* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 23-8. Antibacterials
Public health genomics wikipedia , lookup
Harm reduction wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Compartmental models in epidemiology wikipedia , lookup
Antibiotic use in livestock wikipedia , lookup
Antimicrobial resistance wikipedia , lookup
Focal infection theory wikipedia , lookup
Infection control wikipedia , lookup
Canine distemper wikipedia , lookup
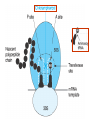
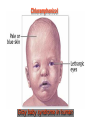
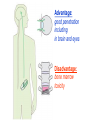
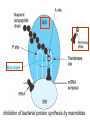
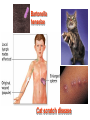
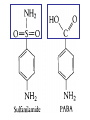
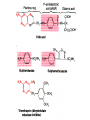
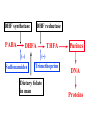
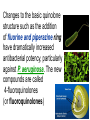
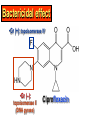
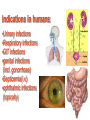
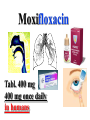
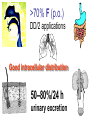
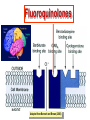
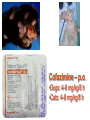
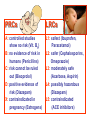
•Amphenicols •Macrolides •Lincosamides •Polymyxins •Streptogramins •Oxazolidinones Sulfonamides Quinolones Metronidazole Oxiquinolones Nitrofurans Others Assoc. Prof. Ivan Lambev (www.medpharm-sofia.eu) AMPHENICOLS •Chloramphenicol •Thiamphenicol (with SH_group instead of nitro functional group (–N02): less ADRs but less antibacterial activity •Florfenicol (analog of thiamphenicol) CHLORAMPHENICOL Originally isolated from Streptomyces venezuelae (1947). It is now produced synthetically because has a very simple structure. Mechanism of action It is a nonionized, highly lipophilic compound that enters bacterial cells by passive diffusion and binds primarily to the 50S ribosomal subunit. Bacterial protein synthesis is inhibited. Chloramphenicol can also bind to the mammalian ribosome (70S) that resembles bacterial ribosomes and interfere with mitochondrial protein synthesis. This is particularly relevant in erythropoietic cells. It is bacteriostatic for most Gram-positive and many Gram-negative aerobic bacteria. Resistance is commonly plasmid-mediated and occurs as a result of enzymatic inactivation by several types of chloramphenicol transacetylase. Chlorampenicol p.o., i.v., i.m., s.c. •Dogs: 50 mg/kg/8 h •Cats: 50 mg/kg/12 h Pharmacokinetics ●Well distributed, incl. CNS and eye. ●Attains higher concentrations for a long time in CSF than other antibacterials (30–50% of plasma concentrations in the absence of meningitis). ●Eliminated by hepatic glucuronide conjugation in the dog: only 5–10% is excreted in the urine. ●In the cat, more than 25% is excreted in the urine because of reduced ability to glucuronidate drugs. ●Chloramphenicol should not be given to young animals (ill-equipped with metabolizing enzymes) and avoided in adults with liver disease. ADRs ●Reversible, dose-related anemia can occur in dogs and cats. Cats may be more susceptible. ●Idiosyncratic fatal aplastic anemia has not been reported in small animals. ●Chloramphenicol inhibits hepatic microsomal enzymes and prolongs effects of drugs such as barbiturates and phenytoin. The inhibition is irreversible and long lasting. Chloramphenicol Gray baby syndrome in human Advantage: good penetration including in brain and eyes Disadvantage: bone marrow toxicity Florfenicol is a structural analog of thiamphenicol with greater activity against pathogenic bacteria than chloramphenicol and thiamphenicol. It is also active against enteric bacteria, resistant to chloramphenicol. Florfenicol can cause dose-related bone marrow suppression but has not been reported to cause fatal aplastic anemia in humans. Currently it is approved for use only in cattle, aquaculture and pigs. In cattle it is used to treat infectious conjunctivitis and respiratory disease caused by Pasteurella and Haemophilus. Florfeniciol ® (Norfenicol ) Florfeniciol (Norfenicol®) Cattle Treatment of respiratory tract infections in