De Putte S, Bacher K, Thierens H. Digital amorphous silicon flat panel detector versus conventional radiography in chest imaging: comparison of patient radiation exposure. AJR 2002; 178 (3, supplement): 47 [5] The Fleischner Society’s 32nd Annual Conference on Chest Disease Brugge, 12-15 V 2002 Bacher K, Smeets P, Van de Putte S, De Hauwere A, Verstraete K, Thierens H. Patient dose reduction with a digital amorphous silicon flat panel detector compared to conventional radiography in chest imaging. Eur Radiol 2002; 12 (10) [6] European Association of Nuclear Medicine Congress 2002 Vienna, 31 VIII 2002 Monsieurs M, Bacher K, Brans B, Vral A, de Ridder L, Dierckx RA, Thierens H. Patient dosimetry after 131-I-lipiodol therapy. Eur J Nucl Med 2002; 29: 172 134 [7] European Association of Nuclear Medicine Congress 2002 Vienna, 31 VIII 2002 Monsieurs M, Brans B, Bacher K, Van De Putte S, Dierckx RA, Thierens H. Patient dosimetry for 131-I-MIBG therapy for neuroendocrine tumors based on MIBG pretherapy scans and 131-IMIBG post therapy scans. Eur J Nucl Med 2002; 29: 172 [8] European Association of Nuclear Medicine Congress 2002 Vienna, 31 VIII 2002 Lahorte C, Burvenich I, Bacher K, Thierens H, Coene E, Schelfhout E, Cuvelier C, Slegers G. Synthesis, biodistribution and dosimetry of the 123I-14C5 MoAb in mice: A potential SPECT-ligand for radioimmunodetection of tumour growth and metastasis in vivo. Eur J Nucl Med 2002; 29: 77 [9] Radiological Society of North America Congress 2002 Chicago, 1-6 XII 2002 Bacher K, Smeets P, De Hauwere A, Verstraete K, Thierens H. Patient dose reduction with a digital amorphous silicon flat panel detector compared to conventional screen-film and phosphor-based computed radiography systems in chest imaging. Radiology 2002; 225 (P): 643 [10] Radiological Society of North America Congress 2002 Chicago, 1-6 XII 2002 Bacher K, Seaux I, De Wolf D, Thierens H. Patient-specific Monte Carlo calculation of the effective dose in pediatric interventional cardiology. Radiology 2002, 225 (P): 644 [11] Radiological Society of North America Congress 2002 Chicago, 1-6 XII 2002 Smeets P, Bacher K, Duyck P, Verstraete K, Thierens H. Digital amorphous silicon flat panel detector versus screen-film and storage phosphor systems in chest imaging: comparison of the entrance skin dose. Radiology 2002, 225 (P): 382 [12] 38th Annual Meeting of the AEPC Amsterdam, 28-31 V 2003 Bacher K, De Wolf D, Thierens H. Patient dose in peadiatric interventional cardiology: effect of additional beam filtration. Cardiology in the Young 2003, 13: 77 135 [13] 225th National Meeting of the American Chemical Society New Orleans, 23-27 III 2003 Vandenbulcke K, De Vos F, Offner F, Philippe J, Apostolidis C, Molinet R, Nikula TK, Bacher K, de Gelder V, Vral A, Lahorte C, Thierens H, Dierckx R, Slegers G. In vitro evaluation of Bi-213rituximab versus external gamma irradiation for the treatment of BCLL patients: Relative biological efficacy with respect to apoptosis induction and chromosomal damage by the micronucleus assay. Abstracts of papers of the American Chemical Society 2003, 225: U261-U261 79-NUCL Part 2 [14] European Association of Nuclear Medicine Congress 2003 Amsterdam, 23-28 VIII 2003 Lambert B, Bacher K, Gemmel F, Defreyne L, Jeong JM, Thierens H, Slegers G, Van de Wiele C, Dierckx RA, De Vos F. 188-Re-Lipiodol for locoregional treatment of hepatocellular carcinoma: a phase I