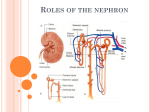
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download L8 Lecture Notes - Moodle
Survey
Document related concepts
Transcript
NAME: BSc Adult Nursing NURS08046 Responding to Ill Health Level 8 Life Science Lecture Notes Responding to Ill Health NURS08046 – Life Science Notes Contents pages Introduction 2 Cardiovascular System 3 Endocrine System 12 Urinary System 18 Respiratory System 28 Cells Pharmacology ` 35 43 Figures/diagrams are for illustrative purposes only and may include more or less detail than is found in the text. All images used are taken from Wikicommons Media or other open source (details given) or produced by UWS staff. Front cover image: US Department of Energy (2005) DNA split https://commons.wikimedia.org/wiki/File:Dna-split.png 1 Responding to Ill Health NURS08046 – Life Science Notes Responding to Ill Health NURS08046 - Level 8 Introduction The aim of the life sciences component of this module is to build on the anatomy and physiology delivered at level 7 to support learning related to health and pathophysiology studied in year two modules. Life Science will be taught through 12 hours of lectures and a 2 hour academic support session. Please note that lectures are not designed to simply repeat the information presented here but rather to communicate ideas and themes underlying some of the concepts of each topic area. Note also that lecturers may decide to include some additional PPt slides beyond that given on Moodle. It is strongly recommended that you look through these notes before lectures as this makes learning easier. You should look at headings, sub headings, diagrams and tables etc. and look up unfamiliar words. The key words are often already highlighted in bold and or italics. You are advised to revise year one Life Science material before the year two sessions as this knowledge will be assumed by the lecturers. Support Materials: The course is supported by a Moodle site. Power Point lecture presentations will be made available as well as revision materials covering each topic. Full details of the assessment will be provided on Moodle. : 2 Responding to Ill Health NURS08046 – Life Science Notes THE CARDIOVASCULAR SYSTEM BRIEF REVISION OF HUMAN CVS The human cardiovascular (or circulatory) system maintains a continuous flow of blood to and from all cells of the body, thus ensuring that all cells receive the nutrients and oxygen they need and that their waste products (e.g. carbon dioxide) are removed. Thus, it has a very important homeostatic function. It is made up of three components: the heart, the blood vessels and the blood itself. The human CVS is a closed double circulatory system – i.e. the blood is contained within the blood vessels and there are two separate systems of blood vessels within it. These are: the pulmonary circulation (concerned ONLY with carrying the blood to and from the lungs for the purposes of gas exchange) and the systemic circulation (concerned ONLY with the supply of oxygen and nutrients to the tissues of the body and the removal of waste materials from these tissues.) These two circulations are anatomically quite separate from each other, but blood passes from one to the other in a defined and ordered manner. In the pulmonary circulation, which is a low pressure system, deoxygenated blood is pumped from the right atrium of the heart into the right ventricle and, from there, via the pulmonary arteries, to the lungs. In the lungs, the blood gives up its carbon dioxide to, and picks up oxygen from, inhaled air; it then returns to the left atrium of the heart via the pulmonary veins and thus enters the systemic circulation. UWS Staff (2015) In the systemic circulation, which is a high pressure system, the newly oxygenated blood in the left atrium is pumped into the left ventricle and, from there, via the systemic arteries, to all the tissues of the body to supply them with oxygen and collect the waste products of their metabolism. After passing through the tissues, the blood, now deoxygenated and carrying waste materials, returns to the right atrium via the systemic veins to re-enter the pulmonary circulation. The operation of these two linked circulatory systems is clearly interdependent and notice that both oxygenated blood (in the left side) and deoxygenated blood (in the right side) is passing through the heart at the same time. The right and left sides of the heart are quite separate from each other so that the two types of blood do not mix. 3 Responding to Ill Health NURS08046 – Life Science Notes CONDUCTING SYSTEM OF THE HEART It is very important that the contraction of all of the muscle cells of the heart wall is co-ordinated and synchronised so that the pumping action of the heart is both effective and efficient. There are small groups of specialised neuromuscular cells within the heart wall that initiate and conduct the electrical impulse that stimulates the heart to beat. Together, these cells are referred to as the conducting (or conduction) system of the heart and may be regarded as a sort of ‘wiring’ system. Normally, the impulse that initiates the heartbeat arises in a small group of specialised neuromuscular cells in the wall of the right atrium - the sino-atrial (SA) node (sometimes called sinus node). From here, the impulse spreads throughout both atria (causing them to contract) and reaches another specialised patch of tissue (node) at the top of wall of muscle tissue that lies between the two ventricles – the atrioventricular (AV) node. There is a brief pause in the conduction of the impulse at this node so that atrial contraction (and thus ventricular filling) is completed before ventricular contraction begins. A mass of specialised fibres originating from the AV node (the AV bundle or Bundle of His) cross the ring of fibrous connective tissue that separates the two atria from the ventricles. O n reaching the upper end of the ventricular septum, the Bundle of His divides into the right and left bundle branches. Then within the myocardium itself the branches break up into fine fibres called the Purkinje fibres. Madhero88 (2009) Electrical_conduction_system https://commons.wikimedia.org/wiki/File:Electrical_conduction_system_of_ the_heart.svg?uselang=en-gb Lynch (2006) Heart right anatomy http://commons.wikimedia.org/wiki/File:Heart_right_anatomy.jpg?uselang= en-gb 4 Responding to Ill Health NURS08046 – Life Science Notes Thus from the AV node the impulse passes down into the wall of muscle tissue between the two ventricles and then passes throughout the walls of both ventricles, causing them to contract. As a result of this arrangement, the beating of the four chambers of the heart is co-ordinated, the two atria contracting first, followed by the two ventricles. It should be appreciated that the fine cellular structure of the heart is also important in the conduction of the impulse that initiates the heartbeat: the cardiac muscle cells of the wall of the heart have a unique structure in that many of them are branched so that they together form an interconnected network. Thus, they are able to act as a single functional unit. It is important to appreciate that all of the cells of the conducting system and of the heart muscle itself can spontaneously depolarise and initiate a heartbeat. However, it is the cells of the SA node that do this more quickly than any other part and thus set the rhythm of the heartbeat. Hence, the SA node is known as the ‘pacemaker’ of the heart and the rhythm of beating that originates here is referred to as ‘sinus rhythm’. Finally, it should be appreciated that no external stimulus needed to initiate or co-ordinate the heartbeat - the heart is said to show ‘autorhythmicity’ – and that the factors (e.g. some hormones and nervous inputs), that regulate the heart rate simply modify this endogenous rhythm. THE CARDIAC CYCLE Defined as the events of one heartbeat. In each heartbeat, the atria and the ventricles each contract and then relax in co-ordinated fashion to ensure that the heart pumps in a correct and coordinated manner and thus that the body’s circulatory requirements are met. Two terms need to be defined: systole - contraction diastole - relaxation The cardiac cycle can be considered in a number of ways; it will be considered here as consisting of four parts. It is very important to notice that the events of this cycle depend crucially on heart structure (especially the location and orientation of the valves within the heart) and the pressure of blood in different heart chambers and the blood vessels associated with the heart at different times. Clearly, as it is a cycle, it doesn’t really matter at which point a description of the cardiac cycle begins. Commonly, and for ease of understanding, the point at which all the chambers of the heart are relaxing (in diastole) and the ventricles of the heart are filling passively is chosen as the starting point: 5 Responding to Ill Health NURS08046 – Life Science Notes OpenStax College ((2013) Phases_of_the_Cardiac_Cycle https://commons.wikimedia.org/wiki/File:2027_Phases_of_the_Cardiac_Cyc le.jpg?uselang=en-gb=en-gb i) complete diastole -passive ventricular filling blood enters right & left atria from pulmonary and systemic veins respectively and then pours passively through open atrioventricular valves into the respective ventricle. 70% of ventricular filling occurs atria and ventricles in diastole atrioventricular valves open, semilunar valves shut ii) atrial systole sino-atrial node discharges and thus causes atrial depolarisation and contraction. remaining 30% of ventricular filling achieved atria in systole; ventricles in diastole atrioventricular valves open; semilunar values shut both atrial and ventricular pressures rise iii) ventricular systole impulse for heartbeat passes to ventricular myocardium from atrioventricular node and thus causes ventricular depolarisation and contraction ventricular pressure quickly rises, causing atrioventricular valves to shut when ventricular pressures exceed blood pressure in systemic and pulmonary arteries, semilunar valves open and blood is expelled into these arteries atria in diastole – filling passively; ventricles in systole 6 Responding to Ill Health NURS08046 – Life Science Notes - ventricular pressure rises dramatically; atrial pressure rises gradually iv) ventricular diastole ventricles relax; pressure in ventricles falls and, when ventricular pressures are lower than pressures in systemic and pulmonary arteries, semilunar valves shut when ventricular pressure falls below atrial pressure atrioventricular valves open and passive ventricular filling occurs atria in diastole; ventricles in diastole atrioventricular valves shut, then open; semilunar valves open, then shut ventricular pressure falls dramatically atrial pressure falls slightly. TIMINGS IN CARDIAC CYCLE The heart typically beats about 75 times per minute at rest; thus, each cardiac cycle takes approximately 0.8 secs to complete. The different parts of the cycle occupy the following periods: atrial systole – 0.1 sec ventricular systole – 0.3 sec atrial and ventricular diastole (relaxation period) – 0.4 sec It is the relaxation period is shortened if the heart rate is required to increase (e.g. in exercise). THE ELECTROCARDIOGRAM (ECG) The generation of the electrical impulse that initiates the heartbeat and the propagation of this impulse through the heart produces electrical activity that can be detected on the body surface using sensitive electrodes. The electrocardiogram (ECG) is a recording of this electrical activity and is thus a record of the changes in electrical activity in different parts of the heart during the cardiac cycle. Anthony Atkielski (2007) SinusRhythmLabels https://commons.wikimedia.org/wiki/File:SinusRhythmLabels.svg?uselang=en-gb There are three clearly recognisable waves/groups of waves in a typical ECG recording, each associated with a particular phase of the cardiac cycle: i) P wave small upward wave related to atrial depolarisation 7 Responding to Ill Health NURS08046 – Life Science Notes ii) QRS complex small downward (Q), very large upward (R), small downward (S) wave but classed as a single “event” related to ventricular depolarisation masks activity related to atrial repolarisation iii) T wave moderate upward wave related to ventricular repolarisation Appearances of some waves and lengths of intervals between waves is altered in some disease states: e.g. enlarged Q wave may indicate a myocardial infarction (MI). ECG can therefore be of great diagnostic value. And, as it is easy to carry out, it is widely used clinically. CORONARY CIRCULATION Like any living tissue, the heart muscle itself requires an adequate blood supply. In fact, as the heart muscle is one of the most consistently active tissues in the body, the heart receives a proportionately greater blood supply than might be expected from its size – it receives about 5% of the total output from the left ventricle. Two large vessels - the left and right coronary arteries – supply the heart muscle with oxygenated blood. The left coronary artery is greater in diameter and supplies the left ventricle, whereas the right coronary artery supplies the right side including the important conducting tissues, the SA and AV nodes. Blausen Medical Communications, Inc. (2013) Blausen 0260 CoronaryVessels Anterior https://commons.wikimedia.org/wiki/File:Blausen_0260_CoronaryVessels_Anterior.p ng Both the coronary arteries arise from the aorta, just above its origin from the left ventricle. 