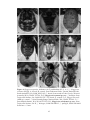
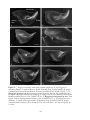
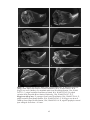
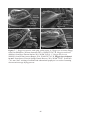
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Conservation of Spiders (Araneae) in the Western Australian
Introduced species wikipedia , lookup
Molecular ecology wikipedia , lookup
Ecological fitting wikipedia , lookup
Island restoration wikipedia , lookup
Theoretical ecology wikipedia , lookup
Occupancy–abundance relationship wikipedia , lookup
Latitudinal gradients in species diversity wikipedia , lookup
Biodiversity action plan wikipedia , lookup
Reconciliation ecology wikipedia , lookup
Habitat conservation wikipedia , lookup
Conservation of Spiders (Araneae) in the Western Australian Rangelands, with Particular Reference to Disturbance by Fire Peter R Langlands (B.Sc. Hons) This thesis is presented for the degree of Doctor of Philosophy of the University of Western Australia School of Animal Biology November 2010 Cover picture: A female Hoggicosa bicolor (Hogg, 1905) SUMMARY Fire is a common disturbance in many ecosystems and an integral part of arid Australia. However, there are many gaps in the current knowledge of how the fauna and flora of the arid zone respond to fire. This is particularly true for invertebrates, including spiders, where there are several obstacles to successful biodiversity conservation. Besides lack of knowledge of the response of spiders to fire, there is also a considerable taxonomic impediment. That is, a majority of species are described long ago or not at all. I set out to improve our knowledge for the conservation of spiders in arid Australia by removing part of this taxonomic impediment and examining the long-term response of spiders to fire. To help remove the taxonomic impediment and facilitate my investigations of the postfire response of spiders, I revise the taxonomy and systematics of a genus of wolf spiders. The Australian wolf spider genus Hoggicosa Roewer, 1960 with the type species Hoggicosa errans (Hogg, 1905), is revised to include ten species. Hoggicosa is comprised of burrowing lycosids, several constructing doors from sand or debris, and are predominantly found in semi-arid to arid regions of Australia. Of nine previously described species, three are synonymised with Hoggicosa castanea (Hogg, 1905) comb. nov., and four new species are described. A phylogenetic analysis including nine Hoggicosa species, 11 lycosine species from Australia and four from overseas, with Arctosa cinerea Fabricius, 1777 as outgroup, supported the monophyly of Hoggicosa. Proposed synapomorphies for Hoggicosa are a larger distance between the epigynum anterior pockets compared to the width of the posterior transverse part in females, and a large number of specialised setae on the tip of the cymbium in males. The analysis found that an unusual sexual dimorphism for wolf spiders (females more colourful than males), evident in four species of Hoggicosa, has evolved multiple times. To examine the post-fire changes in assemblages of spiders I established a chronosequence study in spinifex habitat of central Western Australia. Ground-active spiders were pitfall-trapped over nine months in 27 sites representing experimental fires (0 and 0.5 years) and wildfires (3, 5, 8 and 20 years post-fire). Three questions were examined: 1) Is there a successional pattern following disturbance by fire? 2) If so, what best explains this post-fire successional pattern? and 3) To what extent can the post-fire response of spiders be predicted? i I examine the first question by assessing if the spider assemblage displays a successional pattern, and if this is deterministic (i.e. if the assemblage converges to a long unburnt state). There were significant non-linear changes in species richness and evenness, along with marked changes in composition of spiders with increasing postfire age. For all three measures, the assemblage appears highly deterministic, converging towards the long unburnt state. Similarity in richness, evenness and species composition to the 20-year-old sites all increased with increasing time since fire (3 to 8 years). However, experimentally burnt sites did not fit this sequence neatly. Two alternative hypotheses are proposed to explain this second trajectory: inertia within the system, or the rapid migration and recolonization from nearby surrounding unburnt areas. A vast array of variables can act singly or in combination to influence an area‟s biota. In arid Australia, fire and rainfall are two key and potentially interacting forces that contribute in shaping assemblages of plants and animals. However, the degree to which spatial processes, environmental variables and biotic processes combine with fire and rainfall to shape assemblages of animals remains poorly quantified. As such, I assessed and quantified which variables can best explain the observed post-fire patterns in species richness and composition of spiders. Along with post-fire age, I examined the short-term effect of rainfall, the influence of space, environmental variables (soil, floristics, habitat structure) and biotic processes (predation by scorpions, small insectivorous mammals and reptiles). Predictor variables explained 79% of the variation in species richness and 32% in composition of spiders using distance-based redundancy analysis. Variation partitioning identified post-fire age as explaining the largest proportion of this variation, 61% for species richness and 78% for composition. However, spatially and non-spatially structured vegetation variables (floristics and habitat structure) explained much of this variation. Rainfall explained a large proportion of the explained variation for species richness (59%), but not species composition (30%). There was little variation attributable to predation (0 and 14% for species richness and composition, respectively) or pure spatial structure (5 and 5%). Developing a predictive understanding of how species assemblages respond to fire is a key conservation goal. In moving from solely describing patterns following fire to predicting changes, plant ecologists have successfully elucidated generalisations based ii on functional traits. Using species-traits might also allow better predictions for fauna but there are few empirical tests of this approach. To examine the third question I tested whether species-traits changed with post-fire age for spiders. I made a priori predictions as to whether spiders with ten traits associated with survival, dispersal, reproduction, resource-utilization and microhabitat occupation would increase or decrease with postfire age. I then tested these predictions using a direct (fourth-corner on individual traits and composite traits) and an indirect approach (emergent groups), comparing the benefits of each and also examining the degree to which traits were inter-correlated. For the seven individual traits that were significant in the fourth-corner analysis, three followed predictions (body size, abundance of burrow ambushers and burrowers were greater in recently burnt sites); two were opposite (heavy sclerotisation of cephalothorax and time to maturity were in greater abundance in long unburnt and recently burnt sites respectively); and two displayed response patterns more complex than predicted (abdominal scutes displayed