* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Heat
Compressed air energy storage wikipedia , lookup
Public schemes for energy efficient refurbishment wikipedia , lookup
Regenerative brake wikipedia , lookup
World energy consumption wikipedia , lookup
Energy storage wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
Zero-energy building wikipedia , lookup
International Energy Agency wikipedia , lookup
Low-carbon economy wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Alternative energy wikipedia , lookup
Energy harvesting wikipedia , lookup
Micro combined heat and power wikipedia , lookup
Negawatt power wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Internal energy wikipedia , lookup
Conservation of energy wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
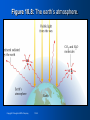
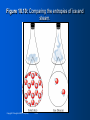
Zumdahl • Zumdahl • DeCoste World of CHEMISTRY Chapter 10 Energy Goals of Chapter 10 • • • • • • • • • • General properties of energy Temperature & Heat Direction of energy flow as heat How energy flow affects internal energy How to measure heat Heat (enthalpy) of chemical reactions Hess’s Law Changes in quality of energy as it’s used World’s energy resources Energy as driving force for natural processes Copyright © Houghton Mifflin Company 10-3 The importance of energy • • • • • • • Huge abundance of fossil fuels Society with huge appetite for energy We have become dependent on oil Has led to tension between countries Supplies are dwindling, prices are rising Need to find alternatives to oil Use relationship between chemistry & energy to find these alternatives Copyright © Houghton Mifflin Company 10-4 Energy: the ability to do work or produce heat • Potential Energy • Energy due to position or composition • Examples: water behind a dam, gasoline • Kinetic Energy • Energy of motion – depends on mass & velocity (KE = ½mv2) • Examples: car driving, thrown baseball Copyright © Houghton Mifflin Company 10-5 Law of Conservation of Energy • Energy can be converted from one form to another but cannot be created or destroyed • Energy of the universe is constant • Can convert from one form to another • Example: roller coasters • State function: property that is independent of pathway • Example: Ball bearing in roller coaster; PE is same at top of first hill regardless of path to bottom Copyright © Houghton Mifflin Company 10-6 Temperature & Heat • Temperature: measure of the random motions of the components of a substance (warm water molecules move faster than cold water molecules) • Heat: flow of energy due to a temperature difference (heat will flow from warmer water to cooler water) Copyright © Houghton Mifflin Company 10-7 Figure 10.2: Equal masses of hot and cold water. Each side contains 1 kg of water Copyright © Houghton Mifflin Company 10-8 Figure 10.3: H2O molecules in hot and cold water. Water molecules move more rapidly in hot water Copyright © Houghton Mifflin Company 10-9 Figure 10.4: H2O molecules in same temperature water. Heat is transferred from the hot water to the cold water until both are the same temperature Copyright © Houghton Mifflin Company 10-10 Final temperature is the average of the two original temperatures Change in temp (hot) ΔT = 90°C – 50°C = 40°C Change in temp (cold) ΔT = 50°C – 10°C = 40°C Copyright © Houghton Mifflin Company 10-11 Exothermic vs. Endothermic • System: part of universe on which we wish to focus • Surroundings: everything else in universe except system • Exothermic: evolution of heat, energy flows out of system • Endothermic: absorbs energy from surroundings, heat flows into system Copyright © Houghton Mifflin Company 10-12 Which is it - Endothermic or Exothermic? 