* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CMV infections
Anaerobic infection wikipedia , lookup
Toxoplasmosis wikipedia , lookup
Meningococcal disease wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Henipavirus wikipedia , lookup
Onchocerciasis wikipedia , lookup
Trichinosis wikipedia , lookup
Herpes simplex wikipedia , lookup
West Nile fever wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Chagas disease wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Marburg virus disease wikipedia , lookup
Leptospirosis wikipedia , lookup
Sarcocystis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Schistosomiasis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Hepatitis C wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Lymphocytic choriomeningitis wikipedia , lookup
Neonatal infection wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
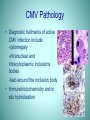
Cytomegalovirus Infection in Renal Transplantation Cytomegalovirus • Member of the herpes virus family (EBV, varicella-zoster, herpes simplex) • Worldwide seroprevalence 30-100% • Found in body fluids – Blood, saliva, urine, breast milk Types of CMV Infection • Primary infection (asymptomatic to mononucleosis like syndrome in immune competent individuals) • Latent infection (presence of viral genome in mononuclear leukocytes, endothelial cells, and organs in the absence of active replication of infectious virus) • Reactivation • Reinfection (new strain of CMV) CMV and SOT • CMV is the most common and single most important viral infection in solid organ transplant recipients. • CMV infection usually develops during the first few months after transplantation • Associated with clinical infectious disease (eg, fever, pneumonia, GI ulcers, hepatitis) and acute and/or chronic graft injury and dysfunction CMV Infection and Transplantation • Approximately 20% to 60% of all transplant recipients develop symptomatic CMV infection. • The patient at highest risk for symptomatic disease is the CMV-seropositive donor/CMVseronegative recipient (D+/R-) who develops a primary infection after transplantation. • Such patients are at particular risk for severe manifestations of CMV infection, including tissue-invasive CMV and CMV recurrence Emovon OE et al, American Society of Nephrology 35th Annual Meeting, 2002 Primary and Secondary Infections • Reactivation infection develops in the patient who becomes CMV-seropositive before transplantation via the traditional routes of transmission and exposure during hemodialysis and blood transfusion, and is more frequent than primary CMV infection. • CMV-seropositive recipients are also at risk for superinfection by CMV from a CMV-seropositive donor, especially in the setting of intense immunosuppression (ie, OKT3 and antithymocyte globulin) Nancy et al Organ Transplant 2003 Avery RK. Medscape Transplantation. 2000. http://www.medscape.com/viewprogram/282 Direct Effects of CMV Infection in the Transplant Recipient • CMV infection is a multifaceted phenomenon with a variety of direct and indirect effects in the organ transplant recipient. • Symptomatology for clinical infectious disease • fever, pneumonia, GI ulcers, hepatitis) ranges from the mild, subclinical case to life-threatening multi-organ disease. • Symptomatic CMV infection can be characterized by • a self-limiting syndrome of episodic fever spikes for a period of 3 to 4 weeks, arthralgias, fatigue, anorexia, abdominal pain, and diarrhea. • CMV infection can disseminate to various organs and can cause death • CMV retinitis can lead to retinal detachment and blindness. Nancy et al Organ Transplant 2003 CMV Infection: Indirect effects • An increased risk for fungal and other opportunistic infections due to additional immunosuppression from CMV infection • A "bidirectional" interaction between CMV infection and the host's immune system. • The type, duration, and intensity of exogenous immunosuppressive therapy influence and enhance CMV infection. • CMV is an immunomodulatory virus, and its effects on the host include enhanced susceptibility to opportunistic infections and, probably, chronic allograft dysfunction. • Acute and/or chronic allograft injury and dysfunction. Rubin R. Infection in organ transplant recipients. In: Rubin RH, Young LS, (eds). Clinical Approach to Infection in the Compromised Host, 3rd ed., Nancy et al Organ Transplant 20 Sequence of Infections After Organ Transplant Fishman, J. A. et al. N Engl J Med 1998;338:1741-1751 CMV and Renal Allograft • CMV causes renal allograft injury that may be indistinguishable from injury caused by rejection or other factors • Results in decreased allograft survival Brennan D. XVIII International Congress of the Transplantation Society. Medscape Transplantation. 