a U-shaped response and dispersal ability a hump shaped curve). However, within a given trait, there were few significant results among post-fire ages. Several traits were inter-correlated and scores based on composite traits used in a fourth-corner analysis found significant patterns, but slightly different to those using individual traits. Changes in abundance with post-fire age were significant for three of the five emergent groups. The fourth-corner analysis yielded more detailed results, but overall the two approaches are considered complementary. These studies make a substantial contribution of new knowledge to assist with the conservation of spiders in the arid zone of Australia. I have removed the taxonomic impediment for a charismatic and common genus of wolf spiders. In addition, I have examined in detail the post-fire response of an assemblage of spiders in spinifex habitat. The high degree of determinism in this assemblage and the strong correlation between post-fire changes in the vegetation and spiders is promising for land managers. It suggests that managers can directly influence the conservation of the spider fauna by actively managing fire effects on habitat structure and floristics. The development of a traits-based approach, which may contribute to predicting post-fire responses of spiders in other habitats, was only moderately successful. However, the development of such a mechanistic approach is novel and deserves further attention. iii CONTENTS SUMMARY ....................................................................................................................... i CONTENTS ..................................................................................................................... iv ACKNOWLEDGEMENTS ............................................................................................. vi STRUCTURE OF THESIS ............................................................................................viii SECTION I: TAXONOMY AND SYSTEMATICS General Introduction ..................................................................................................... 1 CHAPTER 1: Systematic revision of Hoggicosa Roewer, 1960, the Australian „bicolor’ group of wolf spiders (Araneae: Lycosidae) .............................................. 5 Abstract ................................................................................................................. 6 Introduction ........................................................................................................... 7 Materials and Methods .......................................................................................... 9 Results ................................................................................................................. 12 Discussion ........................................................................................................... 14 Systematics ......................................................................................................... 17 References ........................................................................................................... 52 Appendix 1 .......................................................................................................... 83 Appendix 2 .......................................................................................................... 85 Appendix 3 .......................................................................................................... 88 Summary of Section I ................................................................................................ 100 SECTION II: ECOLOGY General Introduction ................................................................................................. 102 CHAPTER 2: Is the successional trajectory of an arid spider assemblage following fire deterministic? .................................................................................................. 109 Abstract ............................................................................................................. 110 Introduction ....................................................................................................... 111 Methods............................................................................................................. 113 Results ............................................................................................................... 117 Discussion ......................................................................................................... 119 References ......................................................................................................... 124 Appendix A ....................................................................................................... 137 Appendix B ....................................................................................................... 148 Appendix C ....................................................................................................... 139 Appendix D ....................................................................................................... 148 iv CHAPTER 3: Untangling the drivers of structure in animal assemblages in arid ecosystems: the influence of fire, rainfall, vegetation, predators and spatial structure on spiders (Araneae) ............................................................................... 151 Abstract ............................................................................................................. 152 Introduction ....................................................................................................... 153 Materials and methods ...................................................................................... 156 Results ............................................................................................................... 160 Discussion ......................................................................................................... 163 References ......................................................................................................... 168 Appendix A ....................................................................................................... 183 Appendix B ....................................................................................................... 184 Appendix C ....................................................................................................... 185 Appendix D ....................................................................................................... 186 CHAPTER 4: Predicting the post-fire responses of animal assemblages: testing a trait-based approach using spiders ......................................................................... 187 Summary ........................................................................................................... 188 Introduction ....................................................................................................... 189 Materials and Methods ...................................................................................... 192 Results ............................................................................................................... 