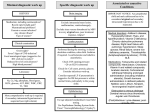
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Complications associated with central venous access device in
Marburg virus disease wikipedia , lookup
Schistosomiasis wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Anaerobic infection wikipedia , lookup
Hepatitis C wikipedia , lookup
Hepatitis B wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Oesophagostomum wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Haemophilia (2015), 1–7 DOI: 10.1111/hae.12665 ORIGINAL ARTICLE Complications associated with central venous access device in children with haemophilia: a nationwide multicentre study in Finland € AI € N E N , * R . L A S S I L A , † M . A R O L A , ‡ P . L AH € T E E N M AK € I , § M . M OT € T ON € EN,¶ K . V E P S AL € A . M AK I P E R N A A † k and P . R I I K O N E N * *Department of Pediatrics Kuopio University Hospital, Kuopio; †Coagulation Disorders Unit, Department of Hematology and Cancer Center, Helsinki University Central Hospital University of Helsinki, Helsinki; ‡Department of Pediatrics Tampere University Central Hospital, Tampere; §Department of Pediatrics and Adolescent Medicine Turku University Central Hospital, Turku; ¶Department of Pediatrics and Adolescence Oulu University Hospital, Oulu; and kChildren’s Hospital Helsinki University Central Hospital, Helsinki, Finland Summary. Children with haemophilia require venous access for regular infusion of coagulation factors. A central venous access device (CVAD) ensures longterm access but associates with infectious and noninfectious complications with proposed risk factors of young age at initial CVAD implantation and presence of an inhibitor. Our aim was to evaluate the incidence and risk factors for complications associated with CVAD usage in a retrospective nationwide multicentre study in five Finnish Paediatric Haemophilia Treatment Centers. Our study investigated 106 CVADs in 58 patients with 137 971 CVAD days. The median access survival was 1159 CVAD days, and most often a malfunction led to CVAD removal after a long survival (median of 1640 CVAD days). We detected a very low bloodstream infection rate (0.12/ 1000 CVAD days). The presence of neutralizing inhibitor was a significant risk factor for infection. Heparin vs. saline flushing did not influence the CVAD outcome. We detected a lower infection rate than previously reported, although 90% of the patients were very young (<2 years) at first insertion (median age = 1.02 year). Port access was frequent after initial implantation: six patients (10%) used the port daily for immune tolerance induction therapy and 74% at least twice weekly for prophylaxis. Young age did not increase the risk of infections, as 59% of the CVAD-related infections were recorded in children over 6 years of age. Our national experience confirms the safety of prophylactic factor concentrate administration via ports even in very young children. Introduction associated with a number of complications, such as CVAD-related bloodstream infection (CRBSI), thrombosis or mechanical failure. According to the literature, infections are the primary complication associated with CVADs and also the most common reason for their removal [1–3]. The frequency of CVAD-related infection is partly related to the maintenance procedures of the device and the quality of caregiver’s education and training. A malfunction of the CVAD is another common reason for CVAD removal [2,4]. Young age at initial CVAD implantation and presence of an inhibitor has been proposed as risk factors for a CVAD infection [2,3,5,6]. The use of heparinized saline solutions to flush and lock CVAD to reduce catheter occlusion has been recommended. It has been also been suggested that heparin flushing Children with severe forms of haemophilia require regular venous access for infusion of coagulation factors. Repeated peripheral vein punctures are technically problematic in very young children and subcutaneous contamination is frequent. Thus, surgically inserted central venous access devices (CVADs) may be required to secure long-term and reliable venous access. Unfortunately, these devices have been Correspondence: Pekka Riikonen, Department of Pediatrics, Kuopio University Hospital, P.O. Box 100, 70029 KYS, Kuopio, Finland. Tel.: +358 17 172443; fax: +358 17 172444; e-mail: [email protected] Accepted after revision 9 February 2015 © 