* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pathogenesis of Disease of the Large Intestine
Lyme disease microbiology wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Infection control wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Schistosomiasis wikipedia , lookup
Chagas disease wikipedia , lookup
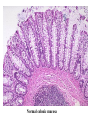
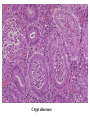
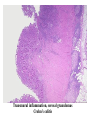
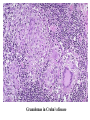
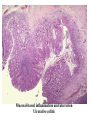
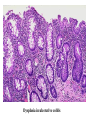
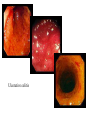
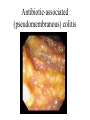
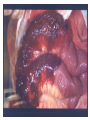
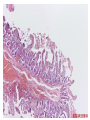
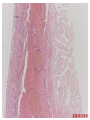
Pathogenesis of Diseases of the Large Intestine Dr Paul L. Crotty Department of Pathology AMNCH, Tallaght October 2008 Large intestine: by aetiology • • • • • • • • • • • Congenital: Anal anomalies, atresia, stenosis, Hirshsprung’s disease Acquired Infection: Infective enterocolitis (viral, bacterial, protozoal) Physical: Obstruction, Diverticular disease, Rectal mucosal prolapse Chemical/Toxic: NSAIDs Circulatory disturbances: Ischaemic bowel disease Immunological disturbance: Iatrogenic: (NSAIDs) Antibiotic-associated pseudomembranous colitis Idiopathic:: Crohn’s disease, ulcerative colitis Psychosomatic: : Pre-neoplastic/ Neoplastic: – Adenoma -> adenocarcinoma – CIBD -> dysplasia -> adenocarcinoma Fluid dynamics Food intake: ~2 litres/d Saliva: ~1 litre/d Gastric secretions: ~2 litres/d Bile: ~1 litre/d Pancreas: ~2-3 litres/d Small intestinal secretions: ~1 litre/d Total 9-10 litres/d Fluid dynamics Re-absorption: Small intestine: ~6 litres/d Large intestine: normally ~2-3 litres/d but with capacity to increase up to ~6 litres/d Average stool weight 200-250g/d of which 65-85% is water Mechanisms of Diarrhoea Secretory: increased secretions: persists after fasting. Examples: cholera, some viral infections Osmotic: some solute present: osmotic retention of fluid in stool, resolves on fasting. Examples: disaccharidase deficiency; some viral infections Exudative: pus present: ulceration in bowel. Examples: invasive bacterial infection: idiopathic chronic inflammatory bowel disease Dysmotility-associated: Examples: Irritable bowel syndrome, hyperthyroidism Malabsorption: Steatorrhoea Chronic Inflammatory Bowel Disease Most (but not all) can be separated into 1 of 2 patterns: (1) Crohn’s disease (2) Ulcerative colitis based on clinical, endoscopic and pathological features Features of both Crohn’s disease and ulcerative colitis Idiopathic chronic inflammatory diseases Both have acute exacerbations and remissions Typically onset 15-40 y: (small second peak ~ 65-70y) - Active inflammation during acute exacerbation - Neutrophils in crypts (cryptitis, crypt abscesses) - Over time: destruction of mucosal architecture Crohn’s disease - Granulomatous inflammation - May involve any part of bowel - Typically small intestine and/or colon (one third each) - Discontinuous: ‘skip lesions’ typically with rectal sparing - Aphthous ulcers early: linear ulcers later - Transmural inflammation - Wall thickening/strictures with luminal narrowing - Deep fissures/fistulas - Extra-intestinal disease - Probable small increased risk of colorectal carcinoma Ulcerative colitis - NOT granulomatous - Colon only involved (no small bowel involvement) - Extends variable distance in continuity from rectum - Rectum always involved - Has well-defined proximal limit - No skip lesions - Broad-based ulcers with pseudo-polyps - Mucosal-based inflammation: NOT transmural - No wall thickening, no strictures, - No fissures , no fistulas - Extra-intestinal disease: also P.S.C. - Significant risk of dysplasia and carcinoma Normal colonic mucosa Crypt abscesses Transmural inflammation, serosal granulomas