197 Discussion ......................................................................................................... 199 Literature cited .................................................................................................. 205 Appendix S1...................................................................................................... 216 Appendix S2...................................................................................................... 218 Appendix S3...................................................................................................... 234 Appendix S4...................................................................................................... 235 Synthesis of Ecological Results and Future Research .............................................. 238 CONCLUSION ............................................................................................................. 243 v ACKNOWLEDGEMENTS Looking over the past years and back to when I started, I feel that I have changed and grown enormously, not just academically, but as a person. Each of my supervisors has contributed to these changes in their own way. I especially thank Bob Black, Barbara York Main, Volker Framenau and Karl Brennan for all they have taught me, and all the time they have spent with me over the years! Particularly, to Bob for his keen eye for detail, to Barbara for her words of encouragement and to Volker for his patience in teaching me taxonomy and systematics. Special thanks to my supervisor and mentor Karl Brennan for his support. Our conversations were so often engaging and stimulating and Karl never failed to suggest solutions when problems arose. Thanks also to his family (Melinda Moir and Bridgette) for their welcoming hospitality during my visits. I doubt I would have reached the end if not for the trips to Kalgoorlie to write. I thank Mark Harvey for providing me with workspace at the Western Australian Museum, along with all those who made my time there an enjoyable one: Julianne Waldock, Jung-Sun Yoo, Karen Edward, Ricardo Ott, Bill Humphreys, Mike Rix, Brian Hanich and Terry Houston. I thank also the many arachnologists who assessed my species identifications: Julianne Waldock, Volker Framenau and Ricardo Ott from the Western Australian Museum; Barbara Baehr and Robert Raven from the Queensland Museum and Barbara York Main from the University of Western Australia. The Western Australian Department of Environment and Conservation provided vital financial support, while staff past and present were invaluable. I thank the following: Bruce Ward and Graeme „tubs‟ Liddlelow for their desert knowledge and support in the field; Neil Burrows, Ian Kealley and Neville Hague for their support of the project; Ray Cranfield for his identification of plants and field assistance as well as Mark Cowan for identification of the vertebrate by-catch and stimulating discussions in the field. Graeme Behn and Li Shu provided GIS assistance that was crucial in determining fire histories. Brad Durrant and Nadine Guthrie generously loaned the pitfall traps. I thank the staff of the Department of Environment and Conservation Kalgoorlie office for their assistance in conducting experimental burns, with field trips and temporary vi office space: particularly Ryan Butler, Ian Kealley, Garry Hearle, Anthony Richardson, Brad Barton, Julie Waters, Steve Toole and Luke Puertoliano. To office companions and students past and present I extend thanks for all the company and coffee breaks: particularly Scott Carver, Sharron Perks, Karen Edward, Mike Rix, Janine Wojcieszek, Rachel Binks, Blair Parsons, Jen Francis, Aimee Silla, Andrew Williams, Sean Tomlinson, Sean Stankowski, Mike Young, Danilo Harms and Bruno Buzatto. I am grateful also to the many staff within the Animal Biology department who have helped over the years: Kerry Knott, Natalie Byrne, Rob Hurn, Tom Stewart, Dale Roberts, Helen Spafford and Graeme Martin. I also thank Mike Johnson and Jane Prince for being on my review panel. I am indepted to all the people who provided field assistance: Karen Debski, Amanda McGinty, Fernanda Ferreira, Christopher Taylor, Eliane „Lica‟ Assis and Filomeno „Phil‟ Cruz. I appreciated the great company and interesting discussions in the field of Tom Bragg and Barbi Hayes. I thank the attendees of Conservation Volunteers Australia for their assistance with the backbreaking work of creating firebreaks on the October 2005 trip (Louise Robertson, Alison Sparey, Graham Plowright, Marie-lina Muller, Andrea Schuster, Karen Lang, Min Kim and Russell Gorton). Additional satellite imagery was generously donated by Peter Sanders and Adrian Allen at Satellite Remote Sensing Services, Department of Land Information. Rainfall data was also provided free of charge by the Bureau of Meteorology, Perth, Western Australia. My Ph.D. research was funded by an Australian Postgraduate Award, with additional funding provided by the School of Animal Biology at the University of Western Australia, and the Department of Environment and Conservation. The Australian Biological Resources Study (ABRS) provided a conference travel grant. Finally, I thank my family (Bob, Natasha, Larissa, Andrew and Loretta plus Christopher) for their unwavering financial, physical and emotional support over the years and Jane for reviving my chirp! vii STRUCTURE OF THESIS There are two sections to my thesis: a taxonomic/systematic section and an ecological section. The primary focus of my work was ecological, but I undertook the taxonomic study to alleviate problems with identifications and to allow the use of the species I described in a detailed population study following fire. Therefore, my supervisors and I envisioned that the two sections would connect neatly, with one or more of the species described in the taxonomic component utilised in the ecological work. In addition, I anticipated that acquiring skills in taxonomy would prove indispensable in my future scientific career. Ultimately, the abundance and density of Hoggicosa wolf spiders was too low for conducting a population study, despite many weeks searching throughout the proposed Lorna Glen Conservation Park. Thus, although the direct connection between the two sections did not eventuate, the broader and underlying importance of taxonomic work for the conservation of spiders is self-evident. Ecological research conducted at the community level requires a sound taxonomic and systematic knowledge of the component species. I therefore consider both sections of this thesis contribute substantially to the broad aim of improving knowledge to conserve spiders in the arid zone of Australia. This thesis is prepared as a series of papers for publication, with each chapter representing a separate paper. For each paper, I have indicated the intended journal and publication status, along with contributions from co-authors. As each journal has slightly different formatting requirements, minor differences in formatting between chapters will be noticeable, along with some repetition in the method parts. However, the advantage of this approach is that I have honed my skills in preparing papers for publication, the currency of modern science, and this format will aid the rapid dissemination of my work. viii Publications arising from this thesis Chapter 1 Langlands, P. R., and V. W. Framenau. 