1. Your hand gets cold when you touch ice. (with respect to your hand) 2. The ice melts when you touch it 3. Ice cream melts 4. Propane burning in a propane torch 5. After swimming water drops evaporate from your skin 6. Two chemicals mixing in a beaker give off heat Copyright © Houghton Mifflin Company 10-13 What happens in an exothermic chemical reaction? • Energy is conserved • Reactants have potential energy • Energy gained by surroundings must equal energy lost by the system • In any exothermic reaction, some of the potential energy stored in the chemical bonds is converted to thermal energy via heat (random kinetic energy) Copyright © Houghton Mifflin Company 10-14 Figure 10.5: The energy changes accompanying the burning of a match. Reactants have > PE than products, difference is heat Copyright © Houghton Mifflin Company 10-15 Thermodynamics • Thermodynamics – the study of energy • First Law of Thermodynamics = Law of Conservation of Energy (energy of the universe is constant) Copyright © Houghton Mifflin Company 10-16 Internal Energy (E) • Sum of kinetic and potential energies of all “particles” in a system • Can be changed by flow of work, heat, or both • ΔE = q + w; change in energy = heat + work • Sign reflects systems point of view • Endothermic – energy flows into system = +q (energy is increasing) • Exothermic – energy flows out of system = -q (energy is decreasing) • Same rules apply to work (w) • +w = work flows into system (surroundings do work) • -w = work flows out of system (system does work) Copyright © Houghton Mifflin Company 10-17 Measuring Energy Changes • Calorie: amount of energy (heat) required to raise the temperature of one gram of water by one degree Celsius • Food “calorie” = kilocalorie = 1000 cal • Joule: SI unit of energy • 1 calorie = 4.184 J • It takes 4.184 J of energy to raise the temperature of one gram of water by 1°C Copyright © Houghton Mifflin Company 10-18 How much energy is required to raise the temperature of 7.40 g of H2O from 29.0°C to 46.0°C? E = specific heat x mass x temp change E = (4.184 J/g°C)x(7.40 g)x(46°C – 29°C) E = 526 J Copyright © Houghton Mifflin Company 10-19 Thermochemistry (Enthalpy) • H = Enthalpy: energy function that indicates how much energy is produced or absorbed in a reaction • ΔHp = energy that flows as heat • ΔH: the change in enthalpy • p: indicates process has occurred under constant pressure • The enthalpy change is the same as the heat of reaction • See Example 10.5 & Self-Check 10.5 (pg. 302) Copyright © Houghton Mifflin Company 10-20 Figure 10.6: A coffee-cup calorimeter. Calorimeter: device used to determine the heat associated with a chemical reaction Run reaction – observe temperature change Heat capacity of calorimeter enables us to calculate the heat energy released/absorbed by reaction Determine ΔH for reaction & calculate ΔH for other reactions Copyright © Houghton Mifflin Company 10-21 Hess’s Law • Enthalpy is a state function – it is independent of the pathway • When going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whether the reaction takes place in one step or a series of steps • See example on page 304 Copyright © Houghton Mifflin Company 10-22 Two characteristics of ΔH for a reaction: • If a reaction is reversed, the sign of ΔH is also reversed • The magnitude of ΔH is directly proportional to the quantities of reactants and products in a reaction. If the coefficients in a balanced reaction are multiplied by an integer, the value of ΔH is also multiplied by that integer • See examples on page 304 & 305 Copyright © Houghton Mifflin Company 10-23 Figure 10.7: Energy sources used in the United States. Copyright © Houghton Mifflin Company 10-24 Figure 10.8: The earth’s atmosphere. Copyright © Houghton Mifflin Company 10-25 Figure 10.9: The atmospheric CO2 concentration over the past 1000 years. Copyright © Houghton Mifflin Company 10-26 Energy as a Driving Force • Two driving forces • Energy spread: concentrated energy is dispersed widely • Matter spread: molecules of a substance are spread out and occupy a larger volume Copyright © Houghton Mifflin Company 10-27 Entropy • Designated by letter S • Measure of disorder or randomness • More disorder (or entropy) means: • More energy spread • More matter spread Copyright © Houghton Mifflin Company 10-28 Figure 10.10: Comparing the entropies of ice and steam. Copyright © Houghton Mifflin Company 10-29 Second Law of Thermodynamics • The entropy of the universe is always increasing • All processes lead to a net increase in the disorder of the universe • We are plunging slowly toward total randomness – the heat death of the universe Copyright © Houghton Mifflin Company 10-30