* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Mayo IMPACT Poster1
Survey
Document related concepts
Transcript
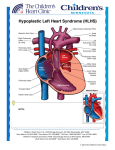
The effects of abnormal MEF2C activity induced by Irregular Folic acid levels and Notch Signaling on Early Heart Development Kwame Opoku Akyeampnog, Okhumhekho Kassim, Uyi Jefferson Imasuen, Ali Eastman Oku Faculty Mentor: Dr. Penny Knoblich Minnesota State University, Mankato, MN, Department of Biological Sciences. Abstract HLHS is a complex heart disease that affects 1 out of every 4,344 babies in the US every year (Facts about Hypoplastic Left Heart Syndrome, 2015). Myocyte enhancing factor (MEF2C) is part of the MEF2 family of transcription factors that has a vital role in regulating gene expression in tissues such as the heart (Black, et. al, 2010).MEF2C binding activity also interacts strongly with an AT-rich sequence of muscle promoters, which plays specific roles in cardiac muscle structure and enables maximum activity interaction It is therefore plausible that a malfunction of this regulator (MEF2C) during critical periods od heart development, would lead to HLHS. The notch signaling pathway is one of the major regulators of the expression of MEF2C (Lui1, 2010). Folic acid has strongly been linked to different congenital heart diseases and plays a key role in function of the notch signaling system. We, therefore, plan to knockout the MEF2C gene using CRISPR-Cas 9 (or siRNA) under varying concentrations of folic acid. The cells with the knocked-out mef2c gene will then be used to develop miniature hearts (using stem cells ) to determine if any abnormal heart phenotypes develops. Hypothesis During critical periods of heart development, abnormal activity of the myocyte enhancer factor 2C (MEF2C) as a result of irregular Notch signaling , folic acid levels, and/or genetic mutations is the underlying cause of HLHS. Figure 1 Figure 3 MEF2C has both trans-activating and DNA binding activities that is significant for cardiac myogenesis. The transcription factor is controls morphogenesis and myogenesis, and can be inhibited by different regulating activity pathway. The team believed the major activities that affects MEF2C regulation of gene expression in cardiac tissues are: Significance • The significance and innovation of our proposed hypothesis comes from our focus on genetics and/or gene expression. The expression of MExF2C and Notch 1 signaling depends heavily on the presence of the genes coding for them as well as transcription factors. We believe that either genetic mutations, or maternal conditions altering gene expression, could underlie the development of HLHS • Our hypothesis can primarily change the timing of the diagnosis of HLHS. Because we have linked genetic disorders specifically (errors in nucleotide sequences) to the MEF2C transcription factor and the Notch signaling cascade, it is possible to begin using embryonic genetic testing to uncover mutations that can lead to HLHS. • This hypothesis is suitable for testing with tools surrounding genetics. Testing this hypothesis would require the development of knockouts of the MEF2C gene, which could be done using siRNA in different embryonic hearts cells at different times. • The CRISPR-Cas 9, a fairly new and recent genomic engineering tool, would be invaluable in this experiment. Cas9 is a protein that comes from a single RNA strand that is then cleaved into a smaller RNA strands known as crRNA which are found in many organisms • Notch signaling: Notch is a regulatory signaling pathway with many receptors. Mutations in the Notch1 receptor has been strongly linked to the onset of HLHS. Several studies have demonstrated a paternally inherited mutation in the gene for Notch signaling and more prevalent in males than females. The down regulation of notch2 receptor affecting MEF2c could result the increase of HLHS in males • Decreased Folic Acid Levels: Folic acid is a key player in ensuring proper cell differentiation and cell proliferation, and reducing heart malformations during fetal development. Folic acid has also been shown to increase notch signaling including notch1 activity. Folic acid deficiencies have also been directly connected to the activity of Hand 1, Flk 1 and VEGF which are important parts of the development of a fetal heart The irregular