8 Responding to Ill Health NURS08046 – Life Science Notes The heart also requires a venous drainage which takes deoxygenated blood away from the muscle. A network of cardiac veins drain blood away from the myocardial muscle tissue and eventually all drain into the coronary sinus – a large vein at the back of the heart – that in turn drains into the right atrium where the deoxygenated blood that it contains joins with deoxygenated blood from the rest of the body and enters the pulmonary circulation. When the heart muscle is contracting, the coronary vessels are compressed and thus blood cannot easily pass through them. The coronary vessels are only open when the muscle is at rest (i.e. during diastole), and thus it is in the relaxation period of the cardiac cycle that blood moves through these vessels. When the heart rate increases, e.g. exercise, then the period of diastole shortens thus tending to decrease blood flow. This is in direct conflict with the needs of the heart, as, like any other muscle, it requires more blood as its workload increases. Despite this, in fit young healthy individuals, the cardiac output can be increased by up to five fold (5 L/min to 25 L/min) and heart rate from ~ 70 b/min to 180 b/min. In other words, the heart muscle receives enough blood during diastole to “see it through” systole even when heart rate is high (short diastole). Blood Pressure Blood pressure is defined as the pressure exerted by the blood on the wall of a blood vessel. It is generated by contraction of the ventricles. Blood pressure varies greatly in different parts of the cardiovascular system (CVS) – in both the pulmonary and systemic circulations, it is highest in those vessels that are closest to the heart (pulmonary trunk and aorta respectively) and falls progressively as the distance from the heart increases. Thus, in the veins it is very low indeed. Typically, in clinical usage, unless otherwise specified, the term “blood pressure” refers to the pressure in the large arteries of the systemic circulation. The blood pressure in the large arteries is not constant but pulsatile, reflecting the alternate contraction (systole) and relaxation (diastole) of the ventricles. The two extremes of the pulsatile blood pressure measured in the larger arteries are referred to as the systolic and diastolic pressures, the systolic clearly being the higher. Blood Pressure Values Typical values for these two pressures are 120 mmHg for the systolic and 80 mmHg for the diastolic pressures in young men, 110 mmHg and 70 mmHg for the same pressures respectively in young women. Blood pressure of a typical young male would be written as: 120/80 mmHg, that of a typical young female as: 110/70 mmHg. (mmHg stands for “millimeters of mercury”) There is, however, a homeostatic range of blood pressure which is perfectly compatible with health; the normal homeostatic ranges for the systolic and diastolic pressures are as follows: 9 Responding to Ill Health NURS08046 – Life Science Notes systolic: diastolic: 90 – 139 mmHg 60 – 89 mmHg. A blood pressure in which both the systolic and diastolic pressures fall within these ranges is described as being normotensive. If the systolic pressure is greater than 139 mmHg and/or the diastolic pressure is greater than 89 mmHg, the subject is said to be hypertensive. If the systolic pressure is lower than 90 mmHg and/or the diastolic is lower than 60 mmHg, the subject is said to be hypotensive. Both hypotension and hypertension are potentially very dangerous conditions, the former because it may bring about an inadequate blood supply to vital organs, especially the brain, the latter because it contributes to blood vessel damage, which can, in turn, together with the high blood pressure, bring about vessel rupture with consequent severe haemorrhaging and interruption of blood supply to tissues. Indeed, in many cases of stroke and heart attack, patients are found to be hypertensive with resultant badly damaged vessels. Note that blood pressure gradually rises as age increases – this is probably due to changes in the blood vessels that occur with increasing age. Determinants of Blood Pressure The precise value of the blood pressure at any one time depends on two main factors: a) b) cardiac output - acts to push blood through the blood vessels peripheral resistance - acts to oppose blood flow through the blood vessels If the value of one (or more) of these factors increases, then blood pressure itself will increase; similarly, if the value of one (or more) of these factors decreases, then blood pressure itself will decrease. Note, long-term regulation of blood pressure also depends on blood volume, which is regulated by the kidney. Cardiac Output This is defined as the volume of blood ejected from the left (or right) ventricle into the aorta (or pulmonary trunk) per minute. It is calculated as follows: cardiac output = heart rate (beats min-1) x stroke volume (ml) The stroke volume is defined as the amount of blood ejected by the relevant ventricle at each systole i.e. how volume of blood that leaves one ventricle following one contraction. At rest, typically, the heart rate is 75 beats min-1 and the stroke volume of each ventricle is about 70 ml. Thus the cardiac output into either the pulmonary or systemic circulation is: 75 x 70 ml/min = 5250ml/min (5.25l/min). 10 Responding to Ill Health NURS08046 – Life Science Notes Both the heart rate and the stroke volume can be readily and rapidly changed (e.g. during exercise), thus causing a change in the cardiac output. Hence, variations in the cardiac output can be used as a rapid mechanism to control blood pressure. Venous return (the amount of blood returning to the heart from the veins) is also important in maintaining blood pressure: cardiac output is largely determined by the degree of filling of the ventricles during diastole and this, in turn, depends on adequate venous return. A reduced venous return will lead to reduced ventricular filling, thus to a reduced cardiac output and reduced blood pressure. Peripheral Resistance This is defined as the sum total of all the vascular resistances that oppose blood flow. Resistance to flow depends on various factors (blood vessel length, blood viscosity), but is only regulated by vessel radius: the smaller the vessel radius, the greater the resistance to flow – this can readily be varied by altering the degree of muscular contraction of the vascular smooth muscle – and thus is the major mechanism by which variation in peripheral resistance is brought about. Most resistance to blood flow occurs in the arterioles and thus these vessels have a very important role in controlling the peripheral resistance and thus, in turn, blood pressure. The peripheral resistance too can be readily and rapidly changed, mainly by varying the diameter of blood vessels (e.g. of the arterioles). Hence, variations in the peripheral resistance can be used as a rapid mechanism to control blood pressure. Factors that Regulate Blood Pressure Many factors can affect the blood pressure. They can act on the heart (to vary cardiac output) and/or on the blood vessels (to vary peripheral resistance) and, if they act on both, will act in complementary ways. They include: i) The autonomic nervous system (ANS): the sympathetic division of the ANS acts to increase blood pressure by increasing both the cardiac output and the peripheral resistance. The parasympathetic division acts to decrease blood pressure by reducing cardiac output (no effect on peripheral resistance). ii) Hormones. e.g. adrenalin – causes an increase in blood pressure by both increasing cardiac output and peripheral resistance. Some endocrine (hormone) systems affect the blood volume and thus can affect blood pressure. They include the renin/angiotensin system and anti-diuretic hormone (ADH). iii) Temperature - increased body temperature (e.g. fever) increases blood pressure as it causes an increase in the heart rate and thus cardiac output decreased body temperature has the opposite effect iv) Emotions – effects from higher brain centres. Emotions (e.g. anger, fear, anxiety) cause an increase in blood pressure by increasing both the cardiac output and peripheral resistance. Others (e.g. grief, depression) cause a decrease in blood pressure by decreasing both the cardiac output and peripheral resistance. 11 Responding to Ill Health NURS08046 – Life Science Notes THE ENDOCRINE SYSTEM Introduction The endocrine system consists of glands widely separated from each other with no direct links (Figure below). Endocrine glands consist of secretory cells surrounded by an extensive network of capillaries that allows effective distribution of hormones from the secretory cells into the bloodstream. The hormone is then carried in the bloodstream to target tissues and organs where they affect cellular growth and metabolism. Homeostasis of the internal environment of the body is maintained by a combination of the autonomic nervous system (see CNS section) and the endocrine system. The autonomic nervous system can rapidly change conditions while hormones of the endocrine system evoke slower more precise adjustments. United States Government (2005) The endocrine system http://commons.wikimedia.org/wiki/Endocrine_syst em?uselang=engb#mediaviewer/File:Illu_endocrine_system.jpg Hormone action Hormones function by binding to hormone-specific receptors in the target cell. Binding of the hormone and receptor acts as a switch, able to alter the chemical or metabolic reactions inside the cell. Receptors may be found on the cell surface or inside the cell. 12 Responding to Ill Health NURS08046 – Life Science Notes The Endocrine Pancreas and Control of Blood Glucose The pancreas is a large dual gland which has both an exocrine (ducted) and endocrine (ductless) components. It consists of a broad head, a body and a tail. The head lies in the curve of the duodenum. The exocrine role of the pancreas involves the generation of digestive juices that enter the small intestine via the pancreatic duct.) There are, however, groups of endocrine cells distributed throughout the gland, known as the islets of Langerhans, which secrete hormones which control the concentration of blood glucose BruceBlaus (2013) PancreaticTissue https://commons.wikimedia.org/wiki/ File:Blausen_0701_PancreaticTissue. png There are no ducts associated with these cells, and hormones are secreted directly into the bloodstream