2015 John Wiley & Sons Ltd Keywords: central venous access device, children, complications, haemophilia, heparin, infections 1 2 € AINEN € K. VEPS AL et al. may prevent catheter-related infections. In a prospective, randomized trial conducted in children with cancer, which investigated 203 tunnelled central venous catheters, there was a higher incidence of catheter occlusion, and an increased bacteraemia rate with saline flushing when compared with heparin flushing [7]. A systematic review provided a weak evidence that heparin flushing could reduce the risk of occlusion but there was no evidence that it could reduce bloodstream infections [8]. After these non-conclusive results, heparin flushing has not been uniformly used. The aim of this study was to evaluate the nationwide incidence and risk factors of CVAD-related complications in children with haemophilia in Finland. Materials and methods Design and setting We designed a retrospective multicentre study to evaluate the incidence and risk factors for CVAD complications during the follow-up of 17 years. Our study was performed in five Finnish Paediatric Haematology-Oncology Centers (Kuopio, Oulu, Turku, Tampere and Helsinki University Hospitals), which serve also as haemophilia treatment centres for children and adolescents. In these centres, CVADs have routinely been used to facilitate prompt primary prophylactic clotting factor therapy even in the youngest patients with haemophilia. It is the standard practice to implant electively a CVAD into children who are initiating prophylaxis or immune tolerance induction (ITI) therapy to ensure reliable venous access and to permit home treatment. Detailed data on patients and treatment history were uniformly collected by the principal investigator (KV) from the medical records of all patients. The registered patient characteristics included date of birth, type of bleeding disorder, inhibitor development, onset and reason for prophylaxis, date of CVAD insertion and removal, indication for CVAD insertion, reason for CVAD removal and incidence of complications associated with CVAD usage, age at transition to peripheral veins and the use of heparinized/non-heparinized saline solution to flush and/or to lock the CVAD. Data were collected between June 1996 and September 2013. The Research Ethics committee of Northern Savo, Finland, provided a favourable opinion for this study (26//2010), and all patients and/or their parents provided written informed consent prior to participation. Patient characteristics and treatment history All children were eligible with the following criteria: severe (FVIII or FIX coagulation activity Haemophilia (2015), 1--7 <0.01 IU mL1) or moderate (FVIII:C or FIX:C 0.01– 0.05 IU mL1) haemophilia A or B born between June 1994 and May 2012 and treated in the participating centres, and who required of CVAD insertion during the follow-up period. Our national policy is to insert CVAD very early for primary prophylaxis. A CVAD was inserted in the majority (87%; 78 of 90; 72 of 74 with severe and 6 of 16 with moderate haemophilia) of our patients. There were 90 patients of which 66 (73%) provided written informed consent for this study. Eight of these 66 children did not require a CVAD. That is the data from a total of 58 patients with 106 CVADs were analysed. Children received a tunnelled subcutaneously implanted CVAD, a ‘port’, to facilitate the administration of factor concentrate. CVADs were inserted through the subclavian or the internal jugular vein by an experienced anaesthesiologist in the operating theatre under strict aseptic techniques. Port access was most often started immediately after implantation. No antibiotic prophylaxis was used in the catheter lock. Maintenance procedures such as hand washing and aseptic techniques were carried out carefully by a specialized trained nursing team or by parents at home. Parents started the specific aseptic training program during the first postoperative days. Under supervision, they learned how to make antiseptic preparation of the skin, proper port needle placement, delivery of the concentrate and flushing the CVAD with heparin or saline. After they were deemed competent to perform sterile techniques during all procedures, home treatment was initiated. In case of febrile episodes, parents were advised to contact the hospital immediately if any fever was detected. Patients were examined