clinically diseased cattle due to Mannheimia haemolytica, Pasteurella multocida and Histophilus somni, susceptible to Florfenicol. Dosage: Intramuscular injection: 20 mg/kg BM to be administered twice at 48 h intervals, using a 16-gauge needle. Subcutaneous injection: 40 mg/kg BM to be administered once only using a 16-gauge needle. The dose volume given at any one injection site should not exceed 10 ml. The injection should only be given in the neck. Florfeniciol – Norfenicol® Swine Treatment of acute outbreaks of respiratory disease caused by strains of Actinobacillus pleuropneumoniae and Pasteurella multocida, susceptible to Florfenicol. Dosage Intramuscular injection: 15 mg/kg BM into the neck muscle twice at 48 h intervals using a 16-gauge needle. The volume administered per injection site should not exceed 3 ml. Florfeniciol (Norfenicol®) Withdrawal Period Cattle Meat and offal: By intramuscular injection (at 20 mg/kg, twice): 39 days By subcutaneous injection (at 40 mg/kg, once): 44 days Milk: Not permitted for use in lactating animals producing milk for human consumption. Swine Meat and offal: By intramuscular injection (at 15 mg/kg, twice): 22 days MACROLIDES and KETOLIDES •Azithromycin (t1/2 40–68 h, tab. 500 mg) •Erythromycin •Clarithromycin (antihelicobacter activity) •Josamycin (saliva excretion) •Midecamycin •Oleandomycin (saliva excretion) •Roxithromycin •Spiramycin (saliva excretion) •Tylosin •Ketolides: Telithromycin Inhibition of bacterial protein synthesis by macrolides Antibacterial spectrum of macrolides ●Active against Gram-positive aerobic bacteria, Mycoplasma and Bartonella henselae (cat scratch disease) with good intracellular distributions. Erythromycin ●Erythromycin is the drug of choice for treatment of Campylobacter jejuni infections. ●It is one of the drugs of choice for treatment of Mycoplasma infections. ●Erythromycin has greater activity against Staphylococcus than lincomycin but not clindamycin. Resistance to erythromycin is plasmid encoded. Bartonella henselae Cat scratch disease Tylosin ●Tylosin is less active against bacteria but more active against many Mycoplasma. ●Tylosin is often prescribed for feline respiratory tract infections, caused by Mycoplasma, Chlamydia and Gram-positive bacteria. ●Tylosin is used to treat bacterial overgrowth of the small intestine and antibiotic-responsive diarrhea (other choice is metronidazole). ●Tylosin should not be used in horses. Spiramycin ●It excretes mainly with saliva and is marketed in combination with metronidazole, for treatment of oral infections in dogs and cats. •Rodogyl® (spiramycin/metronidazole) Azithromycin and clarithromycin are derivatives of erythromycin with greater potency and a wider spectrum of activity, particularly against Gram-negative aerobes: Mycobacteria, Bartonella, Borrelia, Brucella, Leptospira, Campylobacter, Helicobacter, Chlamydia and Toxoplasma. ●They are suitable for p.o. administration once daily. ●Both drugs are concentrated in phagocytes. ●Azithromycin is effective against intracellular tubercle bacilli. Many macrolide-resistant strains are susceptible to ketolides: Telithromycin is active against S. pyogenes, S. pneumoniae, S. aureus, H. influenzae, Moraxella catarrhalis, Mycoplasmas, Legionella, Chlamydia, H. pylori, N. gonorrhoeae, B. fragilis, and T. gondii. Azithromycin •Dogs: 10 mg/kg/24 h •Cats: 5 mg/kg q.24–48 h Erythromycin 10–20 mg/kg q.8–12 h p.o. Clarithromycin 2.5–10 mg/kg/12 h p.o. Tylosin 10 mg/kg/8 h p.o. LINCOSAMIDES Clindamycin is a chlorine-substituted derivative of Lincomycin, an antibiotic that is elaborated by Streptomyces lincolnensis. ●Clindamycin and Lincomycin penetrate well into all tissues, incl. bone, and have been popular for treating osteomyelitis. Clindamycin is more potent than lincomycin especially against obligate anaerobes. ●Clindamycin is indicated for Toxoplasma and Neospora infections although in human medicine sulfadiazine and pyrimethamine remain the drugs of choice for