and circulate around the body There are two main types of cells within the pancreatic islets (alpha) cells secrete glucagon (beta) cells secrete insulin There are other hormones which can affect blood glucose levels – but these will not concern us here. 13 Responding to Ill Health NURS08046 – Life Science Notes The actions of glucagon and insulin are antagonistic, that is the hormones affect blood glucose levels in opposite directions. This often occurs when tight control is needed. glucagon increases blood glucose levels insulin decreases blood glucose levels Some of the main ways in which this is achieved are summarised in the table below: Insulin lowers blood glucose Glucagon increases blood glucose Stimulates uptake of glucose by cells Facilitates release of glucose into blood Increases conversion of glucose to glycogen (glycogenesis) Decrease conversion of glucose to glycogen (glycogenesis) Decreases breakdown of glycogen to glucose (glycogenolysis) Stimulates glycogenolysis Prevents breakdown of protein & fat and formation of new sugar from e.g. protein( gluconeogenesis) Stimulates gluconeogenesis Regulation of Pancreatic Hormones Pancreatic hormone secretion is regulated directly by blood glucose levels. Secretion of insulin is stimulated by increased blood glucose levels Secretion of glucagon is stimulated by decreased blood glucose levels So although insulin and glucagon have opposing effects on blood glucose concentration they work together to ensure that blood glucose levels do not become too high (hyperglycaemia) or too low (hypoglycaemia). Both conditions are detrimental to health. Disorders of pancreatic islets Diabetes mellitus is a very common disorder of the pancreatic islets which results from a malfunction of the hormonal control of glucose metabolism. Briefly, there are two variants of this disorder: Type I or insulin dependent diabetes mellitus (IDDM) This occurs mainly in children and young people, and is characterised by the deficiency or absence of insulin, because of the destruction of cells in the pancreatic islets (often resulting from an autoimmune reaction). 14 Responding to Ill Health NURS08046 – Life Science Notes Type II or non-insulin dependent diabetes (NIDDM) The most common form of diabetes, accounting for about 90% of cases, NIDDM usually occurs in older people many of whom are obese. The causes of NIDDM are complex and multifactorial, but include genetic and lifestyle (poor diet and obesity) components. In NIDDM, insulin levels may be normal or even unusually high. Insulin resistance means that insulin binding to receptors does not invoke the normal changes inside target cells, and responses such as glucose uptake into cells are therefore not stimulated properly. A similar condition to NIDDM arises in 2-5% of pregnancies, called Gestational Diabetes Mellitus (GDM). GDM appears to be characterised by insulin resistance, which in this case may be due to blocking of insulin by pregnancy-related hormones. The condition often disappears following delivery, but approximately 20-50% of women who suffer GDM develop NIDDM in later life. Pituitary Gland The pituitary gland and hypothalamus act as a unit regulating the activity of most of the other endocrine glands. The pituitary is found behind the hypothalamus in the brain and is the size of a pea. It has 3 distinct parts; anterior pituitary, posterior pituitary and intermediate lobe. Role of the anterior pituitary Hormones from the anterior pituitary control secretion by other endocrine glands while others have direct effects on target tissues. EXAMPLES Growth hormone (GH) – most abundant hormone synthesised by the anterior pituitary. Stimulates cell growth and division particularly bone and skeletal muscles. Prolactin – stimulates milk production. Thyroid stimulating hormone (TSH) – stimulates growth and activity of the thyroid gland. Adrenocorticotrophic hormone (ACTH) - stimulates production and release of hormones from the adrenal glands. 15 Responding to Ill Health NURS08046 – Life Science Notes Adrenal Glands The two adrenal glands lie at the upper end of each kidney and are about 4cm long and 3cm wide. They are each composed of two portions, the outer adrenal cortex and the inner adrenal medulla. EEOC (2006) adrenal_gland https://commons.wikimedia.org/wiki/Fil e:Illu_adrenal_gland.jpg Adrenal Cortex The adrenal cortex produces 3 groups of steroid based hormones from cholesterol which are collectively known as adrenocorticoids. 1. Glucocorticoids; e.g. Cortisol (hydrocortisone) which regulates metabolism and response to stress. 2. Mineralocorticoids; e.g. Aldosterone which regulates osmotic balance. 3. Sex hormones; e.g. Androgens - insignificant compared to those produced by testes/ovaries in adulthood. Adrenal Medulla Stimulated by its extensive sympathetic nerve supply to produce adrenaline and noradrenaline. Released into the blood stream from the adrenal medulla after stimulation of the sympathetic nervous system, these two hormones potentiate the fight or flight response by; Increasing heart rate Increasing blood pressure Diverting blood to essential organs e.g. heart, brain and skeletal muscles Stimulating glycogenolysis ( conversion of glycogen to glucose) 16 Responding to Ill Health NURS08046 – Life Science Notes Adrenal disorders Hypo and hyper secretion occurs including: Addison’s disease – hyposecretion of glucocorticoids resulting in serious weakness and collapse under stress. Cushings syndrome-hypersecretion of glucocorticoids resulting in oedema (moon face), hyperglycaemia, hypertension, immunosuppression. N.B these are also side effects of steroid treatments which involve giving glucocortico steroids for their anti-inflammatory effects. Cushing’s disease produces the same syndrome but is caused by the pituitary gland producing excess ACTH (corticotropin). Patients on steroids produce little ACTH due to negative feedback and therefore their own adrenal glands are suppressed. Stopping steroid treatment suddenly leads to adrenal insufficiency and susceptibility to collapse. Such patients should carry ID so that in the event of an accident steroids can be given or even increased to cope with the stress. Conn’s syndrome-hypersecretion of aldosterone leading to increased plasma sodium, water retention, leading to hypertension and potassium depletion. Phaeochromocytoma-is a tumour producing excess adrenaline or noradrenaline leading to hypertension, tachycardia, anxiety etc. Kidney Hormones that influence the function of the kidney, specifically, anti-diuretic hormone (ADH) and renin-angiotensin- aldosterone system are discussed in the next section. The kidneys also produce the hormone erythropoietin when plasma oxygen levels are low and the erythropoietin stimulates red bone marrow to produce erythrocytes (RBC). Athletes train at altitude where oxygen is low deliberately to stimulate erythropoietin EPO this is not cheating but injecting EPO is. Renal failure patients become anaemic which used to require blood transfusion before EPO became widely available for injection 17 Responding to Ill Health NURS08046 – Life Science Notes THE URINARY SYSTEM INTRODUCTION The focus in this second year module will be on physiology of the urinary/renal system. Knowledge of the anatomy and basic functions of the urinary and endocrine systems from previous modules will be assumed. Renal Disease will be dealt with in the associated nursing modules but students should already expect that people with renal disease may develop problems such as: Fluid balance problems, Waste accumulation such as uraemia (excess urea in blood) FUNDAMENTAL CONCEPTS You need to understand basic principles of fluid movement in the body especially osmosis, diffusion, filtration and active transport. You need to understand the concept of concentration and units such as mmol /l (pronounced milli-molar). See early chapters of Life Science text books OVERVIEW OF URINARY SYSTEM FUNCTION The urinary system aids HOMEOSTASIS principally by EXCRETING URINE Water (95% of volume) Nitrogenous Waste: mainly UREA, a little URIC ACID. Ions (salts/electrolytes), such as: Na+, Ca++, K+, Mg++, NH4+, H+, Cl-, PO43-, HCO3Functions of the Urinary System Excretion of nitrogenous wastes. Regulation of blood pH (re. H+) Regulation of Blood Electrolyte balance Regulation of Blood Volume Regulation of Blood Pressure: Renin from kidney also increases BP Production of hormones: Calcitriol which regulates calcium homeostasis and erythropoietin which stimulates red blood cell production. 18 Responding to Ill Health NURS08046 – Life Science Notes Three main processes are involved in the production of urine: 1) FILTRATION 2) SELECTIVE REABSORPTION 3) TUBULAR SECRETION Once formed the urine remains unchanged in composition as it is excreted from the nephron in the kidney to travel to the urinary bladder. Madhero88 (2010) Physiology of Nephron https://commons.wikim edia.org/wiki/File:Physi ology_of_Nephron.png A primary function of the urinary system is the excretion of nitrogenous wastes Protein molecules are made from amino acids which contain Nitrogen Atoms (N) in addition to Carbon, Oxygen and Hydrogen. When the body catabolises (breaks-down) amino acids in addition to CO2 and H2O nitrogenous waste, such as AMMONIA (NH3), is produced. This process is known as DEAMINATION and the ammonia is highly toxic and is converted in the liver to UREA (which is less toxic, but still must be removed. ) The urea from the liver enters the blood, is carried to the kidneys where it is filtered into the glomerular filtrate. Because urea is not selectively reabsorbed most urea passes out into the urine. Patients with advanced Liver disease cannot convert toxic ammonia into safer urea and may suffer from encephalopathy ( a type of brain dysfunction) due to build-up of Ammonia. Patients with advanced Kidney disease can make Urea but cannot excrete it so may die from uraemia (urea in the blood) due to build up of urea. Other nitrogenous wastes include URIC ACID which comes from the breakdown of nucleic acids like DNA. Excess uric acid in blood causes Gout which may be associated with rapid cell destruction in malignancy or failure to excrete uric acid as in kidney disease. 19 Responding to Ill Health NURS08046 – Life Science Notes ANATOMY OF URINARY SYSTEM (see year one notes) Blood Supply Each kidney receives a very profuse blood supply approx. 1200ml/min amounting to one quarter of cardiac output via the RENAL ARTERY which branches as it passes through the pelvis and the medulla on the way to the cortex. In the cortex the arteries form many GLOMERULI, tufts of capillaries where filtration of blood takes place. The blood then flows into the PERITUBULAR CAPILLARIES (which surround the nephrons) and is drained into the RENAL VEIN. OpenStax College (2013) Blood Flow in the Kidneys https://commons.wikimedia.org/wiki/File:2612_Blood_Fl ow_in_the_Kidneys.jpg 20 Responding to Ill Health NURS08046 – Life Science Notes Nephron Structure & Function Each kidney contains around one million nephrons. The nephron, a tubular structure, is responsible for the filtration of the blood to form urine. Nephrons are comprised of 5 sections: 1) Bowman’s capsule Blood is forced through the glomerular capillaries under relatively high pressure. The porous walls of capillaries allow blood plasma (minus the plasma proteins) to pass out of the capillary into the Bowman’s capsule. The FORMED ELEMENTS and plasma proteins remain within the capillary. The fluid that enters the Bowman’s capsule is now called GLOMERULAR FILTRATE or ULTRAFILTRATE. GLOMERULAR CAPILLARY PRESSURE is approx. 