and in the absence of common symptoms and signs of respiratory infection as a cause of fever, blood samples were drawn and cultured before initiation of antimicrobial therapy. Most centres obtained paired blood cultures from a CVAD and a peripheral vein but in some centres blood cultures were collected only from the CVAD. In cases where blood cultures were not percutaneously obtained but culture from CVAD was positive, blood samples were collected again from the CVAD and cultured. Vancomycin or second or third generation cephalosporin based empiric antibiotic therapy was initiated and continued until the CVAD-related bloodstream infection was excluded by culture. When a bloodstream infection was diagnosed, empiric antimicrobial therapy was later modified based on culture results and according to the microbiological sensitivity pattern if needed. In general, the duration of antimicrobial therapy was at least 10 days but a minimum of 14 days in the case of Staphylococcus aureus. CVADs were flushed with heparinized saline solution or non-heparinized saline alone every time the CVAD was accessed. © 2015 John Wiley & Sons Ltd COMPLICATIONS ASSOCIATED WITH CVAD IN CHILDREN 3 Complications Statistical analysis Complications were defined as any complication requiring CVAD removal: CVAD malfunction, mechanical complication, symptomatic deep venous thrombosis (DVT), CRBSI or a local infection such as skin or tunnel infection. A CRBSI was defined according to the guidelines of the Infectious Diseases Society of America [9]. A definitive diagnosis required that the same organism would grow from at least 1 percutaneous blood culture and from a culture of the catheter tip if a port had been removed for suspected CRBSI, or that two positive blood cultures were drawn (one from a catheter and the other from a peripheral vein). A presumed CVAD-related infection was defined with clinical symptoms of infection (fever, chills, or hypotension) and with a recognized pathogen cultured from at least two blood samples (collected from a CVAD on separate occasions) and that the recognized pathogens were not related to an infection at some other site. Thus, febrile episodes not fulfilling the definition of either definitive or presumed CVAD-related infection were not included in this study. Two infections were excluded: Staphylococcus aureus-sepsis and multiple abscesses in a patient with concomitant severe congenital immunodeficiency and Klebsiella pneumonia and Acinetobacter-bacteraemia in another patient who had pneumonia and osteomyelitis. Malfunction was defined as a blockade or an occlusion (difficulty in drawing blood and/or infusing fluids through the catheter) in the absence of documented thrombosis. Mechanical complication was defined as a displacement, that is malpositioning of the catheter tip, disconnection, split or skin erosion requiring CVAD removal. Central venous access device-related thrombosis was defined as a thrombosis with clinical symptoms or signs of venous thrombosis diagnosed by venography or ultrasound. Early complications were those occurring as a consequence of the catheter insertion procedure. These include pneumothorax, arrhythmias and major bleeding complications or CRBSI within the first 2 weeks after CVAD positioning. A major bleeding was defined according to ISTH recommendations [10]. Data on uncomplicated and elective removals with improved venous access via peripheral veins due to ageing were collected. The duration of CVAD placement was calculated from date of positioning until the date of its removal or until the last follow-up for children, whose CVAD remained in place. Exposure days (ED) before CVAD removal were estimated according to the administration frequency of prophylaxis or ITI, usually thrice weekly for haemophilia A, twice for haemophilia B and daily for ITI, and treatment days for major bleeds or surgery. For each CVAD, the total number of catheter days was calculated as the total number of days from CVAD insertion to removal or to the date of last follow-up day. The incidence rate for any complication per 1000 CVAD days was calculated as 1000 times the number of complications divided by the total number of CVAD days. Continuous variables were expressed as median values and ranges. Groups were compared by the log-rank test. CI at the 95% level and exact p values for incidence rates were calculated. A P-value of <0.05 was