toxoplasmosis. ●Clindamycin is one of several suitable drugs for treating chronic rhinosinusitis in cats. Clindamycin 10 – 20 mg/kg q. 12 h Lincomycin – p.o., i.m., i.v., s.c. 12.5 – 25 mg/kg q. 12 h p.o., i.m., i.v., s.c. 10 – 20 mg/kg p.o. q. 8 – 12 h ●Clindamycin is commonly combined with an aminoside in human medicine to treat or prevent mixed aerobic-anaerobic bacterial infections such as those associated with intestinal perforation. ●Clindamycin reportedly has synergistic effects with metronidazole against Bacteroides fragilis. ●Combinations of clindamycin with macrolides or chloramphenicol are antagonistic in vitro. ●Common ADRs are diarrhea and colitis due to C. difficile; nausea, skin rashes; impaired liver function and neutropenia. STREPTOGRAMINS Synercid® are combination of two antibiotics: quinupristin and dalfopristin in a 30:70 ratio. It is rapidly bactericidal for most microorganisms. Synercid® is approved for treatment of infections caused by staphylococci or by vancomycinresistant strains of Enerococus faecium, but not Enterococcus faecalis, which is resistant. The principal toxicities are infusion-related events, such as pain at the infusion site, and an arthralgia-myalgia syndrome. POLYMYXINS (B and E) They are mainly toxic antibiotics derived from strains of the soil bacterium Bacillus polymyxa. Colistin (Polymyxin E) alters bacterial membrane permeability. It is highly active against many Gram-negative bacteria, incl. P. aeruginosa but not Proteus. Polymyxins have nephro- and neurotoxicity in small animal. Therefore they are generally only used in topical or ophthalmic medications, often in combination with bacitracin and neomycin or tetracycline. OXAZOLIDINONES Linezolid is a synthetic antimicrobials, active against Gram-positive organisms including staphylococci, streptococci, enterococci, Gram-positive anaerobic cocci, and Gram-positive rods such as Corynebacteria and L. monocytogenes. It is primarily a bacteriostatic agent except for streptococci, for which it is bactericidal. Linezolid inhibits protein synthesis by preventing formation of the ribosome complex that initiates protein synthesis. Its unique binding site, located on 23S ribosomal RNA of the 50S subunit, results in no cross-resistance with other drug classes. SULFONAMIDES G. Domagk (1895–1964), bacteriologist and pathologist discovered the first sulfonamide in 1935. Nobel prize for Physiology and Medicine in 1939. Mechanism of action •Unlike man, most bacteria cannot utilize external folic acid, a nutrient which is essential for growth, and they have to synthesize it from para-aminobenzoic acid (PABA). Sulfonamides are structurally similar to PABA and inhibit the dihydrofolate synthetase in the biosynthetic pathway for folic acid. •High concentrations of PABA antagonize the effectiveness of sulfonamides. Folic acid is required for purine and pyrimidine synthesis and hence nucleic acid synthesis. Sulfonamides not only block formation of folic acid – they are incorporated into the precursors, forming a pseudometabolite that is reactive and antibacterial. Mammalian cells are not susceptible to sulfonamides. The combination of sulfonamides with trimethoprim potentiates their activity. Trimethoprim enters bacteria and inhibits bacterial dihydrofolic acid reductase, thus acting on the same metabolic pathway as sulfonamides. Therefore the combination has synergistic activity. The binding affinity of trimethoprim is 50 000 times greater for the bacterial enzyme than for mammalian enzyme. DHF synthetase PABA DHF reductase DHFA () Sulfonamides THFA Purines () Trimethoprim Dietary folate in man DNA Proteins Synergism between trimethoprim and the sulfonamide results in up to 40% of bacteria that are resistant to one component being susceptible to the combination. Clinical applications ●Toxoplasma infection (combined with pyrimethamine). ●Chlamydia infection. ●Nocardia infection (with or without trimethoprim). ●Bordetella bronchiseptica infection (kennel cough). ●Prostatic