55mmHg because of the arrangement of the afferent and efferent arterioles. Henry Gray (1918) Gray1130 https://commons.wikimedia.org/wiki/File:G ray1130.svg + UWS Staff (2015) humanphysiology2011.wikispaces.com The above diagram demonstrates some of the opposing forces that are found in Bowman’s capsule – which ultimately lead to formation of glomerular filtrate. . Glomerular capillaries have an extremely high pressure which would normally burst except they are covered by a layer of specialised CAPSULAR EPITHELIUM. The specialised PODOCYTE cells of the capsular epithelium act like an interlocking mesh with slits to allow filtration of the blood. Cells and plasma proteins are too big to go through slits and stay in the blood stream do not pass into the capsule. The capillary walls also have holes, or fenestrations, allowing small plasma components through. Between these layers is a basement membrane, which repels proteins due to their negative charge. 21 Responding to Ill Health NURS08046 – Life Science Notes Damage to the capsular epithelium as caused by hypertension or poisons or infection results in wider “holes” in the filter so plasma proteins pass into the filtrate and are detectable as proteinuria during urine testing. Therefore in normal health glomerular filtrate is virtually identical to plasma except lacking plasma proteins The GLOMERULAR FILTRATION RATE (GFR) is approx. 120ml per minute of which 119ml are reabsorbed. i.e. most of the fluid that is found in the glomerulus will be reabsorbed back into the blood. Reabsorption occurs throughout the remainder of the nephron. The reabsorbed materials are and returned to the blood in the PERITUBULAR CAPILLARIES which surround the nephron. Reabsorption may be passive or active. PASSIVE PROCESSES do not require extra energy. Passive processes depend on concentration or pressure gradients. Fluid moves from higher pressure to lower pressure and substances diffuse from higher concentration to lower concentration. Osmosis is diffusion of water. ACTIVE PROCESSES involves cells “pumping” substances against a concentration gradient. Active transport requires the expenditure of energy. Glucose is actively transported from filtrate back to plasma and sodium is usually pumped out of plasma Note: DIFFUSION is the movement of a substance from an area of high concentration to an area of lower concentration until equilibrium is reached. OSMOSIS is the movement of WATER from an area of HIGH WATER CONCENTRATION (low solute concentration) to an area of LOW WATER CONCENTRATION (high solute concentration) through a SEMI PERMEABLE MEMBRANE until equilibrium is reached. PLASMA PROTEINS play a big role in OSMOSIS because they are not normally filtered through capillaries and therefore the solute concentration is higher in plasma than filtrate and Water concentration is lower in plasma N.B. The concentration of solutes such as glucose and sodium is the same in the blood plasma as it is in the filtrate in the capsule. This means that due to plasma proteins the PLASMA OSMOTIC PRESSURE is higher by 30mmHg and acts to resist filtration as does the pressure of the HYDROSTATIS PRESSURE of the FILTRATE 15mmHg in the capsule. N.B Osmotic pressure is sometime a bit confusing as a high osmotic pressure pulls water. NET FILTRATION PRESSURE = GLOMERULAR BLOOD BRESSURE – (PLASMA OSMOTIC PRESSURE + CAPSULAR FILTRATE PRESSURE) = 10mmHg 22 Responding to Ill Health NURS08046 – Life Science Notes A similar process occurs in TISSUE FLUID which is formed by filtration of plasma through ordinary capillaries into tissue space. Without the plasma proteins exerting an osmotic pressure pulling water back from the tissue spaces the fluid accumulate in the tissue and low plasma protein levels are one cause of OEDEMA. This is seen in starvation when children have low protein intake and their livers have no amino acids to make into plasma protein, liver disease when the liver cannot make plasma protein and nephritic syndrome when the kidney is damaged and allows plasma proteins to be lost in the urine. 2) Proximal Convoluted Tubule (PCT) SELECTIVE REABSORPTION of the filtrate commences here! Useful substances are taken out of the filtrate and returned to the peritubular blood capillaries which surround the PCT. By the end of the PCT about 65% of filtered salt (NaCl) and water are reabsorbed. Most filtered nutrients (amino acids, glucose) are also reabsorbed in the PCT. The substances not reabsorbed such as UREA now pass into the loop of Henle. Glucose and salt are actively transported from the filtrate into the plasma by the cells of the PCT which use a lot of energy. As plasma moves along the peritubular capillaries the plasma concentration of Glucose and salt (solutes) get higher and plasma water concentration in gets lower than that in the filtrate in the PCT. As such Water moves passively by OSMOSIS from the PCT to Plasma in PERITUBULAR CAPILLARIES. In DIABETES MELLITUS so much glucose is filtered into the PCT that the PCT cannot reabsorb all the glucose back into the plasma. This is called exceeding the RENAL THRESHOLD and glucose is passed out in urine (GLYCOSURIA). This means that less water is reabsorbed by osmosis and hence more water passes out in the urine. This POLYURIA is due to OSMOTIC DIURESIS. In fact the word diabetes means fountain and mellitus means honey. So people with diabetes mellitus pass lots of sweet urine. 3) Loop of Henle The loop of Henle is where a further 20%, of the salt and water are reabsorbed from the filtrate. The sodium reabsorbed from the loop of Henle makes the solute concentration in the medulla high and this helps water reabsorption by osmosis from the adjacent collecting ducts. 4) Distal Convoluted Tubule (DCT) Up until now the reabsorption of Na+ and Cl- was fixed i.e. the amount absorbed was independent of the body’s needs. The DCT is responsible for the final absorption of Na+ but it can now be regulated by the hormone ALDOSTERONE. Aldosterone is secreted by the ADRENAL GLANDS (which lie on top of the kidneys). Aldosterone promotes the REABSORPTION of Na+ by the DCT by an active transport mechanism in exchange for secretion of K+. 23 Responding to Ill Health NURS08046 – Life Science Notes ALDOSTERONE secretion is regulated by the kidneys. When Blood Pressure, Blood Volume or Blood Sodium are low the kidney secretes an enzyme called RENIN which activates an inactive plasma protein ANGIOTENSINOGEN to become ANGIOTENSIN 1. ANGIOTENSIN CONVERTING ENZYME found in lungs and other tissues convert angiotensisin 1 to ANGIOTENSIN 2. Collectively this is known as the reninangiotensin system and this plays a vital role in the control of blood pressure. UWS Staff (2015) ANGIOTENSIN 2 is a powerful vasoconstrictor leading to raised blood pressure and also stimulates aldosterone secretion from the adrenal cortex. Increased sodium reabsorption in the DCT leads to increased water reabsorption by osmosis. The combined effects of all this renin angiotensin action increases Blood Pressure. Tubular Secretion also occurs in the DCT which is the active “pumping” of unwanted substances into the urine that would otherwise stay in the blood. Many drugs and toxins are excreted this way Hydrogen (acid) ions (H+) and are removed from the plasma by tubular secretion when the pH of the blood is too low. At the same time Bicarbonate ions HCO3- (alkaline) are reabsorbed into plasma to help neutralise acidity. People with kidney disease have difficulty maintaining the pH of the plasma between 7.35 and 7.5 and often are have acidosis. Summary of events of aldosterone: if the body is short of Na+ then aldosterone blood levels increase which promotes reabsorption of Na + and therefore all or most the Na+ entering the DCT will be absorbed. If the body has too much Na+, then aldosterone levels drop and the absorption of Na + is incomplete and Na+ is lost in the urine. 24 Responding to Ill Health NURS08046 – Life Science Notes 5) Collecting Duct (CD) - receives what is left of the filtrate from the DCTs of many nephrons. The CD passes down through the medulla and drains into the renal pelvis. The CD is responsible for the final reabsorption of water, and like the DCT, this process is regulated by a hormone called ANTIDIURETIC HORMONE (ADH), produce by the POSTERIOR PITUITARY GLAND. ADH works by making the gaps bigger between cells lining the collecting duct making the duct more porous with more osmosis of water from the collecting duct into the peritubular capillaries of the medulla and hence less water leaves the body in the urine. The urine may thus be more concentrated. People lacking sufficient ADH have DIABETES INSIPIDUS a condition in which results in high output of dilute (insipid) urine. Summary of events of ADH: If you drink too much fluid (over-hydrated) - the levels of ADH drop, less water is reabsorbed and large volumes of dilute urine are produced. Conversely, if dehydrated, the body produces - large amounts of ADH and maximal absorption of water occurs. HYPERTENSION may be caused by excess ADH increasing blood volume and/or by excess Aldosterone which causes sodium retention. Hypertension is a both a cause and effect of renal disease and patients with kidney disease may enter a vicious cycle of positive feedback called MALIGNANT HYPERTENSION. Urine Some water has to be lost in the urine to excrete nitrogenous wastes. The minimal daily production of urine is about 1.5 litres. Once the filtrate leaves the collecting duct it is now urine and its composition cannot be altered. In addition to urea, creatinine, potassium, and ammonia, other typical solutes found in the urine include: uric acid, sodium, chloride, magnesium and calcium ions. If disease alters body metabolism or kidney function, traces of other substances not normally present, may appear in the urine e.g. ketone bodies in diabetes. A patient who is drinking normal amounts will produce approx. 1ml of urine per minute (60ml per hour) and therefore approx. 1500ml a day. Any UWS producing less than 30ml per hour is a cause for concern. A healthy bladder holds 300ml to 400ml or more so unless drinking a lot a person should only need to pass urine four or five times a day. Passing urine unnecessarily can lead to reduced bladder capacity and result in frequency and urgency. 25 Responding to Ill Health NURS08046 – Life Science Notes WATER AND ELECTROLYTE BALANCE Around 60% of the average adult male body weight is due to water. This water is distributed throughout the body, inside and outside the cells. Dissolved in the water are many electrolytes (salts, ions) all of which have an important role in contributing to the normal osmotic pressure of body fluids. In addition, most electrolytes play a major role in regulating essential body processes (more details below) UWS Staff (2015) BODY FLUID COMPARTMENTS The Total Body Water (TBW) of a 70Kg male will be around 42 litres (i.e. 60% of 70Kg). Women and obese men have a lower proportion of water because of their tendency to have a body composition with more fat. A 70kg woman may have a 55% body water 38 litres. This is one reason why the recommended alcohol limit has fewer units for women. Intracellular Fluid (ICF). Most of the body water is found in the cytoplasm of the body cells – roughly 28 litres. Extracellular Fluid (ECF). The remaining water (roughly 14 litres) is found outside the cells and is distributed as: -interstitial Fluid (Tissue Fluid) - the fluid in the spaces between the cells (about 10- 11 litres) -Blood Plasma - contained within the circulatory system (about 3 - 4 litres) Please note that there is relatively free movement of water between the fluid compartments i.e. if you were able to trace the movement of an individual water molecule it could be found “moving” around the body in and out of any of these compartments. WATER BALANCE In order to avoid overhydration or dehydration individuals must stay in water balance. Normally water loss and water gain are both approximately equal to 2500ml. 26 Responding to Ill Health NURS08046 – Life Science Notes Water Gain Water Loss Metabolism (200 ml) Food (700 ml) Drinking (1600 ml) Faeces (100 ml), Exhaled (300 ml) Evaporation from skin (600 ml) Urine (1500 ml) Factors leading to Dehydration are (a few examples) inadequate fluid intake, sweating, diabetes mellitus or diabetes insipidus can increase urinary water loss