considered statistically significant. All analyses and figures were performed with SPSS software version 21.0; SPSS Inc. (IBM Corp., Armonk, NY, USA). © 2015 John Wiley & Sons Ltd Results Patients and CVADs A total of 58 patients with 106 CVADs were included in this study, which involved a total of 137 971 CVAD follow-up days. These included 122 053 CVAD days for patients without inhibitor and 15 918 CVAD days with inhibitor. Fifty-one patients had severe haemophilia A and two children had severe haemophilia B. Three patients with moderate haemophilia A and two patients with moderate haemophilia B were included. Eleven patients (19%) of our cohort had an inhibitor, all of them with severe A haemophilia. Eight of the 11 inhibitory positive patients were successfully immune-tolerized and three children had ongoing ITI therapy. The main reason for implanting the first CVAD was the start of prophylaxis (88% of initial insertions). Additional indications for port insertion were the initiation of ITI therapy and difficult venous access (10.3% and 1.7% of initial implantations). The median age at first CVAD insertion was 1.02 years (range = 0.1–9.1 years). The majority (50 of 58; 90%) of patients were very young (≤2 years) at the time of first insertion. Port access was frequent immediately after initial implantation: six patients (10%) were using the port daily for ITI, 30 patients (52%) every second day or three times per week and 13 patients (22%) two times per week for prophylaxis. Twenty-five children (43%) had one CVAD insertion, 23 children (40%) two CVADs. Ten patients (17%) had 3–4 CVAD replacements. The median age at transition to peripheral veins was 8.2 years (range = 2.6– 16.2). Detailed data on patients with CVAD are presented in Table 1. Heparin flushing was carried out with the majority of the CVADs (90/106; 85%). Sixteen CVADs in 10 patients (two of them had an inhibitor) were flushed with saline only after using the device. Haemophilia (2015), 1--7 4 € AINEN € K. VEPS AL et al. Outcome of CVADs Eighty-nine of the 106 CVADs (84%) had been removed and 17 CVADs (16%) were still in place at the end of follow-up period. CVADs remained in place for between 3 and 3778 days (median = 1159 days). The duration of CVADs in patients without inhibitor was longer, 1501 median days than those with inhibitor 782 days (P = 0.002). Median ED before CVAD removal were 550 for all patients, and 600 ED for non-inhibitor patients and 365 ED for patients with inhibitor (P = 0.04). Table 1. Characteristics of patients with CVADs. Number of patients Bleeding disorder Factor VIII deficiency (%) With inhibitor Factor IX deficiency (%) With inhibitor Haemophilia severity Severe Moderate Indication for first CVAD insertion (%) Prophylaxis ITI Difficult venous access Median age at initial CVAD insertion (range), years Age at initial CVAD insertion, years (%) <2 2–6 >6 Median age at transition to peripheral veins (range), years Number of CVADS Number of CVADs per patient (%) One Two Three Four Median duration of CVAD placement (range), days 58 54 (93) 11 4 (7) 0 53 5 51 (88) 6 (10.3) 1 (1.7) 1.02 (0.11–9.14) 52 (90) 2 (3) 4 (7) 8.21 (2.62–16.18) 106 25 (43) 23 (40) 6 (10) 4 (7) 1159/(3–3778) CVAD, central venous access device; ITI, immune tolerance induction. Thirty-nine CVADs (37%) were removed electively after a median of 1175 catheter days because of improved peripheral venous access. Fifty of all the 106 CVADs (47%) required removal because of some complication. The most frequent complication was malfunction (20%, 21 of 106 CVADs). Other reasons were CVAD-related infections (11%), mechanical complications (9%), local skin/tunnel infection (5%) and thrombosis (2%). Port survival according to the reason for removal is shown in Table 2 and Fig. 1. Only two early complications occurred: one Staphylococcus aureus septicaemia was encountered within the first 2 weeks after CVAD positioning and one major bleed in the area of port entry. The bleed was recorded in a patient with inhibitor, and it required CVAD removal 3 days after its insertion. No other complications such as pneumothorax or arrhythmias as a consequence of catheter insertion procedure were recorded. No mortality due to any CVAD-related complication was detected. The majority (66%) of the complications requiring CVAD removal were non-infectious. Malfunction, a catheter blockade or an occlusion in the