infections (concentrations in prostatic tissue are 10-fold greater than in plasma). Co-trimoxazolе (BAN) 30 mg/kg q.12 h p.o., i.v., i.m., s.c Trisulfan tabl. Dogs Cats ●Poorly absorbed sulfonamides can be used to treat GI infections, including coccidiosis (sulfaguanidine), or ulcerative colitis (sulfasalazine). ●Topical sulfonamides are used in ophthalmic and skin preparations. ●Trimethoprim-sulfonamide may be the treatment of choice for Pneumocystis pneumonia. BIOMEZIN® Premix: Mixed with feed 1:500 ratio prophylactically (or 1:250 for treatment) to protect respiratory infections in newborn piglets (twice a day for 10 do 14 days). After 15 days, the prophylactic course may be repeated. ® NORODINE 24: Timethoprim &Sulfadiazine 40 mg/200 mg (Solution i.v.) Norodine® 20 20 mg/100 mg Tablets: p.o. • indicated in the treatment of acute, subacute and chronic respiratory bacterial infections in horses, cattle, pigs, dogs and cats. Sulfacetamidе •collyrium 20% 10 ml For local treatment of bacterial conjunctivitis Resistance is common and due to the production of dihydrofolate synthetase with reduced affinity for binding of sulfonamides, and is transmitted in Gram-negative bacteria by plasmids. •Resistant strains of Staphylococcus aureus can synthesize more PABA than normal. Pharmacokinetics •Most sulfonamides are well absorbed orally. They are widely distributed in the body and cross the BBB and placenta. •Sulfonamides are metabolized in the liver, initially by acetylation which shows genetic polymorphism. The acetylated product has no antimicrobial actions but retains toxic potential. Distribution of sulfonamides in tissue is good. A large number of parent drugs and N-acetyl metabolites are excreted by the kidney. ADRs of sulfonamides Hypersensitivity reactions to sulfonamides include polyarthritis and fever, cutaneous eruptions, thrombocytopenia, leukopenia and hepatitis. They are cytotoxic and capable of binding to protein. Dobermans and pinschers are predisposed to sulfonamide hypersensitivity. This may be because of a reduced ability to detoxify hydroxylamine groups compared with mixed-breed dogs. Also, dobermans and other breeds of dogs commonly affected with von Willebrand’s disease (Scottish terriers, German shepherds) may not tolerate sulfonamides well. These drugs probably should be avoided in dobermans. Keratoconjunctivitis sicca (KCS) may occur with prolonged use of some sulfonamides. It is probably most often associated with sulfasalazine, as this drug is used for long-term treatment of ulcerative colitis. KCS has also been reported within the first week of treatment in a small proportion of dogs treated with trimethoprim-sulfadiazine. ●Crystalluria, hematuria and urinary tract obstruction can occur as a result of concentration of sulfonamides in renal tubules and acid pH. Ensure that animals receiving sulfonamides are well hydrated. Excessive salivation: Cats foam at the mouth if given oral sulfonamide drugs, if enteric-coated tablets are broken. ●Trimethoprim-sulfonamides have been reported to cause idiosyncratic severe hepatic necrosis on rare occasions. ●Aplastic anemia and thrombocytopenia may occur rare. ●It has been postulated but not proven that Co-Trimoxazole is a risk factor for acute pancreatitis. ●Sulfonamides at high doses (30 mg/kg twice daily) may decrease iodinization of colloid and decrease concentrations of thyroxine and triiodthyronine. QUINOLONES (inhibitors of DNA gyrase, resp. inhibitors of topoisomerases) •Nalidixic acid and its derivatives •Fluoroquinolones Nalidixic acid has been available for over 60 years but low activity poor tissue distribution, and adverse effects limited its use to being a second- and thirdline oral treatment for Gram (–) low urinary tract infections. Gr (—): E. coli Salmonella Shigella P. aeruginosa •Nalidixic acid ® (Nelidix ) ® •Gramurin Changes to the basic quinolone structure such as the addition of fluorine and piperazine ring have dramatically increased antibacterial