substantially, diarrhoea - the absorption of water by the large intestine is impaired, diuretics - may be prescribed to reduce systemic or pulmonary oedema or high blood pressure Factors leading to Overhydration: - excessive fluid intake. Especially if taken with high levels of salt (NaCl). - insufficient excretion of water by the kidney. Could be due to partial kidney failure or overproduction of ADH by the pituitary gland. ELECTROLYTE BALANCE Although there are many different electrolytes dissolved in the body fluids, only Sodium (Na+), Potassium (K+) and Calcium (Ca2+) will be considered for the moment. Sodium Correct ECF levels (around 140 -150 millimoles per litre) of sodium are essential for normal nerve and muscle function and for regulating total ECF (including plasma) volume. If too much sodium is retained in the body then extra water is also retained in order to keep the osmotic pressure of the body fluids correct. As a result ECF (and plasma) volume increases leading to an increase in blood pressure (hypertension). Conversely, if there is too little sodium body fluids are lost, again to maintain the correct the osmotic pressure. As a result ECF volume decreases and blood pressure drops (hypotension). Potassium The ECF levels of potassium are usually very low (between 3.5 and 5.0 millimoles per litre). Most of the potassium is inside the body cells and, like sodium, is essential for normal nerve and muscle function. The heart is particularly susceptible to changes in potassium levels and potassium chloride injections are used in judicial executions in USA. Calcium Vitamin D is necessary for Calcium absorption. Most of the body calcium is stored in bone, however, it is found both in the ECF and ICF and plays many important roles: involved in the contraction process of muscles, nerve function. Hypocalcaemia produces muscle spasms (tetany) due increased excitability of nerve and muscle tissue and can also in the long term lead to demineralisation of bone.Hypercalcaemia reduces excitability of nerve and produces muscle weakness. There is also the risk of kidney stones. Parathyroid hormone (from the parathyroid glands) is mainly responsible for regulating calcium levels 27 Responding to Ill Health NURS08046 – Life Science Notes THE RESPIRATORY SYSTEM Introduction The main function of the respiratory system is to enable oxygen to move from air into the blood and carbon dioxide to move in the opposite direction. It also has other functions, such as the metabolism of some compounds, the filtration of toxic materials from the circulation, and to act as a reservoir for blood. Structure of the respiratory system LadyOfHats (2007) Respiratory_system https://commons.wikimedia.org/wiki/File:Respiratory _system_complete_en.svg The respiratory system (or tract) can be divided into upper and lower parts. The nose, mouth, pharynx and associated structures make up the upper part of the respiratory tract; the larynx, trachea, bronchi and lungs make up the lower part of the tract. 28 Responding to Ill Health NURS08046 – Life Science Notes Function of upper respiratory tract structures The nasal, pharyngeal and laryngeal cavities act to filter, heat and moisten air that passes through it. The membranes of these cavities possess a rich blood supply and can produce copious mucous secretions to trap any impurities. These chambers are also intimately involved in the functions of smell and speech (not to be considered here). At the entrance to the trachea and oesophagus there is a small mobile flap of cartilage called the epiglottis that blocks the trachea during swallowing to prevent food entering the windpipe. Structure of the lungs & the pleura Each lung is divided into lobes made up of segments and then subdivided further into lobules. Each lung is covered on its outer surface by a thin layer of very smooth epithelial tissue called the visceral pleura which turns back on itself to form a layer of parietal pleura which lines the inside of the chest wall. Between these two layers is the pleural space or cavity, which contains some watery (serous) lubricating fluid. This arrangement allows the lungs to expand and deflate inside the chest with very little frictional resistance. OpenStax College (2013) 2313 The Lung Pleurea https://commons.wikimedia.org/wiki/File:2313_The_ Lung_Pleurea.jpg 29 Responding to Ill Health NURS08046 – Life Science Notes The airways and air flow The passages that conduct air into and out of the lungs (the airways) consist of a series of branching tubes which become progressively narrower, shorter and more numerous as they go deeper into the lungs. The trachea is the tube that runs down from the pharynx through the neck into the chest; it is about 10cm long, about 1.8cm in diameter and is made of connective tissue and smooth muscle. It is held open by C-shaped rings of strong cartilage. Like most of the respiratory tract below it, it is lined with ciliated epithelium which can transport mucus and trapped particles upwards to the epiglottis for swallowing. 1. Trachea 2. Mainstream bronchus 3. Lobar bronchus 4. Segmented bronchus 5. Bronchiole 6. Alveolar duct 7. Alveolus United States Government (2006) Illu quiz lung. http://commons.wikimedia.org/wiki/File:Illu_quiz _lung05.jpg The trachea divides within the chest into right and left main (or primary) bronchi. The right bronchus is slightly larger and more vertical than the left; hence, it is the right main bronchus that is more likely to become obstructed by an inhaled foreign body. These bronchi are held open by incomplete rings of cartilage and they divide into the smaller secondary and then tertiary bronchi. This branching continues down to the terminal bronchioles which are the smallest airways without alveoli. The airways down to this point in the respiratory tract take no part in gas exchange and are thus referred to as the anatomical dead space; they together contain about 150 ml of air. The terminal bronchioles divide into respiratory bronchioles which have occasional alveoli budding from their walls. These eventually come to form alveolar ducts which are completely lined with alveoli. 30 Responding to Ill Health NURS08046 – Life Science Notes This alveolated region of the lung where the gas exchange occurs is known as the respiratory zone. The respiratory zone of each lung is about 2.5 to 3 litres in volume. Ventilation INSPIRATION: air only flows from a region of high pressure to one of low pressure. During the intake of air into the lungs (inspiration) the volume of the chest (thoracic) cavity is increased; this reduces the intrathoracic pressure to below that of the external atmosphere and thus air is sucked into the lungs (to the level of the terminal bronchioles). At rest, the increase in the volume of the chest cavity is brought about almost completely by the diaphragm contracting and pulling down - this increases the top-to-bottom dimension of the chest cavity. In situations where an increased respiratory effort is required, other muscles can be used to aid expansion of the chest. These include the external intercostal muscles, which pull the ribs upwards and outwards, thus increasing the transverse dimension of the chest and the volume of the chest cavity and some muscles of the head and neck, such as the scalene muscles that lift the first two ribs, and the sterno(cleido)mastoids which raise the sternum. These latter muscles increase the antero-posterior dimension of the chest and thus again the volume of the chest cavity. At the level of the terminal bronchioles, the cross-sectional area of the airways is so enormous that the speed of airflow becomes very slow. At this point diffusion takes over as the main mechanism of ventilation and the distance to the alveoli (which is very short) is then traversed in about a second. EXPIRATION: the lung contains much elastic tissue and so it returns passively to its initial volume when the inspiratory muscles rest. This passive recoil compresses the air in the lungs to a higher pressure than outside and so air flows out of the lungs - this is the means by which expiration is achieved at rest. During exercise, expiration also becomes an active process. The muscles of the abdominal wall contract, increasing intra-abdominal pressure and pushing the diaphragm upwards. The internal intercostal muscles act to pull the ribs down and inwards. Both these actions cause a decrease in the thoracic volume and thus assist expiration. Factors affecting ventilation Compliance refers to how much effort is required to expand the chest wall and inflate the lungs. The lungs are normally very easy to inflate – they are said to have high compliance. Airway resistance is the resistance posed to airflow by the walls of the airways. This is normally low, and thus the pressure required to move air through the airways is also very small. Surface tension of the alveolar fluid: the surface of the alveoli in contact with the air is lined with a watery liquid to permit gas exchange. This liquid layer should cause the alveoli to collapse due to its surface tension. However some of the alveolar cells secrete a material called surfactant which acts like 31 Responding to Ill Health NURS08046 – Life Science Notes a detergent to dramatically lower the surface tension of the alveolar lining layer and thus prevent alveolar collapse. If surfactant is reduced or absent, then many alveoli collapse on expiration and the effort required to expand the lung at each inspiration is greatly increased. An example of this is respiratory distress syndrome, when premature infants do not produce sufficient surfactant to keep a large proportion of alveoli inflated after expiration. Since normally the lungs have high compliance and airway resistance is low, the energy expenditure associated with normal breathing is low. If, however, something occurs to lower lung compliance (e.g. presence of scar tissue in the lung) and/or to raise airway resistance (e.g. inflammatory swelling of the airway epithelium), then the effort required to maintain an adequate respiratory effort can become considerable. Lung volumes The total capacity of the lungs is about 6 litres. The tidal volume is the volume of air moved into and out of the lungs at each inspiration and expiration; at rest this is about 0.5l. The volumes of air that can be inspired or expired over and above the tidal volume are known as the inspiratory and expiratory reserve volumes respectively. The maximal volume that can be exhaled is known as the vital capacity (about 4.8l.). However, some gas remains in the lungs even after a maximal expiration; this is called the residual volume (about 1.2l) and it represents the air in the anatomical dead space and air remaining in the alveoli to keep them open. The minute volume is the total volume of air inhaled and exhaled each minute and is calculated as follows: minute volume (L/min) = tidal volume(L) x respiratory rate (breaths/min) At rest, the average number of breaths per minute is about 12 and the tidal volume is 0.5l; thus the minute volume is 0.5 x 12 = 6 l/min. However, when the dead space (150ml) is taken into account, only (500-150) x 12 = 4.2l/min is actually fresh air available for gas exchange. Note that the tidal and minute volumes can be increased dramatically if the body’s demand for oxygen increases. The blood-air interface & diffusion Oxygen and carbon dioxide move between air and blood by simple diffusion along their concentration (pressure) gradients, i.e. from an area of high to one of low pressure. The rate of diffusion of a gas across a (tissue) surface is directly proportional to both the area of the surface and to the difference in gas pressure between the two sides, but is inversely proportional to the thickness of the surface. The blood-air barrier in the lungs is very thin and has a total area (considering both lungs together) of around 50 to 100 square metres; it is therefore structurally perfect for gas exchange. The large surface area is achieved by