absence of documented thrombosis, was associated with 21 of 106 CVADs (20%) after the long survival, a median of 1640 CVAD days, with a complication rate (CR) 0.15 per 1000 CVAD days. Ten CVADs (9%) were removed because of a mechanical complication (CR 0.07) with a shorter survival (445 CVAD days, median). Three of the mechanical complications were skin erosion over the port, three CVADs had to be removed after displacement (malpositioning of the catheter tip) and four after disconnection or splitting of the catheter. Two clinically significant CVAD-related thrombosis were recorded. One non-inhibitor patient had a thrombosis in the brachiocephalic vein and a patient with inhibitor suffered a thrombosis in the jugularis vein during a bleeding episode while treated with bypassing agents without concomitant ITI. The initial symptom had been a CVAD malfunction. A total of 17 CRBSIs were detected in 14 CVADs and in 12 (71%) of these cases required CVAD Table 2. Outcome of all 106 CVADs. Removed CVADs Infectious complications CVAD-associated bloodstream infection Local skin/tunnel infection Non-infectious complications Malfunction Mechanical Thrombosis in situ Uncomplicated removal (improved venous access) Still in use without any complication Number of CVADs (non-inhibitor patients), n = 86 Number of CVADs (inhibitor patients), n = 20 Total number of CVADs, n = 106 Median duration, days 74 15 89 1227 9 3 3 2 12 5 937 846 19 8 1 34 2 2 1 5 21 10 2 39 1640 445 831 1175 12 5 17 850 CVAD, central venous access device. Haemophilia (2015), 1--7 © 2015 John Wiley & Sons Ltd COMPLICATIONS ASSOCIATED WITH CVAD IN CHILDREN Local skin or tunnel infections (n = 5) requiring CVAD removal (n = 2) were very rare. No CVAD had to be removed because of clinically suspected CVAD-related infection. Complication rates for the different risk groups are summarized in Table 4. removal. The overall rate of bloodstream infection was very low, 0.12 infections per 1000 CVAD days. Five infections developed in three children with inhibitors (three infections during ITI) and 12 infections occurred in 11 children without inhibitors. The infection rate for children with inhibitors was 0.31 per 1000 CVAD days, and for children without inhibitor 0.1 per 1000 CVAD days, P = 0.004. Three (18%) of the 17 CVAD-related infections were detected when the child was under 2 years, 4 (23%) at the age of 2– 6 years and the rest (59%) over 6 years of age. Grampositive organisms were responsible for CVAD-associated bloodstream infections (Table 3). Thirteen CRBSIs were observed in 90 (14.4%) heparinized ports and four infections in 16 (25%) non-heparinized ports. Bloodstream infection rate per 1000 CVAD days was 0.11 for heparinized and 0.25 for non-heparinized ports, P = 0.30 (a total follow-up 121 974 and 15 997 days respectively). Discussion This relatively large nationwide study of 106 CVADs in 58 paediatric patients with 137 971 CVAD followup days reports a very low CVAD-related bloodstream infection rate: 0.12/1000 CVAD days for all and 0.10/ 1000 for non-inhibitor patients. Previous reports have described a wide variety of higher infection rates (0.2– 3.4 infections/1000 CVAD days) [1,2,4–6,11–13]. In a large meta-analysis with 2704 haemophilia patients and 2973 CVADs [3], infection was the most common reason for removal and the incidence of infection was 0.66/1000 catheter days. This might be partly explained by the high number of external CVADs (22.6%), the incidence of infection in the fully implanted CVADs was 31% of that with external CVADs. One small retrospective single centre study with 44 CVADs has reported a similar low infection rate (0.13/1000 CVAD days) as in our study [14], but they concentrated only on those complications which required the CVAD removal. Median age at initial CVAD insertion was higher (22 vs. 12 months) and there were fewer patients with inhibitors (16% vs. 19%). Our low frequency of complications may reflect the meticulous and harmonized techniques, centralized insertion policy, skilled nursing and strong support of the parents who maintain the devices at home. The meta-analysis showed that young age at insertion significantly increased the risk for infections: patients over 6 years were 46% less likely to develop infection than children aged 2–6 years [3]. In contrast, in our cohort a young age did not increase the risk of infections. The majority (59%) of