potency, particularly against P. aeruginosa. The new compounds are called 4-fluoroquinolones (or fluoroquinolones) Bactericidal effect •Gr (+): topoisomerase IV •Gr (–): topoisomerase II (DNA gyrase) Ciprofloxacin Bacterial DNA topoisomerase II and IV) ( ) Fluoroquinolones •Bactericidal effect; very broad antibacterial spectrum •High activity against P. aeruginosa, Salmonella, Shigella, Neisseria, Campylobacter, and Chlamydia •Beta-lactamase stability •Limited and variable activity against streptococci ●Active against 90–100% of bacteria isolates from urine (where concentrations are 10–20-fold higher than in plasma) including methicillin-resistant Staphylococcus (MRSA). ●Active against Brucella, Mycobacterium, and Mycoplasma. ●Penetrate intracellularly, thus potentially effective against intracellular bacteria. ●Concentrate in phagolysosomes, enhancing intracellular killing. Clinical applications of fluoroqinolones in animals ●Urinary tract infections caused by Pseudomonas ●Bacterial prostatitis in dogs ●Serious Gram-negative systemic infections ●Osteomyelitis caused by Gram-negative aerobes ●Saprophytic Mycobacterium infection in cats ●Deep granulomatous pyoderma ●Serious bacterial respiratory tract infections ●Neutropenic, febrile patients with cancer ●Otitis externa due to Gram-negative infections Indications in humans: •Urinary infections •Respiratory infections •GIT infections •genital infections (incl. gonorrhoea) •Septicemia(i.v.) •ophthalmic infections (topically) Moxifloxacin Tabl. 400 mg 400 mg once daily in humans •Enrofloxacin •Gatifloxacin •Levofloxacin (Tavanic®) – S-isomer of ofloxacin 500 mg/24 h p.o. 10 days •Norfloxacin •Lomefloxacin •Ofloxacin •Pefloxacin etc. >70% F (p.o.) DD/2 applications Good intracellular distribution 50–80%/24 h urinary excretion ADRs ●Vomiting, inappetence or diarrhea may occur occasionally. Facial erythema and edema have been reported rarely, as have tremors and ataxia. ●Seizures have been reported rarely in animals with CNS disorders, with high doses and with concurrent use of NSAIDs. ●An apparent species-specific toxicity is acute retinal degeneration in cats treated with enrofloxacin. Blindness often results but some cats may regain vision. It has been postulated that the relatively open BBB of cats combined with the lipophilic properties of enrofloxacin predispose cats to accumulating high concentrations of the drug in the CNS. The risk may be higher in cats with urinary tract infections and concomitant renal failure and care should be taken with dosage in geriatric cats or those with liver or renal impairment. It is not clear whether other fluoroquinolones can also cause blindness. ●Fluoroquinolones should not be used in young animals as they cause erosion of articular cartilage (in dogs more than cats and large dogs especially). The mechanism of cartilage damage may be related to chelation of Mg2+ in joints. Lesions have been documented in dogs given five times the recommended dose and occur within 1–2 days of beginning administration. It is recommended that fluoroquinolones be avoided in large breed dogs up to 18 months of age (12 months for medium breeds, 9 months for small breeds). If a fluoroquinolone must be used because there is no suitable alternative, strict restriction (especially for large breed dogs) and use of chondroprotectives are advised. •Co-administration of ciprofloxacin and theophylline causes eleveted plasma theophylline concentrations due to inhibition of cytochrome P450. •Both drugs are epileptogenic. Fluoroquinolones Adapted from Bennett and Brown (2003) METRONIDAZOLE 10–20 mg/kg q.12–24 h p.o. Metronidazole is bactericidal to anaerobic bacteria, probably in a concentration-dependent manner. After entry into the cell it undergoes reduction to produce metabolites, some of which have antibacterial activity. These cause extensive breakage of DNA strands and inhibit the DNA repair enzyme. This reduction reaction occurs under anaerobic conditions. It is also