activity of the notch signaling system as a result of insufficient levels of folic acids during fetal development could lead to HLHS by affecting the production of MEF2C. Decreased myogenic capability can explain the underdevelopment of tissue on the left side of the heart. Background Figure 3. A picture depicting the function of the CRSPR Cas 9. The CRISPR/Cas9 system for targeted genome editing. (n.d.). Retrieved February 1, 2016, from http://www.clontech.com/US/Products/Genome_Editing/CRISPR_Cas 9/Resources/About_CRISPR_Cas9 Figure 2 Innovation Axis label here There are many surgical procedures and treatments that have improved the prognosis of HLHS, but HLHS patients still undergo later complications in life. HLHS has been linked to various chromosomal disorders including Turner syndrome, Jacobsen syndrome, and Trisomies 9, 11, & 13 (Cole & Eghtesady 2016). Phenotypes ranged from isolated to multiple combinations of cardiovascular malformations including ventricular, vascular, septal, valvular, and other defects. It was concluded that because there were many different phenotypic expressions of HLHS, that there must be a concert of factors working together to cause HLHS as opposed to just a single factor (Cole & Eghtesady, 2016). The vast array of genetic mutations and copy number variants linked to this disease, in addition to the differing number of phenotypes observed, has led us to believe the variants converge on a general signaling/regulatory pathway. One regulatory pathway that is crucial in cardiac development is the Notch signaling cascade. The Notch receptors and ligands can be regulated by a multitude of different factors. One such factor that will be discussed is folic acid levels (Gilbert, 2014). Folic acid has also been shown to significantly reduce congenital defects in children. In zebrafish, it has been shown that a competitor molecule, methotrexate (MTX), diminishes production of tetrahydrofolic acid. This results in decreased transcription levels of HAND 2 and MEF2C, and abnormal heart formation. These findings led us to our hypothesis. Rationale 1. Black, B. L., & Cripps M. R. (2010). Heart development and regeneration: Myocyte enhancer factor 2c transciption actors in heart development and disease. Oxford: Academic Press. Figure 1. Visual representation of transcription factors that interact and are crucial in cardiac development. Heart Development Pathway in Heart Development SuperPath. (n.d.). Retrieved December 20, 2015, from http://pathcards.genecards.org/pathway/74 References Figure 2. A picture outlining early heart development of an embryo. (a)3D cardiac microchamber generated from WTC hiPSCs on a 400-mm pattern, where cardiomyocytes only appeared in the centre and myofibroblasts on the perimeter. Panels above and to the right of the main panel represent the z axis projection images at their respective x and y crosssections Ma, Z., Wang, J., Loskill, P., Huebsch, N., Koo, S., Svedlund, F. L., . . . Healy, K. E. (2015). Self-organizing human cardiac microchambers mediated by geometric confinement. Nature Communications Nat Comms, 6, 7413. Researchers at the University of California have been able to grow tiny human beating hearts from reprogrammed skin derived stem cells. These hearts could be used to test the effects of our knockout or knockdown on heart development ( Ma et. al, 2015). In addition, another study could be conducted in which the amount of folic acid administered to each heart is varied during the course of development. These manipulations can determine whether or not ventricular malformations or other HLHS associated malformations occur in relationship to the timing of the MEF2C knockdown or varied folic acid levels 2. Cole, C. R., & Eghtesady, P. (2016). The myocardial and coronary histopathology and pathogenesis of hypoplastic left heart syndrome. Cardiology in the Young, 26(01), 19-29. doi:10.1017/S1047951115001171 3. Facts about Hypoplastic Left Heart Syndrome. (2015). Retrieved January 15, 2016, from http://www.cdc.gov/ncbddd/heartdefects/hlhs.html 4. Gilbert, S. F. (2014). Developmental Biology. Sunderland, MA: Sinauer Associates, Inc 5. Lui1, H., Huang1, G., Zhang1, X., Ren1, D., & Wilson2, J. X. 2010. Folic Acid Supplementation Stimulates Notch Signaling and Cell Proliferation in Embryonic Neutral Stem Cells.174-180