2002. Available at: http://www.medscape.com/viewarticle/419014 CMV and Renal Allograft • It has been linked to acute rejection and chronic rejection in the form of – bronchiolitis obliterans in lung transplant recipients, – coronary atherosclerosis in heart transplant recipients, – vanishing bile duct syndrome in liver transplant recipients, and – a variety of additional histologic lesions in renal transplant recipients Brennan D. XVIII International Congress of the Transplantation Society. Medscape Transplantation. 2002. Available at: http://www.medscape.com/viewarticle/419014 Detection of CMV Infection • Immune status: serology (IgG) • Active infection (viremia) – Histology – Viral culture – Shell vial culture – Antigenemia assay – CMV PCR (qualitative/quantitative) Cytomegalovirus Which Diagnostic Test Should be Utilized and Why? Clinical Suspicion for CMV • CMV in the immunocompetent host -inapparent infection -mononucleosis-like syndrome • Primary CMV infection in pregnancy -symptoms in neonate range from moderate hepatosplenomegaly with jaundice to fatal illness -sequelae include hearing loss, vision impairment, and varying degrees of mental retardation • CMV in the immunocompromised host -commonly reactivation of latent infection -variable presentation: retinitis, esophagitis, colitis, interstitial pneumonia, hepatitis, meningoencephalitis Diagnostic Tests for CMV • • • • • Serology Cultures Early Antigen Detection CMV Antigenemia Assays Molecular Amplification Serology • Diagnosis of recent or acute CMV probable with: -detection of CMV-specific IgM antibodies -4 fold increase in IgG titers in paired samples at least 2 weeks apart • Paired samples limit the tests utility in establishing a timely diagnosis • Helpful in determining past exposure to CMV infection • Helpful in determining risk of acquisition • CMV Avidity assay measures IgG maturity effective at identifying primary infection Cultures • Isolated from blood, urine, throat washings, CSF, bronchial washings, and biopsy specimens • Takes 1 to 6 weeks to show cytopathic effects on human fibroblast cultures. • Detection of CMV in culture does not confirm active disease: CMV may be shed intermittently for several months after acute infection • May be useful in determining drug resistance Early Antigen Detection (Shell Vial Cultures) • Allows identification of CMV antigens in culture before cytopathic effects are apparent • Cells are exposed to monoclonal antibodies, binding indicative of early CMV replication in cells • Results available within two to three days CMV Antigenemia Assays • Rapid detection (24 hours) of CMV proteins in PMNs • Tagged monoclonal antibodies to p65 lower matrix protein of CMV • Effective for identification of CMV in immunosuppressed • Showed 90% sensitivity and 96% specificity in detecting primary infection in immunocompetent host Molecular Amplification • Fast and reliable • COBAS Amplicor test: PCR assay that amplifies region of CMV polymerase gene • Hybrid Capture System CMV DNA test (CMV Digene): RNA probe that targets 17% of CMV genome. RNA:DNA hybrid is detected with an antibody by signal amplification • Nucleic acid sequence based system : detects early gene IE1 and late gene pp67 expression CMV Pathology • Diagnostic hallmarks of active CMV infection include: -cytomegaly -intranuclear and intracytoplasmic inclusions bodies -halo around the inclusion body • Immunohistochemistry and in situ hybridization QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Cytomegalovirus Definitions and Diagnosis • CMV Infection – isolation of CMV virus, proteins or nucleic acid from any body fluid or tissue (culture, antigenemia, PCR) • CMV Syndrome – CMV infection associated with systemic symptoms (fever, malaise, myalgia) and leukopenia +/- thrombocytopenia • CMV Tissue Invasive Disease – CMV infection plus signs/symptoms of disease and consistent histology – (pneumonitis, GI, hepatitis, CNS, nephritis,myocarditis, pancreatitis etc) Cytomegalovirus • Risk of CMV disease depends on: – D/R serostatus • D+/R-: high risk (primary infection) • D-/R+ or D+/R+: intermediate (reactivation or superinfection) • D-/R-: low risk – Organ – K-P, lung/H-L, small bowel – Net state immunosoppression – ALA therapy – Rejection – Other immunomodulatory viruses (HHV-6) • Prophylaxis: risk decreased, not eliminated Treatment of CMV Infection • Prevention of CMV infection is the standard care • The recent emphasis on prophylaxis has changed the temporal characteristics of CMV, once an early disease (occurring < 3 months after transplantation), to the current pattern of late disease (occurring > 3 months after transplantation) Razonable RR. 40th Annual Meeting of Infectious Diseases Society of America; October 2427, 2002; Chicago, Illinois. Medscape Transplantation. 2002. Available at: http://www.medscape.com/viewarticle/443973 Prevention of CMV Disease Opelz G et al. Am J Transplant. 