active against many protozoa but the mechanisms involved are incompletely understood. Resistance is rare among susceptible bacteria and involves reduced intracellular drug activation. Metronidazole is bactericidal for many Gram (+) and most Gram (–) obligate anaerobes. It has no effect on aerobic bacteria. It is active against Balantidium coli, E. histolytica, Giardia and Trichomonas. Campylobacter are moderately susceptible. Helicobacter pylori are commonly susceptible but the susceptibility of animalderived Helicobacter spp has not been established. Clinical applications ●GI infections with Balantidium coli, Entamoeba histolytica, Giardia, Trichomonas or anaerobic bacteria. ●Mouth infections, periodontal disease, ulcerative gingivitis in combination with spiramycin (e.g. Rodogyl®). ●Bacterial overgrowth of the small intestine and antibiotic-responsive diarrhea. ●Anaerobic soft tissue infections, especially where good tissue penetration is important. ●In combination with a fluoroquinolone for sepsis. ADRs ●Vomiting, nausea. ●Dose-related neurotoxicity has been reported in dogs. Signs included severe ataxia, positional nystagmus (involuntary eye movement), seizures and head tilt. ●Cats often salivate profusely. ●Inappetence has been noted in horses. ●A marginal and contentious carcinogenic effect has been observed in some laboratory studies. As a result metronidazole and other nitroimidazoles are no longer used in food-producing animals in some countries. ●Metronidazole may be teratogenic and therefore should not be used during pregnancy, especially in the first 3 weeks, unless the benefits to the mother outweigh potential risks to the fetus. OXIQUINOLONES They block RNA polymerase. TILBROQUINOL – enteroantiseptic with antiamoebic activity NITROXOLIN – broad spectrum uroantiseptic (p.o.) NITROFURANS – broad spectrum antibacterial agents FURAZOLIDONE, NIFUROXAZIDE – enteroantiseptics (Salmonella etc.) NITROFURANTOIN (p.o.) – uroantiseptic: E. coli, Proteus mirabilis, Staph. aureus, Klebsiella, Serratia, Pseudomonas. It blocks carbohydrate metabolism by inhibiting its acetyl CoA synthesis. Furazolidone: 2.2–20 mg/kg q.8–24 h p.o. ●Nitrofurantoin has broad antibacterial activity but its use in small animals is limited to treatment of lower urinary tract infections. ●Nitrofurantoin is rapidly absorbed from the gut. It is rapidly eliminated (drug appears in the urine within 30 min of administration) and therapeutic blood concentrations cannot be maintained. Approximately 40–50% of the drug is eliminated unchanged in the urine. ●ADRs in small animals include GI disturbances and hepatopathy. Nitrofurantoin ® (Furadantin ): 4 mg/kg q.6–8 h RIFAMPICIN (RIFAMPIN – USAN) This semisynthetically modified antibiotic product of Streptomyces mediterranei has been an important component of the treatment of tuberculosis in humans. Rifampicin acts by inhibiting RNA polymerase, which catalyzes the transcription of DNA to RNA. It is bactericidal and has a wide spectrum: ●Brucella, Staphiloccocus spp. (incl. MRSA) ●Gram (+) and (–) anaerobic bacteria, incl. B. fragilis. ●Chlamydia and Rickettsia ●Mycobacterium tuberculosis and leprae Clinical applications ●Rifampicin is primarily used in small animal practice to treat chronic granulomatous skin infections in dogs. ●It is used in combination with erythromycin to treat Rhodococcus equi pneumonia in foals. Pharmacokinetics Absorption after oral administration is good. Rifampicin is effective against intracellular bacteria. Penetration into CSF is poor but enhanced by inflammation. Penetration into phagocytic cells is excellent. Its t1/2 in dogs is 8 h. Rifampicin is acetylated in the liver to a bioactive metabolite which is excreted in bile. Rodococcus equi is a Gram (+) coccobacillus. It is found in dry and dusty soil. Rifampicin (Rifampin) 10–20 mg/kg/12 h Rifampicin can induce hepatic microsomal enzymes, which may result in increased elimination rate with time. The metabolism of other drugs (barbiturates, ketoconazole, theophylline, hormonal contraceptives, and GCS), may be increased. Urine, feces, sweat and tears may be colored red-orange. Adverse effects Approximately 20% of dogs develop increases in hepatic serum enzyme concentrations and may progress to clinical hepatitis. This may be fatal in dogs with a history of liver disease. Clofazimine Clofazimine binds to DNA and may inhibIts function as a template. It is used in humans as part of multidrug protocols to treat leprosy. It is used in cats to treat Mycobacterium lepraemurium and other nontuberculous mycobacterial infections. Hepatoxicity has been reported in dogs. Cofazimine – p.o. •Dogs: 4–8 mg/kg/8 h •Cats: 4–8 mg/kg/8 h PRINCIPLES OF RATIONAL ANTIBACTERIAL THERAPY (Adapted from Laurence et al., 1997 & others) The following principles, many of which apply to drug therapy in general, are a guide to good clinical practice with antimicrobial agents. (1) Make a diagnosis precisely: – defining the site of action; – defining the microorganism(s) responsible and their sensitivity to drugs (antibiogram!); – biological samples for laboratory must be taken before treatment is begun. (2) Aims of therapy The goal of antibacterial therapy is to help the body eliminate infectious organisms without toxicity to the host. It is important to recognize that the natural defense mechanisms of a patient are of primary importance in preventing and controlling infection. Examples of natural defenses against bacterial invasion are: ●the mucociliary escalator in the respiratory tract ●the flushing effect of urination ●the normal flora in the GIT. All such mechanisms can be affected by disease or therapeutic interventions. Once microbial invasion occurs, various host responses serve to combat the invading organisms, including: ●the inflammatory response ●cellular migration and phagocytosis ●the complement system ●antibody production. The difficulty of controlling infections in immunocompromised patients emphasizes that antibacterial therapy is most effective when it supplements endogenous defense mechanisms rather than when acting as the sole means of control. (3) Consider factors affecting the success of antibacterial therapy •Bacterial susceptibility Various factors need to be considered in susceptibility testing. The minimum inhibitory concentration (MIC) is the concentration of drug that must be attained at the infection site. In general, if bacteria are not susceptible to a drug in vitro they will be resistant in vivo. •Distribution to the site of infection To be effective, an antibacterial agent must be distributed to the site of infection and come into contact with the infecting organism in adequate concentrations. Bacteria that locate intracellularly (Bartonella, Brucella, Chlamydia, Mycobacterium, Rickettsia) will not be affected by antibacterial agents that remain in the extracellular space. Staphylococcus is facultatively intracellular and may sometimes resist treatment because of intracellular survival. Drugs that accumulate in leukocytes and other cells include fluoroquinolones, lincosamides, sulfonamides and macrolides but aminoglycosides and β-lactams do not achieve effective intracellular concentrations. An infectious process often adversely affects the distribution of a drug in vivo. An exception is inflammation of the meninges (meningitis), which reduces the normal barrier between blood and CSF, so that antibacterial agents may cross this barrier. This breakdown of barriers by inflammation does not occur to an appreciable extent with the blood–prostate and blood–bronchial barrier. Effective antibacterial concentrations may not be achieved in poorly vascularized tissues, e.g. the extremities during shock, sequestered bone fragments or heart valves. (4) Remove barriers to cure (e.g. lack of free drainage of abscesses, obstruction in the urinary or respiratory tracts). (5) Decide whether therapy is necessary. As a general rule, acute infections require therapy whilst chronic