the CVAD-related infections were recorded when the child was above 6 years of age and only 18% of the infections were Fig. 1. Port outcome according to the reason for removal. *One with concomitant major bleeding event. Table 3. Pathogens responsible for CVAD-related bloodstream infections. Pathogen Staphylococcus epidermidis Staphylococcus aureus Bacillus cereus Enterococcus faecium n (%) 5 10 1 1 CVAD removal required (29) (59) (6) (6) 5 3 8 1 0 CVAD, central venous access device. Table 4. Complications associated with CVAD and Influence of inhibitor. Number of CVADs Total number of CVAD days Median duration of CVAD placement (range), days Complication Malfunction CVAD-associated bloodstream infection Mechanical Local skin/tunnel infection Thrombosis ALL patients, n = 58 Non-inhibitor patients, n = 47 Patients with inhibitor, n = 11 P 106 137 971 1159 (3–3778) 86 122 053 1501 (3–3507) 20 15 918 782 (21–3778) 0.002 Incidence rate (per 1000 CVAD days)/(CI) Incidence rate (per 1000 CVAD days)/(CI) Incidence rate (per 1000 CVAD days)/(CI) 0.15/(0.10–0.23) 0.12/(0.08–0.20) 0.16/(0.10–0.24) 0.10/(0.06–0.17) 0.13/(0.03–0.50) 0.31/(0.13–0.75) 0.669 0.004 0.07/(0.04–0.13) 0.03/(0.02–0.09) 0.01/(0.00–0.06) 0.07/(0.03–0.13) 0.02/(0.01–0.08) 0.01/(0.00–0.06) 0.13/(0.03–0.50) 0.13/(0.03–0.50) 0.06/(0.01–0.45) 0.638 0.025 0.062 CVAD, central venous access device; CI, 95% confidence interval per 1000 CVAD days. © 2015 John Wiley & Sons Ltd Haemophilia (2015), 1--7 6 € AINEN € K. VEPS AL et al. detected when the child was below 2 years, and 23% at the age of 2–6 years. When considering the patients’ very young age at CVAD insertion (90% of children were under 2 years of age at the first port implantation), our results with the very low infectious CR must be considered encouraging. Inhibitors enhanced the CVAD-related infection rates by 3-fold. This is consistent with a previous meta-analysis, which concluded that inhibitors at insertion significantly increased the infectious risk [2,3,5,6,15]. The reason for that is mostly due to more frequent, usually daily device usage during ITI. We recorded similar infection rates with heparinized and non-heparinized ports. However, as in our study, the use of heparin was left to the discretion of the treating physician and not systemized, the comparison remains descriptive. The preventive influence of heparin flushing on catheter-related infections still remains unresolved and prospective evaluation of this practice should be conducted in the future. Central venous access device care guidelines have been as uniform and congruent as possible in our country. Handling scheme recommendations have been agreed frequently by our national haemophilia expert team. However, the retrospective survey with a long study period for 17 years has its limitations of data integrity and the ability to detect all changes with the port handling and teaching techniques to the parents among the different centres along the follow-up time. Non-infectious complications were the most common reason for CVAD removal. Incidence of mechanical complications is similar to previously reported [2], but the malfunction rate seems to be higher than that previously reported [4,5,14,15]. When comparing to the findings of a meta-analysis [3], CVADs remained in situ for twice as long; the median life span was 1159 days (3.2 years) in our cohort compared to 578 days (95% CI 456–733 days) in the meta-analysis. Indwelling duration was even longer before malfunctions: they were recorded after a median of 1640 CVAD days. Some malfunctions may be explained by increasing the age of the patient and growth, e.g. the catheter tip may be dislodged during the growth of the child. References 1 Ljung R. The risk associated with indwelling catheters in children with haemophilia. Br J Haematol 2007; 138: 580–6. 2 Titapiwatanakun R, Moir C, Pruthi RK, Stavlo PL, Schmidt KA, Rodriguez V. Central venous access devices for paediatric patients with haemophilia: a single-institution experience. Haemophilia 2009; 15: 168–74. 