study. Eur J Nucl Med 2003, 30 (Suppl 2): S219 [15] European Association of Nuclear Medicine Congress 2003 Amsterdam, 23-28 VIII 2003 Bacher K, Lambert B, De Vos F, Gemmel F, Van de Wiele C, Defreyne L, Jeong JM, Slegers G, Dierckx RA, Thierens H. Patient dosimetry after 188-Re-Lipiodol therapy. Eur J Nucl Med 2003, 30 (Suppl 2): S219 [16] European Association of Nuclear Medicine Congress 2003 Amsterdam, 23-28 VIII 2003 Ravier M, Hamerlynck E, Bacher K, Lambert B, Gemmel F, De Vos Fff, Dierckx RA, Thierens H. 188Re-Lipiodol post-therapy scintigraphy: influence of the collimator on the image quality. Eur J Nucl Med 2003, 30 (Suppl 2): S244 [17] Radiological Society of North America Congress 2003 Chicago, 30 XI - 4 XII 2003 Smeets P, Bonnares K, Bacher K, Duyck P, Thierens H, Verstraete K. High resolution viewing monitors: comparison of low-contrast phantom image quality. [18] Dimond III International Workshop Leuven, 25-27 III 2004 De Hauwere A, Bacher K, Smeets P, Verstraete K, Thierens H. Analysis of Image Quality in Digital Chest Imaging 136 [19] European Association of Nuclear Medicine Congress 2004 Helsinki, 4-8 IX 2004 Bacher K, Lambert B, De Vos F, Van de Wiele C, Dierckx RA, Thierens H. Comparison of the real-life radiation burden to relatives of patients treated with I-131-Lipiodol or Re-188-HDD/Lipiodol. Eur J Nucl Med Mol Imaging 2004, 31 (Suppl2): S234-S234 [20] European Association of Nuclear Medicine Congress 2004 Helsinki, 4-8 IX 2004 De Vos F, De Decker M, Bacher K, Lambert B, David B, Thierens H, Slegers G, Dierckx RA. Development, pharmaceutical validation and radiation protection properties of a new cocept of 188W/188Re generator. Eur J Nucl Med Mol Imaging 2004, 31 (Suppl2): S234-S234 [21] International Conference on Education and Training in Radiological Protection 2005 Brussels, 23-25 XI 2005 Thierens H, Bogaert E, Lemmens K, Bacher K. Towards a specific education and training programme in radiological protection for practitioners in interventional cardiology. National communications [1] Xth Triennal Symposium of the Belgian Society of Nuclear Medicine Knokke, 18-20 V 2001 Bacher K, Thierens H, Monsieurs M, Brans B, Defreyne L, Vanlangenhove P, Dierckx RA. Evaluation of radiation exposure during the administration of 131-I Lipiodol. Tijdschr Nucl Geneeskd 2001; 23: 112 [2] Xth Triennal Symposium of the Belgian Society of Nuclear Medicine Knokke, 18-20 V 2001 Bacher K, Brans B, Troisi R, Monsieurs M, Defreyne L, Vanlangenhove P, Colle I, de Hemptinne B, Thierens H, Dierckx RA. 131I-Lipiodol therapy: influence of potassium iodide on thyroidal uptake and dose. Tijdschr Nucl Geneesk 2001; 23: 151-152 [3] Xth Triennal Symposium of the Belgian Society of Nuclear Medicine Knokke, 18-20 V 2001 Bacher K, Thierens H, Van De Putte S, Monsieurs M, Brans B, Dierckx RA. 188Re-Lipiodol: prediction of the patient dose based on 131I-Lipiodol bi-planar scanning and Monte-Carlo simulation. Tijdschr Nucl Geneesk 2001; 23: 153 137 [4] Belgian Day on Biomedical Engineering Brussels, 18 X 2002 Bacher K, De Hauwere A, Smeets P, Verstraete K, Thierens H. Patient dose reduction with a digital amorphous silicon flat panel detector compared to conventional screen-film and phosphor-based computed radiography systems in chest imaging [5] XIth Triennal Symposium of the Belgian Society of Nuclear Medicine Knokke, 2002 Bacher K, Lambert B, De Vos F, Gemmel F, Van de Wiele