wrapping blood capillaries densely and intimately around the alveoli: 32 Responding to Ill Health NURS08046 – Life Science Notes LadyOfHats (2007) Alveolus diagram https://commons.wikimedia.org/wiki/File:Alve olus_diagram.svg The pressure of O2 in the blood just entering a pulmonary capillary is about 40mm Hg, (5.3kPa) while only 0.3m away in the alveolar air the pressure of O2 is 100mm Hg (13.3Kpa) therefore O2 rapidly flows down this considerable pressure gradient into the blood in the pulmonary capillary. Similarly, the pressure of CO2 in the pulmonary capillary is 44mm Hg (5.9kPa), whilst the pressure of this gas in the alveolar air is 40mm Hg (5.3kPa) thus CO 2 flows down its pressure gradient into the air in the alveolar spaces. If the driving pressure between air and blood is reduced (e.g. at high altitude) or the blood-gas barrier is thickened (e.g. by fibrosis) or overall lung surface area is reduced (e.g. emphysema) then diffusion can be impaired, with possible serious consequences. Control mechanisms and regulation Breathing can be automatic or conscious. Normally, we are unaware of control mechanisms changing our respiratory rate to suit our needs; however, in many activities, such as playing a musical instrument or singing, breathing has to be consciously controlled. As in other systems, the basic principle in the control of respiration is one of negative feedback, and there are three main elements involved: 1. sensors - gather information 2. control unit (in the brain) – analyses the information from the sensors and initiates the appropriate response 3. effectors - produce the required change There are many different sensor systems involved in normal automatic respiratory control. The most important are sensors that respond to chemicals (chemoreceptors). The chemoreceptors involved in respiratory control 33 Responding to Ill Health NURS08046 – Life Science Notes respond to changes in O2, CO2 and H+ levels in the blood. The normal partial pressure values for O2 and CO2 in arterial blood are as follows: O2 – 100mmHg (13.3 kPa); CO2 – 40mmHg.(5.3kPa) Chemoreceptors in the brain sense changes in pCO2 and H+ concentration in the cerebrospinal fluid surrounding the brain; those in some of the major arteries (the aorta and the carotid arteries) detect changes in arterial pO2, pCO2 and H+ levels. Clearly, increased arterial pCO2 and increased H+ levels lower pH or decreased arterial pO2 levels will bring about an increased respiratory effort, whilst the opposite changes in these levels will bring about a reduction in this effort. It is the pCO2 that is the principal parameter in determining the activity of the respiratory system on a minute-by-minute basis. NB high H+ concentration is the same as saying low pH or acidity. Whenever the blood pH falls respiration increases as in diabetic ketoacidosis or renal acidosis. Other sensors include stretch receptors in the lung: these modify the respiratory process in response to the degree of stretching of the lungs increased stretching causes a reduction in the respiratory rate by increasing the time taken for expiration, whilst decreased stretching stimulates inspiratory muscle activity. The lung also has receptors which lie in airway cells, the nose, nasopharynx, larynx and trachea which are stimulated by noxious gases, cigarette smoke, inhaled dusts and cold air. Stimulation of these receptors produces reflex constriction of the airways - this is thought to be important in asthma. All these control processes are mediated by the autonomic nervous system. Voluntary control of respiration is initiated in the cerebral cortex and can override the above automatic controls to some extent, though not completely – e.g. you cannot stop yourself breathing for any length of time. 34 Responding to Ill Health NURS08046 – Life Science Notes CELLS (Genetic Inheritance & Cancer) Revise your knowledge of the cell: LadyofHats (2006) Animal Cell Structure http://commons.wikimedia.org/wiki/File:Ani mal_cell_structure_en.svg Introduction Note that the nucleus is a vital cellular structure which contains the genetic material (DNA). DNA carries information in coded form which enables the transfer of characteristics from one generation to the next. When the cell divides, DNA is visible as rod-like structures known as chromosomes. Each chromosome contains many genes, (think of chromosomes as long chains of beads, with each bead representing a gene) Each gene is different from its neighbours and each gene will act as template to code for a protein (e.g. enzyme, hormone) to be synthesised by the ribosomes of the cells. Since there are many thousands of genes making up all of our chromosomes it therefore follows that there are many thousands of different proteins all with different functions. The normal development, growth and functioning of the human body requires that all genes are expressed in the right amounts by the right cells at the right times. Chromosomes & Normal Karyotype The nuclei of all normal human cells (except egg and sperm cells) have 46 chromosomes. The chromosomes exist as homologous pairs (= same pairs), 35 Responding to Ill Health NURS08046 – Life Science Notes one member of each pair is inherited from the mother and the other from the father. Thus we have two copies of each chromosome. The chromosomes can be stained and photographed and then arranged with each member of a homologous pair laid beside its partner to display the karyotype (the sum total of the different chromosomes we possess). All normal individuals, male or female, possess 22 pairs of autosomes (chromosome pairs 1 to 22). Chromosomes in pair 23 are the sex chromosomes, which among other things; determine the sex of an individual. Females possess a pair of large sex chromosomes (X chromosomes); males have one X chromosome and a smaller chromosome (Y chromosome). The normal male karyotype is described as 46XY and then normal female is 46XX. The figure below shows a typical karyotype - note homologous chromosomes are arranged in pairs. National Cancer Institute (2007) Karyotype_(normal). https://commons.wikimedia.org/wiki/File:Karyotype_(normal).jpg 36 Responding to Ill Health NURS08046 – Life Science Notes Alleles and Genes As the nucleus has two copies of each chromosome, it therefore follows that there are two copies of each gene, one on each of the homologous chromosomes. One homologous chromosome from each pair is paternal in origin and the other maternal, therefore one copy of each pair of genes comes from the mother and the other from the father. Each chromosome has many different genes at different sites (loci. The singular is locus) e.g. genes A and B in the figure below. The same site (locus) on each of the homologous pairs has a version of a particular gene coding for the same trait (characteristic). Different versions of the same gene are known as alleles e.g. allele a or A. e.g. the trait may be eye colour, dimples, freckles etc. One allele may code for freckles the other allele codes for “clear” skin. gene Allele A UWS Staff (2015) A a B B Allele a Dominant & Recessive Alleles Some alleles may dominate over others. The dominant allele is usually represented by a capital letter and the recessive allele by the same letter, but lower case. For example, Ear lobes may be unattached (fig A) or attached (fig B): Jomegat (2006) Earlobes_free The gene coding for unattached earlobes (E) https://commons.wikimedia.org/wiki/Fil is dominant. The allele for attached earlobes e:Earlobes_free_attached.jpg (e) is recessive. A person with two copies of the dominant allele (EE) will have unattached earlobes, a person with two copies of the recessive allele (ee) will have attached earlobes, a person with one of each (Ee), will have unattached earlobes because E is dominant. 37 Responding to Ill Health NURS08046 – Life Science Notes Genotype & Phenotype The genotype refers to the genes present in an individual e.g. EE, Ee and ee in the example above. The phenotype describes the appearance of the individual e.g. Phenotype unattached earlobes. A homozygous individual has a genotype with two copies of the same allele e.g. ee and EE. A heterozygous individual has a genotype with two different alleles e.g. Ee. Cell Division and Reproduction The only physical links between parents and their children are the gametes – the male sperm and the female ovum. Sperm and ova are produced by a type of cell division known as meiosis and have only half of the number of chromosomes of the parent cell. We say that the gametes are haploid (n) and the parent’s body (somatic) cells are diploid (2n). As a result of fertilization a diploid cell (zygote) is formed with 23 pairs of chromosomes, one member of each pair coming from the sperm and one from the egg. The tiny zygote (no larger than a “full stop” on a page) will divide many times until eventually it becomes a mature individual. This type of cell division is called mitosis and produces daughter cells with exactly the same number and kinds of chromosome as the parent cell. Punnett Square If the genotypes of the parents are known it is possible to work out the probability (chance) of having a child with a particular genotype. This can be done by constructing a Punnett square. The concept of probability is straightforward- what is the chance (probability) of tossing a coin and getting tails? This can be expressed as 50% or 0.5 or ½. Continuing with the example of attached and unattached earlobes If a father’s genotype is Ee what is his phenotype? ………… Which types of gamete will he produce?................................. If a mother’s genotype is ee what is her phenotype?.............. Which types of gamete will he produce? =………… In the Punnett square below- fill in the parental gametes and the genotypes of the possible children Mother Gametes Father What is the probability of this couple having a child with attached earlobes? .............................. 38 Responding to Ill Health NURS08046 – Life Science Notes Dominant and Recessive Disorders In some cases a gene may mutate and no longer properly code for its gene product. Many mutations are harmful and usually result in some loss of function. This loss of function may range from minor to fatal. Mutations A change in the sequence of the bases of the DNA (mutation) can alter the template which is used to make the proteins. When new proteins are synthesised using the ‘faulty’ template they may change their properties e.g. an enzyme may lose its activity. Mutations can arise because of: Errors in the copying process during the replication of DNA as cells prepare to divide. environmental factors: such as radiation or certain chemicals Mutations that occur in Sex cells can be passed on to future generations body cells cannot be inherited Mutations may occur in the sex chromosomes or in any of the 22 pairs of “non sex” chromosomes (autosomes) Autosomal Dominant Disorders The mutation occurs in a dominant allele of a “non-sex” chromosome heterozygotes have the condition, there is no carrier state as only one allele is required. Half the children of a sufferer will probably develop the condition Examples; Neurofibromatosis – gene located on chromosome 17. Tumour-like growths on skin (termed café au lait spots) and nervous system. The symptoms of the disease are variable from mild to severe (i.e. show variable expressivity). See work book. Example A man suffering from neurofibromatosis with genotype Nn marries a woman without the disorder and they plan to have several children. Father’s genotype - Nn. His phenotype= ………… Different types of gametes produced ………… Mother’s genotype …….. Her phenotype = ………… Types of gamete produced ………… (Fill in the gametes and genotypes of the possible children) Mother Gametes Father What is the probability of this couple having an affected child? ......................... 