Crohn’s colitis Granulomas in Crohn’s disease Fissure in Crohn’s disease Normal Crohn’s disease Crohn’s disease Crohn: 1932 [Morgagni: 1761: “ileal passion”] initially termed terminal/regional ileitis - later identified could also have colonic involvement - later still recognised colonic-only pattern of disease “Idiopathic”: but what do we know about its causes? Crohn’s disease Genetic predisposition: Sibling risk: 15-40x risk of general population MZ twin concordance: 40-50% DZ twin concordance: 3-7% Linkage to loci on 16 (IBD1) also chromosome 3, 12 Crohn’s disease linkage to locus on chromosome 16: high LOD score ~ 5.8 2001: NOD2 (nucleotide-binding oligomerisation domain) - normal function as signalling protein in macrophages - activates NFkB in response to bacterial LPS - 40% of Crohn’s disease patients: NOD2 polymorphism - but polymorphism also in ~15% of general population - heterozygous 2-4x risk/ homozygous 40x risk Crohn’s disease Smoking: 2-3X increased risk of Crohn’s disease counterbalanced by decrease in risk of ulcerative colitis Urban > Rural “Good” hygiene > Poor ? Theory: Delayed exposure to antigens/bacteria Crohn’s disease Is there an infectious agent? Animal models do not develop disease if kept in a strict germ-free environment Candidates ??Atypical mycobacteria ??Measles virus Crohn’s disease Is there immune dys-regulation? Is there a defect in the normal mechanisms of suppression of the inflammatory response to normal gut flora? New NOD2 data supportive of this theory Crohn’s disease Present with pain, variable diarrhoea, fever Diagnosis: Clinical, endoscopy, mucosal biopsies, barium Complications: Strictures: obstruction Fissures: abscesses Fistulas: bladder, vagina, skin, entero-enteric Peri-anal disease Malabsorption (terminal ileal disease, blind loops) Slight increased risk of cancer Ulcerative colitis Pseudopolyps in ulcerative colitis Mucosal-based inflammation and ulceration Ulcerative colitis Dysplasia in ulcerative colitis Ulcerative colitis Ulcerative colitis Wilks: 1859 claim on first distinction from dysentery 1888: RSM in London debate on aetiology of the disease ? Diet ? Infection ? Psychosocial Genetic: MZ concordance HLA association ? Infection ? Allergy ? Immune dys-regulation Ulcerative colitis Mucosal inflammation leading to ulceration Chronicity leads to mucosal destruction, regeneration 40% rectum/ recto-sigmoid only 40% extends from rectum to point x 20% pan-colonic Presents with diarrhoea, pain, weight loss Diagnosis: Clinical, endoscopy, biopsy Ulcerative colitis Complications: Fulminant colitis: Toxic megacolon Extra-intestinal manifestations Including primary sclerosing cholangitis Significant risk of dysplasia and malignancy especially with pan-colitis, long duration Large intestine: by aetiology • • • • • • • • • • • Congenital: Anal anomalies, atresia, stenosis, Hirshsprung’s disease Acquired Infection: Infective enterocolitis (viral, bacterial, protozoal) Physical: Obstruction, Diverticular disease, Rectal mucosal prolapse Chemical/Toxic: NSAIDs Circulatory disturbances: Ischaemic bowel disease Immunological disturbance: Iatrogenic: (NSAIDs) Antibiotic-associated pseudomembranous colitis Idiopathic:: Crohn’s disease, ulcerative colitis Psychosomatic: : Pre-neoplastic/ Neoplastic: – Adenoma -> adenocarcinoma – CIBD -> dysplasia -> adenocarcinoma Infective organisms causing diarrhoea World-wide: mortality 5 million /year, most children Mechanisms by which infectious agents cause diarrhoea: (1) Pre-formed toxin in food no live organisms ingested e.g. C botulinum, some S. aureus (2) Live organisms: Non-invasive: (a) organisms colonise gut and produces toxin e.g. V. cholerae, C. difficile, some E. coli (b) organisms bind to brush border e.g. Cryptosporidium Invasive: (a) mucosal e.g. Shigella, most Salmonella, some E. coli (b) deeper layers e.g. S. typhi, Yersinia Viral