2010. Systematic revision of Hoggicosa Roewer, 1960, the Australian 'bicolor' group of wolf spiders (Araneae: Lycosidae). Zoological Journal of the Linnean Society 158:83-123 See enclosed CD for pdf Chapter 2 Langlands, P. R., K. E. C. Brennan, and B. Ward. Is the successional trajectory of an arid spider assemblage following fire deterministic? Accepted for Austral Ecology pending minor revisions Chapter 3 Langlands, P. R., K. E. C. Brennan, and B. Ward. Untangling the drivers of animal assemblages in arid ecosystems: the influence of fire, rainfall, vegetation, higher predators and spatial structure on spiders (Araneae). In preparation for Ecography Chapter 4 Langlands, P. R., K. E. C. Brennan, V. W. Framenau, and B. Y. Main. Predicting the post-fire responses of animal assemblages: testing a trait-based approach using spiders. Accepted for the Journal of Animal Ecology ix Declaration of contributions For each manuscript, I was the main contributor and responsible for the experimental design, data collection, analysis and interpretation of results and writing. Three of my supervisors are co-authors (VW Framenau, KEC Brennan and BY Main) along with a colleague from the Department of Environment and Conservation (B Ward). My coauthors contributed to the experimental design, interpretation of results and writing. Author contributions to each manuscript: Chapter 1: PRL = 85%, VWF = 15% Chapter 2: PRL = 90%, KECB + BW = 10% Chapter 3: PRL = 90%, KECB + BW = 10% Chapter 4: PRL = 90%, KECB + VWF + BYM = 10%. Each author has given permission for all work to be included in this thesis. Signatures: _____________________________ Peter R Langlands (candidate) _____________________________ Adj. Prof Bob Black (co-ordinating supervisor) x SECTION I: TAXONOMY AND SYSTEMATICS SECTION I: TAXONOMY AND SYSTEMATICS General Introduction Australia has an extremely rich and diverse fauna of spiders with 78 families and approximately 3300 currently described species (Raven et al. 2002, Yeates et al. 2003, Chapman 2009). However, this represents only a fraction of the spider fauna, with the vast majority, estimated at 67%, awaiting formal description (Chapman 2009). Wolf spiders (Lycosidae) typify this scenario. Only 168 species of an estimated 400+ species have been described (V. W. Framenau pers. comm.). This lack of taxonomic knowledge is a major problem for ecologists wishing to study spiders, as sound taxonomy and species descriptions form the basis of ecology and conservation (Wilson 1971, Kim 1993, Kim and Byrne 2006). This problem, often termed the taxonomic impediment (New 1984, Wheeler et al. 2004, Evenhuis 2007), arises through two mechanisms, lack of species descriptions (or species descriptions that are old or inaccessible) and lack of taxonomic expertise (Kitching 1993, Brennan et al. 2004). Others however, have argued that the use of parataxonomists and the identification of morphospecies, recognizable taxonomic units or functional groups can address taxonomic impediments (Oliver and Beattie 1993, 1996 a, b, Pik 1999), but this has also been hotly debated (Brower 1995, Goldstein 1997, Oliver and Beattie 1997, Goldstein 1999, Beattie and Oliver 1999). The stance I have taken is to sort to species level for all spider families and have these identifications confirmed by taxonomic experts (See Acknowledgments). When no scientific names could be found or recent taxonomic references were lacking, for example the family Gnaphosidae, I assigned numerical codes for species. To reduce the risk of over-splitting species I chose to exclude females and juveniles and identify males only (Derraik et al. 2002). I set out to help remove this impediment for a common genus of wolf spiders and in the process gain skills to allow me to identify spiders more confidently to species level for my ecological work, where not surprisingly most species (82%) were undescribed. Wolf spiders were an ideal choice, as they are ubiquitous and abundant across all habitats in Australia (Main 1976). The family has also received recent attention in Australia, which provided a broad subfamily and generic framework (Framenau and Vink 2001, Framenau 2002, Framenau 2006 a, b, c, Framenau and Yoo 2006, Murphy et al. 2006, Framenau and Baehr 2007). Using this information, I selected a group, the „bicolor group‟, which was thought to represent a distinct genus (McKay 1973, 1975, 1 Murphy et al. 2006). The „bicolor group‟ was also suitable as it contained a limited number of species that was manageable in scope. Additionally, they are large, robust spiders that are easy to handle and are common throughout the arid zone of Australia. References Beattie, A. J. & Oliver, I. (1999) Biodiversity buzzwords: another reply to Goldstein. Conservation Biology, 13, 1514-1514. Brennan, K. E. C., Moir, M. L. & Majer, J. D. (2004) Exhaustive sampling in a Southern Hemisphere global biodiversity hotspot: inventorying species richness and assessing endemicity of the little known jarrah forest spiders. Pacific Conservation Biology, 10, 241-260. Brower, A. V. Z. (1995) Taxonomic minimalism. Trends in Ecology & Evolution, 10, 203-203. Chapman, A. D. (2009) Numbers of Living Species in Australia and the World, 2nd edition, Australian Biological Resources Study, Canberra, Australia. Derraik, J. G. B., Closs, G. P., Dickinson, K. J. M., Sirvid, P., Barratt, B. I. P. & Patrick, B. H. (2002) Arthropod morphospecies versus taxonomic species: a case study with Araneae, Coleoptera and Lepidoptera. Conservation Biology, 16, 1015-1023. Evenhuis, N.L. (2007) Helping solve the “other” taxonomic impediment: completing the Eight Steps to Total Enlightenment and Taxonomic Nirvana. Zootaxa, 1407, 3-12. Framenau, V. W. (2002) Review of the wolf spider genus Artoria Thorell (Araneae: Lycosidae). Invertebrate Systematics, 16, 209-235. Framenau, V. W. (2006 a) Knoelle, a new monotypic wolf spider genus from Australia (Araneae: Lycosidae). Zootaxa, 1281, 55-67. Framenau, V. W. (2006 b) Revision of the wolf spider genus Diahogna Roewer, 1960 (Araneae, Lycosidae). Journal of Natural History, 40, 273-292. Framenau, V. W. (2006 c) Revision of the Australia wolf spider genus Anomalosa Roewer, 1960 (Araneae: Lycosidae). Zootaxa, 1304, 1-20. Framenau, V. W. & Baehr, B. C. (2007) Revision of the Australian wolf spider genus Dingosa Roewer, 1955 (Araneae, Lycosidae). Journal of Natural History, 41, 1603-1629. Framenau, V. W. & Vink, C. J. (2001) Revision of the wolf spider genus Venatrix Roewer (Araneae: Lycosidae). Invertebrate Systematics, 15, 927-970. 2 Framenau, V. W. & Yoo, J.-S. (2006) Systematics of the new Australian wolf spider genus Tuberculosa (Araneae : Lycosidae). Invertebrate Systematics, 20, 185202. Goldstein, P. Z. (1997) How many things are there? A reply to Oliver and Beattie, Beattie and Oliver, Oliver and Beattie, and Oliver and Beattie. Conservation Biology, 11, 571-574. Goldstein, P. Z. (1999) Functional ecosystems and biodiversity buzzwords. Conservation Biology, 13, 247-255. Kim, K. & Byrne, L. (2006) Biodiversity loss and the taxonomic bottleneck: emerging biodiversity science. Ecological Research, 21, 794-810. Kim, K. C. (1993) Biodiversity, conservation and inventory: why insects matter. Biodiversity and Conservation, 2, 191-214. Kitching, R. L. (1993) Biodiversity and taxonomy: impediment or opportunity. Conservation Biology in Australia and Oceania (eds C. Moritz & J. Kikkawa), pp. 253–268. Surrey Beatty and Sons, Chipping, Norton, Australia. Main, B. Y. (1976) Spiders, Collins, Sydney. McKay, R.J. (1973) The wolf spiders of Australia (Araneae: Lycosidae): 1. The bicolor group. Memoirs of the Queensland Museum, 16, 375-398. McKay, R.J. (1975) The wolf spiders of Australia (Araneae: Lycosidae): 5. Two new species of the bicolor group. Memoirs of the Queensland Museum, 17, 313-318. Murphy, N. P., Framenau, V. W., Donnellan, S. C., Harvey, M. S., Park, Y.