39 Responding to Ill Health NURS08046 – Life Science Notes Autosomal Recessive Disorders In these disorders the recessive allele is abnormal. Only individuals who receive two copies of the abnormal allele will express the abnormal protein and suffer from the disease. Heterozygous individuals (one copy of dominant allele and one of recessive) have normal phenotypes but are carriers of the disorder. See work book. Examples: Cystic fibrosis – the most common recessive human disorder affecting about 1 in 2000 births, approximately one person in 22 is a carrier. Gene located on chromosome 7. Clinical symptoms are mainly associated with thick viscous mucus secretions throughout the body, but particularly in the lungs. At a cellular level the problem is that chloride ions fail to pass through plasma membrane channel proteins. Normally, after chloride ions have passed through the plasma membrane, water follows. Lack of water gives rise to thick viscous mucus. PKU - phenylketonuria occurs once in 5,000 births, and is the most commonly inherited metabolic disorder to affect nervous system development. Gene located on chromosome 12. Affected individuals lack an enzyme phenylalanine hydroxylase which converts the amino acid phenylalanine to tyrosine. Accumulation of unmetabolised phenylalanine causes severe mental retardation. Other recessive conditions include: autism, albinism. Example: A man and woman, who are both carriers of PKU marry and also plan to have several children. Complete and answer the following: Father’s genotype………… His phenotype =………… Different types of gametes ………… Mother’s genotype ………… Her phenotype =…………......... Different types of gametes ………… Mother Gametes Father What is the probability of their having? An affected child ? ......................................................................................... A carrier child ?............................................................................................... A child who is neither affected nor a carrier ?................................................. 40 Responding to Ill Health NURS08046 – Life Science Notes Sex Linked Disorders Both X and Y chromosomes carry genes which code for sexual characteristics, but the X chromosome also carries many genes coding for other traits. Alleles on the X chromosome often do not have an equivalent allele on the Y chromosome (simply because the Y chromosome is so small). Thus a man (XY) has only one X chromosome and therefore has only one copy of the alleles found on it. He must therefore express these alleles whether they are dominant or recessive. On the other hand a woman (XX) has two copies of the X chromosome and the alleles “behave” in the same way as autosomes i.e. a woman needs two copies of an X-linked recessive allele to express it, a man needs only one. An example would be red /green colour blindness. Example: A man with normal vision marries a carrier woman and they also plan to have several children. Father’s genotype………… His phenotype=………… Different types of gamete produced ………… Mother’s genotype ………….. Her phenotype………… Types of gamete produced………… XC Y XC Xc Use the Punnet square to work out the chance of a child being A colour blind boy............................................ A carrier girl .................................................... Note: with X-linked disorders: Men cannot be carriers but women can. Mutations and Cancer occurs Many more women aremutation, likely to be Cancer when,men as athan result of gene theaffected growth by andthe division of disorder. cells becomes uncontrolled. 41 Responding to Ill Health NURS08046 – Life Science Notes Mutations and Cancer A mutation is any event that changes genetic structure or sequence of a cell. Cancer occurs when, as a result of gene mutation, the growth and division of cells becomes uncontrolled. It is likely that several mutations are needed over many years for a cancer to develop from normal tissue, in a process known as carcinogenesis. Carcinogens (agents which predispose to tumour development) cause mutations in the genes controlling cell division: These agents may be: chemical physical (e.g. radiation, ultra violet light) viral (NB recent publicity HPV & cervical cancer) Classification and Behaviour of Tumours The word tumour (neoplasm) is used to describe a mass of tissue which grows more rapidly than normal, in an uncoordinated way, and continues to grow when any initial stimulus is removed. Tumours may be may be benign or malignant, A malignant tumour is a cancer. Tumours are also classified according to their tissue of origin. e.g., carcinoma malignant tumour of epithelial tissue adenoma – benign tumour of epithelial tissue This classification can be quite complex Benign and malignant tumours have very different characteristics –and these are summarised in the table below. Benign Slow growth Cells well differentiated (resemble tissue of origin) Usually encapsulated No distant spread (metastases) Recurrence is rare Malignant rapid growth Cells poorly differentiated (do not resemble tissue of origin) Not encapsulated Spreads (metastases) via: Lymph. Blood, Body cavities, Local infiltration Recurrence is common Some tissues more affected by metastatic spread than others: E.g. LUNGS pulmonary circulation LIVER hepatic portal circulation BRAIN, KIDNEY, BONE – large blood supplies 42 Responding to Ill Health NURS08046 – Life Science Notes PHARMACOLOGY Recall from 1st year notes that pharmacology is the study of the interactions between chemicals (drugs) and biological systems. Generally drugs are given therapeutically to: prevent, cure or control various disease states (e.g. antibiotics to treat bacterial infection; insulin to control diabetes) or modify normal body functions Also recall that pharmacology can be divided into two parts 1) pharmacokinetics – this is what the body does to the drugs and 2) Pharmacodynamics – this is what effect(s) the drugs have on the body. Pharmacokinetics Drugs are absorbed, usually into the blood, and distributed into the various tissues of the body. They may then be excreted unchanged, but usually they are metabolised (their chemical structure is altered) and then excreted. Absorption Absorption describes the process by which drugs enter the body proper and is affected by many factors such as The chemical nature of the drug. and Route of administration. Most drugs are taken orally but these are liable to be (partly) destroyed by the first-pass effect” . Distribution After a drug has been absorbed into the body proper it is then dispersed around the various tissues of the body by the blood. We must ensure that the drug reaches its target tissue at a sufficient concentration to have the desired therapeutic effect. A number of factors affect this distribution such as: the chemical nature of the drug; if drug binds to plasma proteins, the size of drug molecules and how lipid soluble the drug is. Metabolism The body contains a large number of enzymes which are capable of. This process, known as metabolism, usually (though not always!) terminates a drug’s action and makes it more water soluble and thus more readily excreted from the body. Metabolism refers to changing the chemical structure of the drugs which we take. This usually reduces action of drug. Drug metabolism can take place in almost any tissue but is mostly carried out in the liver where the levels of metabolic enzymes are highest. Excretion This is the process whereby a drug and/or its metabolites are eliminated from the body. Drugs may be excreted by a variety of routes: urine, exhaled gases, faeces, saliva, sweat, milk. 43 Responding to Ill Health NURS08046 – Life Science Notes Plasma levels, half-life and steady-state levels The levels (concentration) of a drug in the plasma over time will depend on a combination of the route of administration (how much enters the blood and how fast) and rates of metabolism and excretion (how fast the drug is broken down and removed from the body). How fast a drug is metabolised and excreted from the body is an extremely important clinical consideration as it will affect our choice of dose and dosing schedule (how often the drug is administered). A drug which is quickly metabolised and excreted will have to be administered at a higher dose and perhaps more frequently than a drug which is slowly metabolised and excreted. We therefore need a measure of the speed of removal of a drug from the plasma - this measure is the half-life. 140 120 UWS Staff (2015) Plasma Drug Concentration 100 80 60 40 Half-life 20 0 0 20 40 60 80 100 120 140 Time After a drug has been administered, its concentration in the plasma will rise to a maximum level and then begin fall as it is metabolised and removed from the plasma. The half-life of a drug is the time taken for its concentration in the plasma to fall by half. The shorter a drugs half-life, the more rapidly it is metabolised and excreted and the shorter is its duration of action in the body. Different drugs have different half-lives. Drugs which are slowly metabolised or excreted have long half-lives, whereas drugs which are rapidly metabolised or excreted have short half-lives. The figure below allows you to see a comparison of three drugs with differing half-lives. The half-life helps determine how often (frequency) a drug must be administered. Drug Dosage Regimes (How do we know how much drug to give and how often?) The therapeutic properties of drugs can only be properly achieved if the dose of drug is correct and the blood levels are maintained within those correct limits for the duration of the course of treatment. 44 Responding to Ill Health NURS08046 – Life Science Notes Therapeutic Range It is important to ensure that the amount of drug given produces a plasma concentration which falls within the correct range to be effective (therapeutic range). If the plasma concentration is too low the drug may have little or no effect, conversely if it is too high there may be adverse or toxic effects. E.g. a single large dose of drug may initially have no effect then may ‘overshoot’ into the toxic range then ‘drop-off’ back into having no effect (see figure below). Therapeutic Range effect of single large dose Plasma concentration of drug Toxic effects Therapeutic Range No effect 0 Drug given 12 Time (hours) 24 UWS Staff (2015) Repeated Drug Doses Ideally the plasma concentration of the drug should be maintained between set limits (i.e. within the therapeutic range). To achieve this, the administration of the drug must occur at regular intervals. These intervals are influenced by the half-life of the drug and its therapeutic range (see figure below). Drugs with a short half-life require frequent administration and long half-life drugs less frequent (this is why different drugs have to be taken at different frequencies e.g. two times or 6 times a day etc). Notice that drug concentration accumulates with repeated doses. As drug molecules accumulate in the body the plasma concentration eventually ‘levels off’ to reach a plateau called the Steady-State level (since drug input and excretion/metabolism are roughly equal). However if dosing frequency is not great enough then the plateau stage is never achieved. 45 Responding to Ill Health NURS08046 – Life Science Notes Therapeutic Range effect of single large dose Plasma concentration of drug Toxic effects With reference to the graph - which dosing frequency is best? Why? Therapeutic Range No effect 0 Drug given 12 Time (hours) 24 UWS Staff (2015) Some drugs take a relatively long time to reach their steady-state level. In such cases a larger initial dose may be given (loading dose or bolus) followed by smaller repeated or continuous doses in order to reach the steady-state more quickly. The pharmacokinetics of a drug can be affected by a number of “patientspecific” factors such as age (the very young and the elderly often show differences in drug metabolism and renal function), genetics (people may have differences in the enzymes which metabolise drugs) and disease (liver and kidney problems in particular may affect drug pharmacokinetics). Another factor which may affect the pharmacokinetics of a drug is that of drug interactions (more on this later). If someone is taking more than one drug, they may interfere with each other’s metabolism. Pharmacodynamics i.e. what a drug does to the body. This is often referred to as the “mechanism of action”. Most drugs act by interacting with macromolecules (usually a protein but may be a nucleic acid) on or inside cells. The macromolecule a drug interacts with is known as its receptor or target. An agonist is a drug molecule activates a receptor to induce some change in activity of the cell or tissue. An antagonist binds to a receptor but does not activate it, instead it prevents other agents from activating the receptor and hence are sometimes called “blockers”. Recall that most drugs act by one of the following basic mechanisms: 1. Mimicking or inhibiting a neurotransmitter or hormone e.g., blockers, anti-histamines, oral contraceptives. 