enterocolitis Rotavirus infects enterocytes lining villi in small intestine near-normal/minimal shortening of villi main effect is absence of lactase => osmotic diarrhoea Norwalk virus Adenovirus Astrovirus Vibrio cholerae Toxin production includes binding units, catalytic unit => binds to glycolipid on surface of enterocyte => catalytic unit taken up into enterocyte => activated intracellularly => stimulates G-protein => increases intracellular cAMP => actively stimulates secretion of Na, Cl, water Shigella => stimulates its own endocytosis => proliferates within cell => rapid cell death, lysis => infects adjacent cells Large intestine: by aetiology • • • • • • • • • • • Congenital: Anal anomalies, atresia, stenosis, Hirshsprung’s disease Acquired Infection: Infective enterocolitis (viral, bacterial, protozoal) Physical: Obstruction, Diverticular disease, Rectal mucosal prolapse Chemical/Toxic: NSAIDs Circulatory disturbances: Ischaemic bowel disease Immunological disturbance: Iatrogenic: (NSAIDs) Antibiotic-associated pseudomembranous colitis Idiopathic:: Crohn’s disease, ulcerative colitis Psychosomatic: : Pre-neoplastic/ Neoplastic: – Adenoma -> adenocarcinoma – CIBD -> dysplasia -> adenocarcinoma Antibiotic-associated (pseudomembranous) colitis • Clostridium difficile in normal flora • When other bacteria eradicated by antibiotics, C. difficile proliferates • Selection of toxin-producing forms • Enterotoxin (A) and cytotoxin (B) • Disrupt cytoskeleton, inflammation • Cell death, confluent ulceration Antibiotic-associated (pseudomembranous) colitis Large intestine: by aetiology • • • • • • • • • • • Congenital: Anal anomalies, atresia, stenosis, Hirshsprung’s disease Acquired Infection: Infective enterocolitis (viral, bacterial, protozoal) Physical: Obstruction, Diverticular disease, Rectal mucosal prolapse Chemical/Toxic: NSAIDs Circulatory disturbances: Ischaemic bowel disease Immunological disturbance: Iatrogenic: (NSAIDs) Antibiotic-associated pseudomembranous colitis Idiopathic:: Crohn’s disease, ulcerative colitis Psychosomatic: : Pre-neoplastic/ Neoplastic: – Adenoma -> adenocarcinoma – CIBD -> dysplasia -> adenocarcinoma Ischaemic bowel disease • • • • SMA, IMA -> mesenteric arcades collateral supply watershed areas: splenic flexure venous drainage • acute, subacute, chronic Ischaemic bowel disease • Arterial thrombosis – atherosclerosis, dissection, hypercoagulation • Arterial embolism – atherosclerosis, arrhythmias, SBE • Venous thrombosis – hypercoagulation • Generalised hypoperfusion – hypotensive shock, CCF Ischaemic bowel disease • Transmural infarction – acute occlusion (arterial or venous, thrombotic or embolic) -> acute abdomen – perforation/gangrene if untreated • Mucosal/submucosal infarction – acute/subacute hypoperfusion – can mimic acute colitis • Fibrosis and mucosal atrophy – chronic, strictures: can mimic Crohn’s Large intestine: by aetiology • • • • • • • • • • • Congenital: Anal anomalies, atresia, stenosis, Hirshsprung’s disease Acquired Infection: Infective enterocolitis (viral, bacterial, protozoal) Physical: Obstruction, Diverticular disease, Rectal mucosal prolapse Chemical/Toxic: NSAIDs Circulatory disturbances: Ischaemic bowel disease Immunological disturbance: Iatrogenic: (NSAIDs) Antibiotic-associated pseudomembranous colitis Idiopathic:: Crohn’s disease, ulcerative colitis Psychosomatic: : Pre-neoplastic/ Neoplastic: – Adenoma -> adenocarcinoma – CIBD -> dysplasia -> adenocarcinoma Diverticular disease • • • • • Diverticulum: blind pouch off GI tract Incidence: 40-50% in >60 years in West Diet low in fibre -> decreased stool volume Chronic increased intraluminal preseeure Acquired ‘blow-out’ diverticuli – at points of focal weakness in wall of colon where vessels cross muscle layer Diverticular disease • • • • • Complications Inflammation Abscess formation Perforation Bleeding