-C. & Austin, A. D. (2006) Phylogenetic reconstruction of the wolf spiders (Araneae: Lycosidae) using sequences from the 12S rRNA, 28S rRNA, and NADH1 genes: Implications for classification, biogeography, and the evolution of web building behavior. Molecular Phylogenetics and Evolution, 38, 583-602. New, T. R. (1984) Insect Conservation: An Australian Perspective, Junk, Dordrecht, The Netherlands. Oliver, I. & Beattie, A. J. (1993) A possible method for the rapid assessment of biodiversity. Conservation Biology, 7, 562-568. Oliver, I. & Beattie, A. J. (1996 a) Designing a cost effective invertebrate survey: a test of methods for rapid assessment of biodiversity. Ecological Applications, 6, 594-607. Oliver, I. & Beattie, A. J. (1996 b) Invertebrate morphospecies as surrogates for species: a case study. Conservation Biology, 10, 99-109. 3 Oliver, I. & Beattie, A. J. (1997) Future taxonomic partnerships: reply to Goldstein. Conservation Biology, 11, 575-576. Pik, A. J., Oliver, I. & Beattie, A. J. (1999) Taxonomic sufficiency in ecological studies of terrestrial invertebrates. Australian Journal of Ecology, 24, 555-562. Raven, R. J., Baehr, B. C. & Harvey, M. S. (2002) Spiders of Australia: Interactive identification to subfamily. CSIRO Publishing, Canberra. Wheeler, Q. D., Raven, P. H. & Wilson, E. O. (2004) Taxonomy: impediment or expedient? Science, 303, 285. Wilson, E. O. (1971) The plight of taxonomy. Ecology, 52. Yeates, D. K., Harvey, M. S. & Austin, A. D. (2003) New estimates for terrestrial arthropod species-richness in Australia. Records of the South Australian Museum Monograph Series No.7, 231-241. 4 CHAPTER 1 Systematic revision of Hoggicosa Roewer, 1960, the Australian ‘bicolor’ group of wolf spiders (Araneae: Lycosidae) Peter R Langlands Volker W Framenau Zoological Journal of the Linnean Society, 2010, 158, 83–123 5 ABSTRACT The Australian wolf spider genus Hoggicosa Roewer, 1960 with the type species Hoggicosa errans (Hogg, 1905) is revised to include ten species: Hoggicosa alfi sp. nov.; Hoggicosa castanea (Hogg, 1905) comb. nov. (= Lycosa errans Hogg, 1905 syn. nov.; = Lycosa perinflata Pulleine, 1922 syn. nov.; = Lycosa skeeti Pulleine, 1922 syn. nov.); Hoggicosa bicolor (Hogg, 1905) comb. nov.; Hoggicosa brennani sp. nov.; Hoggicosa duracki (McKay, 1975) comb. nov.; Hoggicosa forresti (McKay, 1973) comb. nov.; Hoggicosa natashae sp. nov.; Hoggicosa snelli (McKay, 1975) comb. nov.; Hoggicosa storri (McKay, 1973) comb. nov.; and Hoggicosa wolodymyri sp. nov. The Namibian Hoggicosa exigua Roewer, 1960 is transferred to Hogna, Hogna exigua (Roewer, 1960) comb. nov. A phylogenetic analysis including nine Hoggicosa species, 11 lycosine species from Australia and four from overseas, with Arctosa cinerea Fabricius, 1777 as outgroup, supported the monophyly of Hoggicosa, with a larger distance between the epigynum anterior pockets compared to the width of the posterior transverse part. The analysis found that an unusual sexual dimorphism for wolf spiders (females more colourful than males), evident in four species of Hoggicosa, has evolved multiple times. Hoggicosa are burrowing lycosids, several constructing doors from sand or debris, and are predominantly found in semi-arid to arid regions of Australia. 6 INTRODUCTION Australia possesses a rich fauna of wolf spiders with 156 currently described species in 24 genera and four subfamilies: Artoriinae Framenau, 2007; Venoniinae Lehtinen & Hippa, 1979; Zoicinae Lehtinen & Hippa, 1979; and Lycosinae Sundevall, 1833 (Framenau, 2007). The latter is the most diverse subfamily in Australia with 109 described species in 17 genera, although the current count represents possibly fewer than half of the species present in collections (V. W. Framenau, unpubl. data). The Lycosinae are defined by two synapomorphies of the tegular apophysis of the male pedipalp, i.e. its transverse orientation, with ventrally directed spur and a sinuous channel on its dorsal surface (Dondale, 1986). Knowledge of Australian Lycosinae is poor, although there have been several recent generic revisions, including Dingosa Roewer, 1955, Knoelle Framenau, 2006, Mainosa Framenau, 2006 and Tuberculosa Framenau & Yoo, 2006 and Venatrix Roewer, 1960 (Framenau & Vink, 2001; Framenau, 2006a, b; Framenau & Yoo, 2006; Framenau & Baehr, 2007). Molecular studies have suggested two distinct clades of Australian Lycosinae; one has an oriental origin (Venatrix and Tuberculosa) and the remaining genera represent a Gondwanan clade with the closest relative in the taxon sampled, Pavocosa gallopavo (Mello-Leitão, 1941), from South America (see Murphy et al., 2006; Gotch et al., 2008). Hoggicosa Roewer, 1960 clearly conforms to the subfamily diagnosis of the Lycosinae (Dondale, 1986). The genus was initially proposed as nomen nudum by Roewer (1955). Later it was formally described with the Australian Lycosa errans Hogg, 1905 as type species (Roewer, 1960) along with a second species from Namibia, Hoggicosa exigua Roewer, 1960. Roewer distinguished Hoggicosa by the shape of the labium (as long as wide) in combination with the curvature of the anterior eye row (straight). The somatic characters that Roewer (1959, 1960) used to diagnose many of his genera have since been found to be of limited value for wolf spider classification compared with more recently employed genitalic characters (see Vink, 2001; Stratton, 2005). Hoggicosa was later considered a junior synonym of Arkalosula Roewer, 1960, which is itself a junior synonym of Arctosa C. L. Koch, 1847 (Guy, 1966: 64). Guy‟s (1966) taxonomic changes, however, were not accepted by later cataloguers (e.g. Brignoli, 1983; Platnick, 2009). In a review of a well-defined Australian group of wolf spiders that he called the „bicolor group‟ based on the striking colour combination of some females (see Fig. 1), McKay 7 (1973) transferred Hoggicosa errans back to Lycosa without providing a taxonomic justification. However, McKay (1973) suggested close affinities of his „bicolor group‟ with Geolycosa Montgomery, 1904. Later McKay (1975) added two more species to the „bicolor group‟ and described males, apparently less conspicuously coloured than females, for the first time. Sexual dimorphism in wolf spiders is evident in a multitude of forms, with most differences between males and females being attributed to reproductive roles (sexual selection). Females of ground dwelling spiders, including lycosids, are generally larger than their male counterparts, explained by a fecundity advantage of larger females (Prenter, Montgomery & Elwood, 1997; Prenter, Elwood & Montgomery, 1998, 1999). Sexual dimorphism in wolf spiders also exists in relative size of chelicerae and venom glands (Walker & Rypstra, 2001, 2002) and relative length of legs (Framenau, 2005). Some dimorphic colour patterns