* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Clotting factors and eicosanoids protect against nematode infections
Marine microorganism wikipedia , lookup
Bacterial cell structure wikipedia , lookup
Urinary tract infection wikipedia , lookup
Human microbiota wikipedia , lookup
Schistosomiasis wikipedia , lookup
Hepatitis B wikipedia , lookup
Bacterial morphological plasticity wikipedia , lookup
Neonatal infection wikipedia , lookup
Infection control wikipedia , lookup
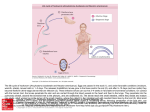
Clotting factors and eicosanoids protect against nematode infections Pavel Hyršl1, 2, Pavel Dobeš1, 2, Zhi Wang1 and Ulrich Theopold1 1 Department of Molecular Biology and Functional Genomics, University of Stockholm, 10691 Stockholm, Sweden 2 Department of Animal Physiology and Immunology, Institute of Experimental Biology, Masaryk University, 61137 Brno, Czech Republic INTRODUCTION Entomopathogenic nematodes (EPN’s) of the genera Heterorhabditis and Steinernema are obligate and lethal insect parasites, in recent years they have been used increasingly as biological control agents for pest insects. Animals from the third developmental stage of nematodes are called dauer juvenile (DJ) or infective juveniles (IJ). IJs occur free living in the soil and are capable of seeking out hosts and penetrate them through either the cuticle or natural orifices (Fig. 1). EPN’s are symbiotically associated with bacteria of either of the genera Photorhabdus and Xenorhabdus. The bacterial symbionts are essential to kill the insect host (usually within 24-48 hours) and to digest host tissues. Drosophila larvae are more resistant to nematode infection than Galleria mellonella where all larvae can be killed with low dose of nematodes. To test whether clotting factors other than TG are required for the response against nematodes and their bacteria we infected knock down (RNAi) lines for previously identified clotting factors including Hemomucin, GP150, FBP1, Tiggrin, and Fondue. Using known information on the expression of these factors, driver lines with specificity for either the fat body or hemocytes were used. Significant increases in susceptibility were observed when GP150 expression was reduced in hemocytes (Fig. 4A). Similarly knock down of Fondue in the fat body (Fig. 4B) increased mortality after nematode infection. In contrast, knock down of the phagocytic receptor Nimrod C1 in hemocytes had no effect adding further support to the idea that phagocytosis does not play a major role (Fig. 4A). Neither of other used clotting factor knock downs showed any effect (Fig. 4A, B). A 100 w1118 Mortality (%) 80 Tiggrin ** 60 GP150 40 Hemomucin 20 NimC1 0 24 hrs B w1118 Fig. 1: The lifecycle of the entomopathogenic nematode Heterorhabditis bacteriphora and its bacterial symbiont Photorhabdus luminescens (according to ffrench-Constant et al., 2003). B A 80 Mortality (%) Tiggrin ** GP150 60 Hemomucin ** 40 Fondue 20 FBP1 0 24 hrs 48 hrs Fig. 4A, B: Identification of clot components that contribute to the defense against Heterorhabditis/Photorhabdus. RNAi lines for individual clotting factors were crossed either with a hemocyte- (A) or a fat body-specific driver (B), infected with Heterorhabditis/Photorhabdus and mortality scored after 24 and 48 hours. Since eicosanoids are well-established regulators of blood clotting and have been implied in insect immunity (Stanley et al., 2009), we speculated that they might also play a role during the Heterorhabditis/Photorhabdus infection. To test this we injected wild type larvae with 0.05 µl of eicosanoid biosynthesis inhibitors dissolved in PBS (5 µg/µl) using automatic nanoliter injector Nanoject II (Drummond Scientific, PA, USA). As control, untreated larvae and larvae injected with the same volume of PBS were used. The larvae were exposed to nematode infection 12 hrs after injection (Fig. 5). All three inhibitors (esculetin, dexamethasone and indomethacin; all from Sigma-Aldrich, St. Louis, MO, USA) led to an increase in mortality 48 hrs post-infection, which suggests that eicosanoids or related lipids do play a role and agrees with the observation that Photorhabdus induces immunosuppression in its host by inhibiting phospholipase A2 (PLA2), the most upstream enzyme in the eicosanoid biosynthesis pathway (Kim et al., 2005). 