infections may not. Chronic abscess or empyema respond poorly. Even some acute infections such as gastroenteritis are better managed symptomatically than by antimicrobials. (6) Choose the most suitable route of administration of antibacterial drug(s) ● Topical administration is valuable for disorders of eye and ear and some skin or gut infections. High drug concentration may be achieved locally in this way and some drugs too toxic for routine systemic administration (bacitracin, neomycin, polymyxins) can be useful topically. ● Oral administration is adequate in most infections and is usually preferable for home treatment. Some owners find it easier to administer drugs orally with food. If in doubt, administration on an empty stomach (no food for 1–2 h before and after dosing) is recommended, as the most common outcome of drug–ingesta interactions is impaired systemic drug availability. ●Parenteral administration is not routinely advantageous but can be useful for fractious, unconscious or vomiting patients, or those with oral/pharyngeal/esophageal pain or dysfunction. (7) Select the best drugs. This involves consideration of: – specificity (the antimicrobial activity of a drug must cover the infecting organisms); – pharmacokinetic factors (the chosen drug must reach the site of infection (e.g. by crossing BBB); – the patients (who may previously had allergic reactions to antimicrobials or whose routes of drug elimination may be impaired, e.g. by renal disease). 8. Client consent and compliance As with all drug therapy, antibacterials will not be effective unless administered correctly to the patient. It is important to maximize the likelihood that a client will administer drugs at the right dose and dosing interval. (9) Indications for combination therapy: – to avoid the development of resistance in chronic infections (e.g. FIV, tuberculosis). – to broaden the antibacterial spectrum: a) in a unknown mixed infection; b) unusual pathogens, including Mycobacterium, Rhodococcus and fungi. c) if the microorganism cannot be predicted (septicemia complicating neutropenia); – to obtain potentation (e.g. penicillin plus gentamicin for enterococcal endocarditis) (10) Antimicrobial therapy in pregnancy or lactation PRC B have: •Azithromycine •Erythromycine •Penicillins •Most cephalosporines PRCs LRCs A: controlled studies show no risk (Vit. B9) B: no evidence of risk in humans (Penicillins) C: risk cannot be ruled out (Bisoprolol) D: positive evidence of risk (Diazepam) X: contraindicated in pregnancy (Estrogens) L1: safest (Ibuprofen, Paracetamol) L2: safer (Cephalosporins, Omeprazole) L3: moderately safe (Acarbose, Aspirin) L4: possibly hazardous (Diazepam) L5: contraindicated (ACE inhibitors) (11) Administer the drug in optimum dose and frequency – Inadequate dose may encourage the development of microbial resistance. – Intermittent dosing is preffered to continual infusion. – Plasma concentration monitoring can be applied to optimize therapy with aminosides, fluoroquinolones, co-trimoxazole, and cephalosporins, in patients with kidney disease. (12) Continue therapy until apparent cure has been achieved. – Most acute infections are treated for 5 to 10 days. There are many exceptions to this, such as typhoid fever, tuberculosis, and infective endocarditis, in which relapse is possible long after apparent clinical cure and so the drugs are continued for a long time, determined by clinical experience. (13) Test for cure. In some infections, microbiological proof of cure is desirable because disappearance of symptoms and signs occurs before the microorganisms are eradicated, e.g. urinary tract infections (examinations must be done after withdrawal of drug therapy). (14) Prophylactic therapy for surgical and dental procedures should be of very limited duration. It should be started at the time of surgery to reduce the risk of producing resistant microorganisms. (15) Remember that the most important carriers of cross infections are your 10 fingers.