augment body size and condition and have been argued to play an important role in the mating behaviour of wolf spiders (Moya-Laraño, Taylor & Fernandez-Montraveta, 2003). Male wolf spiders generally have more conspicuous colour patterns than females, although the patterns are not pronounced in many species. This may play an important role in mate choice by females; for example, fore leg ornamentation of male lycosids was reviewed by Framenau & Hebets (2007). More cryptic colours of females may also have evolved through natural selection; wolf spider females exhibit prolonged brood care and cryptic colouration may protect the females and their clutch from predators such as birds, reptiles, and predatory insects. Some Hoggicosa species, however, show a remarkable reversed colour dimorphism. Females of Hoggicosa bicolor (similarly coloured as penultimate males, Fig. 1A), Hoggicosa storri (similarly coloured as penultimate males, Fig. 1D), Hoggicosa castanea, and Hoggicosa forresti (McKay, 1973, fig. 1F) are much more colourful than their male counterparts (Fig. 1B, E), but the evolution of these striking patterns has not been studied. This paper revises the taxonomy and systematics of Hoggicosa, which is here reestablished as a valid genus. We test the monophyly of this genus with a phylogenetic analysis including 11 Australian representatives of the subfamily Lycosinae and representatives of the northern hemisphere genera Lycosa and Geolycosa, with which Hoggicosa was previously associated. This phylogenetic analysis also allowed us to test 8 the origin of the striking colour dimorphism of males and females, evident in four species of Hoggicosa. MATERIAL AND METHODS This study is based on an examination of more than 20 000 records of wolf spiders in all Australian museums as part of an extensive revision of the Australian Lycosidae. Descriptions are based on spiders stored in 70% ethanol, which were studied under a Leica MZ6 stereo microscope. For examination of female internal genitalia, epigynes were placed in lactic acid overnight. Digital images were taken using a Leica DFC 500 digital camera attached to a Leica MZ16A stereo microscope. To increase depth of field, multiple images were merged using the software program AutoMontage Pro Version 5.02 (p). These images were then placed into Adobe Illustrator CS3 and electronic drawings compiled by manually tracing over the structures in the image. For clarity, setae without diagnostic value have been omitted from all drawings. Male pedipalps mainly differ by details of the median apophysis and palea, but these details are not discernable in retrolateral view; therefore, although generally illustrated in taxonomic papers of Lycosidae, these views are omitted here. All measurements are in mm. Scanning electron micrographs (SEMs) were taken using a Philips XL30 ESEM at the Centre for Microscopy and Microanalysis, University of Western Australia. In preparation for SEMs, specimens were placed in 100% ethanol overnight. They were then cleaned ultrasonically for 2 min and critically point dried. Specimens were then mounted on stubs using carbon tabs and coated with 10–15 nm of gold. Morphological nomenclature follows Framenau & Baehr (2007), but some terms are introduced here for the first time. We identify three parts of the tegular apophysis: a „ventral spur‟, an „apical point‟, and „ridge‟ joining these (see Fig. 5A). The pars pendula is a membranous flap accompanying the embolus on its apical edge (in relation to the pedipalp) (see Dondale & Redner, 1983), which often joins the palea basally. We found a secondary structure basal to the terminal apophysis in all Hoggicosa, which we call the „subterminal apophysis‟ (see Figs 7A–D, 13B). This structure was erroneously labelled pars pendula in the recent description of the genus Knoelle (Framenau, 2006b, fig. 8). 9 Abbreviations Anatomy AE, anterior eyes; ALE, anterior lateral eyes; AME, anterior median eyes; OL, opisthosoma length; OW, opisthosoma width; PL, prosoma length; PW, prosoma width; PE, posterior eyes; PLE, posterior lateral eyes; PME, posterior median eyes; TL, total length. Institutions AM, Australian Museum, Sydney; ANIC, Australian National Insect Collection, Canberra; NMV, Museum Victoria, Melbourne; NTMAG, Northern Territory Museum and Art Gallery, Darwin; SAM, South Australian Museum, Adelaide; QM, Queensland Museum, Brisbane; WAM, Western Australian Museum, Perth; Distribution; NSW, New South Wales; NT, Northern Territory; Qld, Queensland; SA, South Australia; Vic, Victoria; WA, Western Australia. Phylogenetic Analysis Taxa A number of factors determined which taxa in addition to nine Hoggicosa species were included in the analysis (Hoggicosa natashae sp. nov. was excluded as only females are known). 1. We included most Australian genera that were part of a Gondwanan clade, to which Hoggicosa belonged, in a molecular phylogeny of the Lycosidae (Murphy et al., 2006): Hogna crispipes L. Koch, 1877, Lycosa godeffroyi L. Koch, 1865, Lycosa leuckartii (Thorell, 1870), Knoelle clara (L. Koch, 1877), and the „Grey Wolf Spider‟ („New Genus 6‟ in Murphy et al., 2006; see also Framenau & Baehr, 2007). This allowed testing of the relationships found by Murphy et al. (2006) with our morphological character set. 2. Based on morphological and behavioural characters, two putative Australian sister genera were included in the analysis. Males of the monotypic genus Knoelle also possess backwards bent macrosetae on the cymbium tip, but in a larger patch than Hoggicosa (Framenau, 2006b). The undescribed „Grey Wolf Spider‟ has similar somatic morphology and burrowing behaviour as Hoggicosa species. 3. Two Australian lycosine genera were recently reviewed, which allowed an appropriate consideration within our phylogenetic analysis: Mainosa Framenau, 2006 and Dingosa Roewer, 1955 (Appendix 1). 10 4. We included the type species of the Australian lycosine genus Venator Hogg, 1900, V. spenceri Hogg, 1900, and a putative conspecific with very similar somatic and genitalic morphology, Hogna immansueta (Simon, 1909). 5. Species of Hoggicosa were previously associated with two genera, Lycosa (Mediterranean; Zyuzin & Logunov, 2000) and Geolycosa (Holarctic; Wallace, 1942; Zyuzin & Logunov, 2000) and we included representatives of both to test their association with Hoggicosa. Male specimens of Lycosa tarantula (Linnaeus, 1758) and Lycosa praegrandis (C. L. Koch, 1836) were unavailable for study, so male characters were scored from secondary sources (Zyuzin & Logunov, 2000; Álvares, 2006). The limits of the Lycosinae are unclear, and this subfamily may include the traditional Pardosinae and Hippasinae (see Murphy et al., 2006). Therefore, we chose the Holarctic species Arctosa cinerea (Fabricius, 1777) (type species of Arctosa C. L. Koch, 1847) as outgroup, as this genus is basal to a wider concept of the Lycosinae (Murphy et al., 2006). Characters There are only a limited number of phylogenetic, cladistic, or phenetic studies exploring characters in the morphologically conservative family Lycosidae: Vink, 2002; Vink & Paterson, 2003; Stratton, 2005; Framenau & Yoo, 2006; Yoo & Framenau, 2006 (see also Roewer, 1959; Casanueva, 1980). We tested these characters and explored many new ones as part of our phylogenetic analysis. A total of 27 characters remained parsimoniously informative for the 25 species included in our analysis (Table 1; Appendix 2). Eight characters were based on somatic morphology, 16 on genitalic morphology (13 male and three female), and three on burrowing behaviour. We could not score characters relating to the subterminal apophysis of the male pedipalp when relying on secondary sources, therefore these were scored „?