2004;4:928-936. Strategies for Prevention of CMV Disease • Screening of blood and organ donors – Reduction of risk with leukocyte-filtered whole blood • Active immunization—not yet • Passive immunization – Immunoglobulin (Ig)—non-selected polyclonal IgG – CMV hyperimmune polyclonal globulin (pooled antibody) – Monoclonal antibody to CMV • Prophylaxis with antiviral agents • Reduction in the use of anti-lymphocyte sera and in the overall intensity of immune suppression Opelz G et al. Am J Transplant. 2004;4:928-936. Prophylaxis: How to Best Prevent Disease? • Universal prophylaxis: administration of antiviral agents to all individuals at risk for a fixed duration – May increase cost, toxicity, risk of resistance • Preemptive therapy: administration of antiviral therapy in response to a positive microbiologic assay (eg, viral load measurements) or clinical scenarios (eg, use of lymphocyte-depleting antibodies or treatment of graft rejection) – Requires careful monitoring, close patient contact, and use of highly sensitive, quantitative assay Hart GD, Paya CV. Rev Med Virol. 2001;11:73-81. Emery VC. Rev Med Virol. 2001;11:83-86. Preemptive Therapy: CMV Viral Load Testing in Predicting CMV Disease in D+/R- Solid OrganTransplant Recipients • Plasma viral load measurements determined in 364 D+/R- organ transplant recipients receiving oral prophylaxis: CMV disease in 64 (17.6%) patients by 12 months • Using a positive cutoff of >400 copies/mL, sensitivity was 38%, specificity was 60%, positive predictive value was 17%, and negative predictive value was 82% for prediction of CMV disease • Routine monitoring would have predicted disease in only 24 of 64 (38%) patients Humar A et al. Am J Transplant. 2004;4:644-649. Valacyclovir for the Prevention of CMV Disease After Renal Transplantation • 208 seronegative recipients of seropositive kidneys; 408 seropositive recipients • Valacyclovir po 8 to 12 g/day vs placebo for 90 days • Reduced incidence of HSV, CMV viruria and viremia, CMV disease • Reduced incidence of graft rejection in both groups (D+/R-, D+/R+) Lowance D et al. N Engl J Med. 1999;340:1462-1470. Prophylaxis of CMV Disease With Oral Ganciclovir in High-Risk (D+/R-) Transplant Recipients Renal Transplant Recipients Prophylaxis (3 mo) Postprophylaxis (6 mo) Placebo Valacyclovir 45% 45% 3% 16% Hepatic Transplant Recipients Placebo Ganciclovir (po) Prophylaxis (3 mo) 44% 5% Postprophylaxis (6 mo) 44% 15% Lowance D et al. N Engl J Med. 1999;340:1462-1470., Gane E et al. Lancet. 1997;350:1729-1733. Razonable RR et al. J Infect Dis. 2001;184:1461-1464. Paya C et al. Am J Transplant. 2004;4:611-620. Valganciclovir for Prophylaxis in Solid Organ Transplantation • 364 CMV D+/R- patients received valganciclovir 900 mg qd or oral ganciclovir 1000 mg tid through 100 days • CMV disease developed in 12.1% of valganciclovir- and 15.2% of ganciclovir-treated patients by 6 months (some difference in the relative efficacy of agents between organs) and 17.2% and 18.4%, respectively, by 12 months • CMV rate viremia during prophylaxis was lower with valganciclovir (2.9% vs 10.4% for ganciclovir; P=.001) and comparable by 12 months (48.5%, valganciclovir, vs 48.8%, ganciclovir) Paya C et al. Am J Transplant. 2004;4:611-620. Effects of Antiviral Agents on Allograft Injury: Prevention of Indirect Effects? • Valacyclovir in kidney recipients 50% in rejection • Oral ganciclovir in heart, liver, kidney recipients trend in rejection Lowance D et al. N Engl J Med. 1999;340:1462-1470. Valantine HA et al. Circulation. 1999;100:61-66. Ahsan N et al. Clin Transplant. 1997;11:633-639. Valganciclovir for Prophylaxis in Solid Organ Transplantation • Times to onset of CMV disease and to viremia were delayed with valganciclovir; rates of acute allograft rejection were generally lower with valganciclovir • Higher incidence of neutropenia with valganciclovir than with ganciclovir (8.2% vs 3.2%, respectively) • “Once-daily oral valganciclovir was as clinically effective and well tolerated as oral ganciclovir tid for CMV prevention in high-risk SOT recipients.” . Paya C et al. Am J Transplant. 2004;4:611-620. Possible Prophylaxis for CMV • D+/R-: IV ganciclovir in hospital, po valganciclovir x 3 months (6 months with anti-lymphocyte-antibody induction) – Repeat prophylaxis for ALS or antirejection therapy • D-/R-: acyclovir or similar x 3 months (herpes simplex, VZV) • D-/R+ : IV ganciclovir in hospital, po valganciclovir x 3 months (6 months with anti-lymphocyte-antibody induction) – IV ganciclovir for ALS or graft rejection – May substitute routine quantitative monitoring after 3 months • Active disease: treat until assay is negative, then 1 to 2 weeks beyond; prophylaxis with po x 3 months minimum CMV, cytomegalovirus; IV, intravenous; ALS, antilymphocyte serum; VZV, varicella-zoster virus.