46 Responding to Ill Health NURS08046 – Life Science Notes 2. Inhibiting an enzyme e.g., NSAIDs, ACE inhibitors 3. Interfering with a transport molecule e.g., local anaesthetics, antidepressants, anti-ulcer drugs. 4. Most antibiotics and anti-cancer drugs interfere with the function of DNA or RNA either by one of the above mechanisms or by directly interfering with the DNA or RNA. We will now look at some details of how drugs work by considering two classes of drugs: Analgesics and Antibiotics Analgesia (An- “without”; algesia- “the sensation of pain”) Analgesics are therefore drugs which relieve pain. Pain is generated through activation of a specific receptor type called nociceptors. These nociceptors are found in great abundance in the skin, blood vessels, joints and areas of the gut but are sparse in other areas of the body (e.g. there are very little nociceptors present in the brain tissue and headaches are generated through activation of receptors in blood vessels and membranes). Pain serves an essential protective function to warn of injury and minimise damage to the body but excessive or prolonged pain can be very debilitating. Nociceptors are activated through chemical (e.g. prostaglandins, histamine, etc); mechanical (e.g. physical disturbances) or thermal (heat or cold) means. Following activation, nociceptors lead to generation of a “pain message” i.e. the sending of action potential impulses along peripheral pain nerves to the spinal cord where they synapse with ascending sensory neurones and then on up to the thalamus in the brain, where there is a synapse with neurones leading to the sensory cortex in the parietal lobe and sensation is experienced. Therefore any mechanism or drug that reduces the number of action potential impulses reaching the sensory cortex will act to reduce pain sensation. The body has the ability to reduce pain sensation endogenously through the release of endorphins, dynorphins and encephalins. These “natural” painkillers are in fact peptide neurotransmitters produced by the brain which act to block pain transmission. The circumstances underlying the release of these agents is not well understood but collectively they are known as the opioid system and are extremely effective at supressing pain signals from the peripheral nerves. Further endogenous means of reducing pain sensation is through a mechanism called gate control, which suggests that (usually mild-moderate) pain perception can be reduced by simultaneously activating non-pain receptors in a way the “closes the gate” to pain sensation. The physiological processes underlying this are complex but this is a system that can often be utilised effectively e.g. touch or gentle rubbing of a painful area can ease the pain by stimulating touch receptors which are also able to block the passage of the pain signals at synapses. Similar mechanisms are thought to 47 Responding to Ill Health NURS08046 – Life Science Notes underlie TENS machines (transcutaneous electrical nerve stimulation) used for musculoskeletal or labour pain. In terms of drugs used to alleviate pain, there are two major pharmacological groups of analgesics: Opioids, which act centrally (on the central nervous system to mimic the opioid system) and those classed as non-opioids which can either act centrally or at the site of tissue damage in the periphery. Adjuvants are agents which can boost the efficiency of analgesics although on their own do not necessarily possess any pain relieving action (e.g. caffeine can promote the action of paracetamol). In terms of clinical pain management, analgesic use tends to be split into a 3-step ladder that progresses with the level and persistence of pain. The steps start with nonopioid use (e.g. NSAIDS or paracetamol ± adjuvant) then progresses to weak opioid use (e.g. codeine ± adjuvant) and finally to strong opioid (e.g. morphine) ± non-opioid ± adjuvant. This system will be discussed in more detail by HNM staff. NSAIDs (non-steroidal anti-inflammatory drugs) and paracetamol When tissue damage occurs, a rapid non-specific defence mechanism is triggered- the inflammatory response. The body activates a series of mechanisms to limit further damage and prevent infection: the tissue swells, reddens and becomes hot to the touch. The body uses many chemical messengers to bring about the inflammatory response, one such group of messengers are the prostaglandins. One of the actions of the prostaglandins is to make the nociceptors in the damaged area more sensitive. The process the body uses to make the prostaglandins depends on enzymes called cyclo-oxygenases or COX. NSAIDs (such as aspirin and ibuprofen) inhibit these COX enzymes, and so decrease the amount of prostaglandins which are produced- this reduces the sensitivity of the nociceptors, and so reduces pain. Paracetamol is also used to treat inflammatory-type pain but because it has very little actual anti-inflammatory action it is not classed as an NSAID. NSAIDS prevent nociceptors sending pain impulses but even if pain impulses are sent the message pathway can be interrupted. The passing of the message from the peripheral nerves to the spinal cord nerves can be blocked. This blocking of the signal causes a reduction in the amount of pain which the brain “feels”- the pain hasn’t gone away, the brain is just less aware of it. This blocking of the signal in the spinal cord can be done in a number of ways; Local anaesthetics (like lidocaine given at the dentist) generally act as sodium channel blockers, preventing sodium entering neurones, thus blocking the peripheral action potentials reaching the CNS. Such drugs can also be used to inhibit large groups of peripheral nerve as they enter the spinal cord by injecting them in the epidural space around the cord. This type of administration is useful for preventing pain during childbirth or certain types of surgery. Opioid analgesics such as morphine, diamorphine (heroin), pethidine and codeine mimic the action of the brains peptide opioid neurotransmitters and so block the pain signal. The original drug of this type was morphine which is the active chemical in opium (hence the name of the group). In addition to 48 Responding to Ill Health NURS08046 – Life Science Notes being very good analgesics, the opioids have a number of side effects: euphoria and sedation (both of which can be helpful), confusion, respiratory depression in overdose, and other side effects such as nausea and constipation which are caused by the presence of opioid receptors in other parts of the body. They also have the potential to induce dependence (addiction) in patients; this effect is more likely with the ‘stronger’ opioids such as morphine and diamorphine. The opioids are particularly useful during and after surgery, and in the management of severe chronic pain, especially in terminal illness. Pethidine and diamorphine are also used during labour. N.B this is different from epidural anaesthesia described earlier. Not all drugs described as analgesics act through the mechanisms described above (e.g. flupirtine, amitriptyline and gabapentin). The actions of such agents are not described here but serve to highlight just how complicated the physiological mechanisms underlying pain really are. Antibiotics Antibiotics (more accurately referred to as antibacterials) are drugs which are used to treat bacterial infections. Some of these drugs actually kill the bacteria (bacteriocidal), whereas others only inhibit the growth of the bacteria (bacteriostatic). Antiseptics kill bacteria but would usually also kill human cells if taken internally. Antibiotics have to target bacterial cells where they differ from human cells also they are not effective against viruses. There are two major concerns in terms of the use of antibacterial drugs; the first is specificity- how to ensure that the drug is toxic to the bacteria but as safe as possible for the patient. The second issue is bacterial resistance, bacteria are developing the ability to survive drug treatment and as such pose a grave danger to patients. There are a large number of antibiotics, with various mechanisms of action; the diagram below is for illustration and is not intended to be memorised Kendrick Johnson (2011) Antibiotics Mechanisms of action https://commons.wikimedia.org/wiki/File:Antibiotics_Mechanisms_of_action.png 49 Responding to Ill Health NURS08046 – Life Science Notes A major group of antibiotics impair cell wall formation in the bacteria (e.g., the penicillins and the cephalosporins). Animal cells do not have a cell wall, so these drugs should be very specific for the bacteria- unfortunately this does not mean they are harmless to humans, many people are allergic to these drugs. Some bacteria have also become resistant to the penicillins and cephalosporins, they produce an enzyme (-lactamase) which is able to break down part of the drug molecule and render it useless. The remainder of the antibiotics act to inhibit DNA (or RNA) replication or protein manufacture. These drugs prevent the bacteria dividing and are therefore mostly bacteriostatic. The commonest side-effects of antibacterial drug treatment are nausea and rash. Drug Interactions (how one drug can affect the action of another drug) Beneficial Interactions A ‘cocktail’ of different antibiotics may prevent development of antibiotic resistant strains. The ‘cocktail’ of cytotoxic drugs in chemotherapeutic treatment of tumours are more effective at reducing tumour growth. Halothane is a good anaesthetic but a poor analgesic therefore, during halothane induced general anaesthesia, analgesics (e.g. opioids or nitrous oxide) may also be used. Adverse Interactions Before administration drugs should not be mixed together (unless otherwise directed) because the chemical properties of the drugs or other constituents may interact and change the activity or the physical properties of the drugs e.g. solubility which in turn could reduce absorption of the drug. After administration drugs may interact with each other or foodstuffs to produce a variety of undesirable actions. The potential for interactions is so great that it is impossible to discuss them in any detail here; however, one example is given below. Drug molecules may bind to plasma proteins (mainly albumin). Bound drugs, such as warfarin (an anti-coagulant), are inactive and it is only when they are free are they active. The binding of the drug is reversible and as the free form of the drug is metabolized or eliminated, bound drug is released from the albumin thus keeping the plasma free (active) drug level relatively constant. Thus albumin can act as drug reservoir. Administration of another drug, such as sulphonamides (an antibiotic), to someone who has already taken warfarin will cause the warfarin to be ‘pushed off’ the albumin thus increasing the free form of anti-coagulant and as such the clotting ability of the blood could be enormously compromised resulting in enhanced risk of haemorrhage. Sources of Information on Drugs British National Formulary- www.bnf.org Patient Information Leaflets, Textbooks 50