3 Valentino LA, Ewenstein B, Navickis RJ, Wilkes MM. Central venous access devices in haemophilia. Haemophilia 2004; 10: 134–46. Haemophilia (2015), 1--7 In the meta-analysis, the CR for CVAD-related thrombosis was 0.056 per 1000 CVAD days [3]. This incidence reported may be an underestimate, because most CVAD-related thrombi are probably clinically silent [16]. Two clinically significant thrombotic complications were observed in our cohort. The initial symptom was CVAD malfunction. We were unable to assess the prevalence of silent thrombosis because venograms, ultrasound or MRI-angiography were not routinely performed. A recent study investigating 20 children screened by MRI after the removal of CVAD, found a high number of silent DVT (25% of patients had abnormal MRI, consistent with DVT corresponding to CR 0.10/1000 CVAD days) [17]. In conclusion, we report a significantly lower CVAD-related bloodstream infection rate than previously described, despite the fact that the majority (90%) of our patients were very young (≤2 years) at the time of first insertion, and the port access was frequent and long standing (lasting on median 1159 days). Our experience of CVAD emphasizes the safety of undertaking prophylactic factor concentrate administration via ports in very young children enabling home treatment. The meticulous handling of the insertion and subsequent management with guidance appear to be important and favour a good outcome with these types of ports. Acknowledgements This study was supported by a grant from Blood Disease Research Foundation, Finland and Foundation for Pediatric Research, Finland (to KV). Authorship KV collected the data, performed the statistical analyses and wrote the article. RL contributed to writing of the article. PR designed the research study, analysed the data and wrote the article. All authors read, edited and approved the final manuscript. Disclosures The authors stated that they had no interests which might be perceived as posing a conflict or bias. 4 Yeoh ZH, Furmedge J, Ekert J, Crameri J, Curtis N, Barnes C. Central venous access device-related infections in patients with haemophilia. J Paediatr Child Health 2013; 49: 242–5. 5 Mancuso ME, Mannucci PM, Sartori A, Agliardi A, Santagostino E. Feasibility of prophylaxis and immune tolerance induction regimens in haemophilic children using fully implantable central venous catheters. Br J Haematol 2008; 141: 689–95. 6 Van Dijk K, Van Der Bom JG, Bax KN, Van Der Zee DC, Van Den Berg MH. Use of implantable venous access devices in children with severe hemophilia: benefits and burden. Haematologica 2004; 89: 189– 94. 7 Cesaro S, Tridello G, Cavaliere M et al. Prospective, randomized trial of two different modalities of flushing central venous catheters in pediatric patients with cancer. J Clin Oncol 2009; 27: 2059–65. 8 Mitchell MD, Anderson BJ, Williams K, Umscheid CA. Heparin flushing and other interventions to maintain patency of central venous catheters: a systematic review. J Adv Nurs 2009; 65: 2007–21. © 2015 John Wiley & Sons Ltd COMPLICATIONS ASSOCIATED WITH CVAD IN CHILDREN 9 Mermel LA, Allon M, Bouza E et al. Clinical practice guidelines for the diagnosis and management of intravascular catheterrelated infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49: 1–45. 10 Schulman S, Angeras U, Bergqvist D et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010; 8: 202–4. 11 Bollard CM, Teague LR, Berry EW, Ockelford PA. The use of central venous catheters (portacaths) in children with haemophilia. Haemophilia 2000; 6: 66– 70. © 2015 John Wiley & Sons Ltd 12 McMahon C, Smith J, Khair K, Liesner R, Hann IM, Smith OP. Central venous access devices in children with congenital coagulation disorders: complications and long-term outcome. Br J Haematol 2000; 110: 461–8. 13 Tarantino MD, Lail A, Donfield SM et al. Surveillance of infectious complications associated with central venous access devices in children with haemophilia. Haemophilia 2003; 9: 588–92. 14 Upadhyaya M, Richards M, Buckham S, Squire BR. Long-term results of central venous access devices in children with haemophilia. Pediatr Surg Int 2009; 25: 503– 6. 7 15 Jeng MR, O’Brien M, Wong W et al. Monthly recombinant tissue plasminogen activator administration to implantable central venous access devices decreases infections in children with haemophilia. Haemophilia 2009; 15: 1272–80. 16 Kamphuisen PW, Lee AY. Catheter-related thrombosis: lifeline or a pain in the neck? Hematology Am Soc Hematol Educ Program 2012; 2012: 638–44. 17 Ranta S, Kalajoki-Helmio T, Pouttu J, Makipernaa A. MRI after removal of central venous access device reveals a high number of asymptomatic thromboses in children with haemophilia. Haemophilia 2012; 18: 521–6. Haemophilia (2015), 1--7