C, Slegers G, Dierckx RA, Thierens H. Patient dosimetry after 188Re-Lipiodol therapy [6] XIth Triennal Symposium of the Belgian Society of Nuclear Medicine Knokke, 2002 Lambert B, Bacher K, Gemmel F, Defreyne L, Jeong JM, Thierens H, Slegers G, Van de Wiele C, Dierckx RA, De Vos F. 188Re-Lipiodol therapy: phase I study [7] XXIst Annual Symposium of the Belgian Hospital Physicist Association Gent 20-21 I 2006 Bacher K, Smeets P, De Hauwere A, Voet T, Duyck P, Verstraete K, Thierens H. Image quality performance of LCD devices: influence of display resolution, magnification and window settings on contrastdetail detection [8] XXIst Annual Symposium of the Belgian Hospital Physicist Association Gent 20-21 I 2006 Bacher K, Smeets P, De Hauwere A, Voet T, Duyck P, Verstraete K, Thierens H. Image quality and radiation dose in digital chest imaging: comparison of an amorphous silicon and amorphous selenium flat-panel system Invited lectures [1] Postgraduaat Interne Geneeskunde – Nieuwe aanwinsten in de radiotherapie, Gent, 16 X 2002 Radioprotectieve aspecten bij radionuclidenbehandelingen [2] Wetenschappelijke vergadering BVS – Stralingsbescherming en kwaliteitszorg in de medische sector, Brussel, 18 X 2002 Towards individual patient dosimetry in metabolic radiotherapy 138 [3] Wetenschappelijke vergadering BVS – Stralingsbescherming en kwaliteitszorg in de medische sector, Brussel, 18 X 2002 Image quality in correlation with patient dose in radiology [4] Wetenschappelijke vergadering BVS – Nieuwe toepassingen van radionucliden in de geneeskunde, Brussel, 13 XII 2002 Application of alpha-emitters in nuclear medicine: facts and fiction [5] BHPA Symposium 2004, Brussel, 30-31 I 2004 A new era in diagnostic radiology: implementation of digital detectors. [6] DIMOND III International Workshop – Optimisation of dose and performance in interventional and digital imaging, Leuven, 25-27 III 2004 Patient doses in pediatric interventional cardiology [7] BHPA Symposium 2005, Anhée, 21-22 I 2005 Basics of dosimetry and image quality in CT [8] PRVU – Postgraduaat Radiologie van de Vlaamse Universiteiten, Gent, 17 II 2005 Thoraxradiologie: Conventioneel of Digitaal? [9] KVI Workshop – New Dimensions in Medical Imaging, Groningen, 10-11 III 2005 Towards patient-specific dosimetry in radionuclide therapy [10] BHPA Spring Continual Education 2005, Leuven, 19 IV 2005 Image quality analysis using CDRAD [11] Avondsymposium Vereniging Medische Beeldvormers, Gent, 25 V 2005 Digitale radiografie [12] MedicalPHIT Seminar – Digitale mammografie, Gent, 28 V 2005 Overzicht van technologie voor digitale mammografie [13] Postgraduaat Interne Geneeskunde – Interventionele radiologie in de oncologie, Gent 9 VI 2005 Fysische aspecten bij intra-arteriële radionuclidentherapie [14] Colloquium 131I/188Re-Lipiodol bij hepatocellulair carcinoom, Groningen, 8 VII 2005 Dosimetrie en radioprotectie bij radionuclidentherapie voor het hepatocellulair carcinoom [15] BHPA Symposium 2006, Gent, 21-22 I 2006 Image quality and patient dose with digital radiology [16] XXIIe NVKF-conferentie, Doorwerth, 7-8 IV 2006 Beeldkwaliteit van LCD monitoren 139 [17] BGNG/BVS conference – Radionuclide therapy and radiation protection, Leuven 6 V 2006 Patient dosimetry: research tool or routine practice [18] MedicalPHIT Seminar – Trends en kwaliteitin digitale radiologie, Gent, 20 V 2006 Analyse beeldkwaliteit in digitale radiologie 140