100 *** 80 non-injected Mortality (%) MATERIAL and METHODS The tripartite model (Drosophila, nematodes, bacteria) was recently established by Hallem et al. (2007) and we have previously shown an immune function for transglutaminase, a conserved clotting factor (Wang et al., 2010). Infective juveniles (IJs) of Heterorhabditis bacteriophora (H222, isolated from Pouzdřany, Czech Republic) were collected after multiplying on G. mellonella larvae, released IJs were stored in sterilized tap water. D. melanogaster RNAi lines from the Vienna collection (Dietzl et al., 2007) were used. To account for differences in knock down due to position effects, we used all lines available for a given gene. These included lines for Tiggrin (stock 28255 and 100036), GP150 (stock 150899,150900 and 100134), Hemomucin (stocks 1278, 1279 and 100286) and FBP1 (stock 37881). The RNAi line targeting Fondue had been characterized before (Lindgren et al., 2008). The RNAi line for NimC1 was a kind gift from I. Ando. Gal4 expressing driver lines with specificity for either the fat body (pplGal4), hemocytes (HeGal4) or ubiquitously expressed Gal4 (ActinGal4) were used. w1118 - wild type and Bc mutant flies were obtained from the Bloomington fly stock center (Bloomington, IN), and Imd mutant flies were a kind gift from B. Lemaitre. Second and third instar D. melanogaster larvae were rinsed briefly in water and placed in alternating wells of a 96-well microtiter plate containing approx. 1 cm2 of tissue paper. IJs (Fig. 2A) were washed in PBS and diluted to 25 IJs/10µl PBS; 10 µl of nematode suspension were added to each well containing a larva. The assay plate was covered with Parafilm® and infections were conducted at different temperatures (22°C for injections of eicosanoid inhibitors, 25°C for Fig. 3 or 29°C for Fig. 4, 5) in the dark. S urvival was quantified under a dissecting microscope after 24 and 48. Survival was determined based on movement, either spontaneous or in response to gentle prodding with forceps and typical coloration of dead larvae. All experiments were run in triplicates using 48 larvae per group. H. bacteriophora can infect D. melanogaster larvae and cause their death within 24-48 hrs. Cadavers initially show yellow-orange and later red coloration (Fig. 2B). Larvae going into pupation can be also infected; then the pupae are dead and also typically colored (Fig. 2C). Symbiotic P. luminescens ssp. kayaii is naturally bioluminescent, so cadavers of invaded insect are also bioluminescent. 48 hrs 100 C PBS 60 DEX ** 40 IND 20 ESC 0 24 hrs 48 hrs with nematodes Fig. 2: To demonstrate the role of symbiotic bacteria we exchanged the natural symbiont with TT01GFP expressing strain. The bacteria are localised in the gut of IJs and cause septicaemia after release into the insect body (A). Typically colored larvae (B) and pupa (C) of D. melanogaster after H. bacteriophora infection 100 Mortality (%) * 60 w1118 Bc BcImd Imd 40 w/o nematodes Fig. 5: Eicosanoids contribute to immunity against Heterorhabditis/Photorhabdus. Infection experiments were performed after previous injection of eicosanoid biosynthesis inhibitors (dexamethasone, indomethacine and esculetin). Mortality was scored after the indicated times. Control larvae were injected with the inhibitors alone without subsequent infection with nematodes and survival scored after 48 hours. CONCLUSION We show that hemolymph clotting protects Drosophila melanogaster against infection with entomopathogenic nematodes and their symbiotic bacteria; we demonstrated an immune function for known clotting substrates GP150 and Fondue during nematode infection. We also provide biochemical evidence for an involvement of eicosanoids in the same infection model. Taken together our results confirm the conserved nature of the immune function of clot formation. RESULTS and DISCUSSION 80 48 hrs Acknowledgements: We thank Z. Mráček for H. bacteriophora and T. Ciche for the GFP-expressing symbiotic strain of P. luminescencens. Our research is supported by grants from the Carl-Tryggers Foundation (U.T. and P.H.) and Grant Agency of Czech Republic (P.H., GA206/09/P470). References: 20 0 24 hrs 48 hrs Fig. 3: Imd pathway contribute to immunity against Heterorhabditis/Photorhabdus. Infection of control larvae, Imd mutants and Bc, Imd double mutants reveals an influence of the Imd pathway in the absence of melanization (data represent average ± S.D., confidence levels for this and subsequent figures are: *: < 0.05; ** : < 0.01 and ***: < 0.001) Dietzl G, Chen D, Schnorrer F, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 2007;448:151-6. ffrench-Constant R, Waterfield N, Daborn P, Joyce S, Bennet H, Au C, Dowling A, Boundy S, Reynolds S, Clarke D: Photorhabdus: towards a functional genomic analysis of a symbiont and pathogen. FEMS Microbiology Rev 2003;26:433-56. Hallem EA, Rengarajan M, Ciche TA, Sternberg PW: Nematodes, bacteria, and flies: a tripartite model for nematode parasitism. Curr Biol 2007;17:898-904. Kim Y, Ji D, Cho S, Park Y: Two groups of entomopathogenic bacteria, Photorhabdus and Xenorhabdus, share an inhibitory action against phospholipase A2 to induce host immunodepression. J Invertebr Pathol 2005;89:258-64. Lindgren M, Riazi R, Lesch C, Wilhelmsson C, Theopold U, Dushay MS. Fondue and transglutaminase in the Drosophila larval clot. J Insect Physiol 2008;54:586-92. Stanley D, Miller J, Tunaz H. Eicosanoid actions in insect immunity. J Innate Immun 2009;1:282-90.