‟ for L. tarantula and L. praegrandis. Analyses The character matrix was edited using Nexus data editor (Page, 2001) and exported for phylogenetic analysis to TNT version 1.1 (Goloboff, Farris & Nixon, 2003, Willi Hennig Society version). We used the „traditional search‟ option with the following settings: number of replicates („repls‟) 1000, „trees to save per replication‟ at 10, „collapse trees after search‟ with default collapsing rule (min. length = 0), „replace 11 existing trees‟, and maximum trees in memory set to 100 000. A strict consensus tree was exported to Win-Clada vers. 1.00.08 (Nixon, 2002) for character exploration. Bremer support (Bremer, 1994) and relative Bremer support (Goloboff & Farris, 2001) values were calculated using TNT. RESULTS Hoggicosa now includes ten species of which four are described as new (Table 2). The type species Lycosa errans, but also Lycosa perinflata Pulleine, 1922 and Lycosa skeeti Pulleine, 1922 are synonymized with Hoggicosa castanea comb. nov. The males of Hoggicosa bicolor comb. nov., Hoggicosa castanea, Hoggicosa storri comb. nov., and Hoggicosa forresti comb. nov. are described for the first time. All Hoggicosa species construct burrows, some with trap doors, and are predominantly found in the more arid parts of Australia (Table 2). Phylogeny The phylogenetic analysis generated 26 equally parsimonious trees [length (L) = 96, consistency index (CI) = 38, retention index (RI) = 64]. The resulting strict consensus tree (L = 113, CI = 32, RI = 53) had little resolution in resolving relationships amongst genera; however, Hoggicosa was monophyletic (Fig. 2). Although Bremer support values for Hoggicosa are low, it is supported by one unambiguous synapomorphy: the distance between the anterior pockets of the female epigynum is greater than the width of the posterior transverse part (character 24). The genus is also unified by five homoplasious characters: the cephalic region of the carapace is elevated, strongly sloping away from the eyes in lateral view (1), the cymbium tip has ten to 30 macrosetae (10), the cymbium tip macrosetae are curved dorsally (11), the tip of terminal apophysis comes to a sharp point (17), and the subterminal apophysis is beneath the terminal apophysis and difficult to see in ventral view (19). Hoggicosa wolodymyri sp. nov. was found to be sister to all other Hoggicosa, which were defined by three homoplasious characters: the colour of the ventral abdomen of females is uniformly dark (8), the ridge connecting the ventral spur and apical tip on the tegular apophysis is curved (14), and trapdoors are present on burrows (26). Likewise, H. forresti was found to be sister to the remaining Hoggicosa species, which were defined by the presence of a light coloured cardiac or chevron mark on the dorsal abdomen of females (6) (Fig. 2). 12 The remaining Hoggicosa species formed two groups. One containing Hoggicosa alfi sp. nov., H. castanea, and Hoggicosa duracki comb. nov. is defined by the nonhomoplasious character of a thick and opaque pars pendula (20). The group containing H. bicolor, Hoggicosa brennani sp. nov., Hoggicosa snelli comb. nov., and H. storri is defined by two homoplasious characters: the absence of a radial pattern on the carapace (2) and the subterminal apophysis is easily visible next to the terminal apophysis in ventral view (19). Hoggicosa species with a distinct colour dimorphism between adult males and females (H. bicolor, H. castanea, H. forresti, and H. storri, character 4) did not form a monophyletic group, suggesting this colour pattern has evolved multiple times. Under fast (ACCTRAN) character optimization this trait appears independently three times and is lost once (Fig. 2), whereas under slow (DELTRAN) optimization the character appears four times independently (Fig. 2). When the analysis was repeated without this character included there was no difference in the resulting topology. Lycosa was found to be paraphyletic, with the Australian representatives (Lycosa godeffroyi and Lycosa leuckartii) not grouping with the type species L. tarantula (Fig. 2). Lycosa tarantula and L. praegrandis formed a monophyletic group based on six homoplasious characters. The two species of Dingosa and Geolycosa, along with Mainosa longipes (L. Koch, 1878) formed a monophyletic group supported by two nonhomoplasious characters, the absence of macrosetae on the cymbium tip (9) and the presence of a burrow palisade (27). Two of the Hogna species (Hogna crispipes and Hogna kuyani Framenau, Gotch & Austin, 2006) were monophyletic based on the nonhomoplasious character of the ventrally curved cymbium macrosetae. The third Hogna species (Hogna immansueta) was found to group with the type species of Venator based on eight homoplasious characters (Fig. 2). The representative of an undescribed Australian genus „Grey Wolf Spider‟ and the monotypic Knoelle clara (L. Koch, 1877) were both placed in the unresolved base of the tree. 13 DISCUSSION The Australian wolf spider genus Hoggicosa Roewer, 1960 with the type species H. errans (Hogg, 1905) is revalidated and revised to include ten species. The revision of Hoggicosa is significant in relation to the taxonomy of the Australian wolf spider fauna. Prior to this revision, 33 Australian species were misplaced in the putative Mediterranean genus Lycosa. Nine of these species are treated in this revision and removed from Lycosa, reducing the species of this genus in Australia by nearly a third. Still, the Australian Lycosinae remain poorly known with many undescribed species and genera, particularly from the arid interior, present in Australian collections (V. W. Framenau, unpubl. data). Monophyly of the genus Hoggicosa The results of the phylogenetic analysis show clearly that Hoggicosa is a monophyletic genus unambiguously supported by the shape of the female epigyne (distance between anterior pockets greater than the width of the posterior transverse part, character 24). The monophyly of Hoggicosa confirms the results of a recent molecular phylogeny that included two Hoggicosa species (H. bicolor and H. forresti), the grouping of which was found to be strongly supported (L. bicolor and L. castanea in Murphy et al., 2006). Our morphological examination suggests a second synapomorphy for Hoggicosa, which is not evident in our phylogenetic analysis as it combines two characters. This second synapomorphy consists of ten to 30 cymbium macrosetae (character 10) which are bent dorsally (character 11). Relationships of Hoggicosa to other Lycosine genera The systematic analysis resulted in an overall poor resolution of relationships amongst genera, which appears to reflect the conservative nature of wolf spider morphology (e.g. Vink, 2001; Stratton, 2005). Indeed, resolving relationships within the Lycosinae poses a number of problems. A recent molecular attempt found that Lycosinae contain paralogous copies of the 28S rRNA gene, making this gene unsuitable for analysis (Murphy et al., 2006). A combined effort employing morphological character sets and molecular markers may solve relationships in this morphologically conservative group, as exemplified for the New Zealand Artoriinae (Vink & Paterson, 2003). Additional phylogenetic signal may also be provided by morphological characters that we were not able to explore as part of the current study. For example, spinneret morphology has been used in phylogenetic analyses of other entelegyne spiders (e.g. Scharff & 14 Coddington, 1997; Griswold et al., 2005) and shows promising intergeneric differences within wolf spiders (Townley & Tillinghast, 2003). We did not find an unambiguous sister group to Hoggicosa based on our character and taxon sample. Based on similarities in morphology and burrowing behaviour we included Knoelle clara and a representative of an undescribed Australian genus „Grey Wolf Spider‟ as potential sister taxa. But neither of these nor McKay‟s (1973) previous suggestion of Geolycosa were supported as sister group by our phylogeny (Fig. 2). Molecular studies suggest that Australian Hogna may be closely related to Hoggicosa. The maximum parsimony analysis of molecular data by Murphy et al. (2006) found Hogna crispipes as sister to two Hoggicosa representatives. Adding to these results, Gotch et al. (2008) found that Hogna kuyani was sister to Hoggicosa, although the posterior probability of this relationship was low (54%), and Hogna crispipes was basal to both. These two Hogna species (Hogna crispipes and Hogna kuyani) were included in our phylogeny and found to form a monophyletic group, but a sister group relationship with Hoggicosa was not supported. In addition, a third species currently listed in Hogna (Hogna immansueta) was found to group with the type species of Venator, suggesting additional taxonomic problems within the six Australian species currently placed in Hogna. Our analysis clearly shows that some Australian species currently listed in Lycosa do not form a monophyletic group with the type species of the genus, L. tarantula and a congeneric L. praegrandis. Lycosa godeffroyi and L. leuckartii were placed separately in the unresolved base of the tree, contrary to the molecular results of Murphy et al. (2006) who found very strong support for the monophyly of these two species. They represent a very diverse clade of common Australian Lycosinae characterized by a typical „Union-Jack-colour pattern‟ on the cephalothorax with alternating dark and light radial lines (character 2). Tasmanicosa tasmanica (Hogg, 1905), the type species of Tasmanicosa, is part of this group and the genus Tasmanicosa is currently under revision by the junior author. The grouping of the North American representatives of Geolycosa with the Australian genera Dingosa and Mainosa was surprising and may indicate a case of convergence. This group was defined by the lack of cymbium macrosetae and the presence of burrow palisades, which is an uncommon burrow modification in lycosids. All these species 15 live in sandy habitats and this shared burrowing behaviour and putative associated morphology may be a result of adaptations to live in this substrate. Neither Dingosa nor Mainosa were represented in Murphy et al. (2006), but Geolycosa missouriensis which was included, was found to be the basal most lycosine (parsimony analysis) or sister to Rabidosa (Bayesian analysis). The revalidation of Hoggicosa places L. exigua (Roewer, 1960) from Namibia back into Hoggicosa. A critical review of Roewer‟s (1960: 773–774, fig. 432A, B) original description of Hoggicosa exigua clearly shows that the epigyne is not like that of Hoggicosa or Lycosa (e.g. Zyuzin & Logunov, 2000), but much more reminiscent of Hogna (e.g. Fuhn & Niculescu-Burlacu, 1971 for the type species of Hogna, Hogna radiata Latreille, 1817 and Framenau et al., 2006 for Australian Hogna). We therefore transfer Hoggicosa exigua to Hogna, Hogna exigua (Roewer, 1960) pending a revision of the African Lycosidae related to Hogna. Unfortunately, we were not able to include Hogna exigua in our phylogenetic analysis to confirm this transfer. Evolution of sexual dimorphism in colour pattern Originally distinguished by McKay (1973) as the „bicolor group‟ because of the striking leg and body colouration of females in several species, the phylogenetic analysis under both fast and slow optimization suggests this trait to have evolved multiple times. The four species which display marked difference in colouration between adult males and females (H. bicolor, H. castanea, H. forresti and H. storri, Fig. 1A, B, D, E) did not form a distinct group. It is proposed that this trait has originated multiple times within Hoggicosa suggesting high evolutionary pressure on conspicuous female colour patterns or reduction in colouration of males. Whether this dimorphism is a result of sexual or natural selection (or both) remains unresolved and requires detailed studies into the mating behaviour and natural history of these species. If the striking colour pattern is a result of sexual selection and subject to male mate choice, this would be a remarkable example in sexual role reversal in spiders. For example, alternating light and dark bands on the ventral side of the male wolf spider L. tarantula have been found to correlate highly with body size and condition (Moya-Laraño et al., 2003). However, it is also perceivable that a lack of colouration in males provides camouflage in their active search for a female mate, although Hoggicosa appear to be predominantly active at night (V. W. Framenau, pers. observ.). 16 Biogeography of Hoggicosa All Hoggicosa species display broad geographical distributions, with some found over all of inland Australia (H. castanea, H. alfi, and H. bicolor, Figs 10, 12, 14). This is not surprising as wolf spiders are capable of ballooning (Greenstone, 1982) and are highly mobile. With a distribution typical of Eyrean fauna (Heatwole, 1987) it is possible that speciation in Hoggicosa occurred following the aridification of Australia, as seen in many other Australian groups of fauna and flora (see review by Byrne et al., 2008). SYSTEMATICS FAMILY LYCOSIDAE SUNDEVALL, 1833 SUBFAMILY LYCOSINAE SUNDEVALL, 1833 GENUS HOGGICOSA ROEWER, 1960 Hoggicosa Roewer, 1955: 247 (nomen nudum) – Roewer, 1960: 772. Type species: Lycosa errans Hogg, 1905 (by original designation). Gender feminine. Diagnosis: Hoggicosa differs from other Lycosinae by a combination of genitalic and somatic characters. A putative synapomorphy is proposed based on the female epigyne, with the distance between the anterior pockets greater than the width of the posterior transverse part (Figs 8C, 11C). An undescribed genus from Brazil shares this trait; however, the anterior pockets extend along the anterior margin of the epigyne and join in a groove, forming somewhat of an „m‟ shape (see Álvares, 2006: figs 127–150). A second synapomorphy is a patch of ten to 30 dorsally curved macrosetae on the tip of the cymbium in males (Fig. 1I). In lateral view, the shield of the prosoma is highest in the cephalic region sloping gently towards posterior end (Fig. 3A). The prosoma is uniform in colour or with indistinct radial pattern; median or lateral bands are absent. Females of several species show striking contrast in the colouration of the legs and opisthosoma (similarly coloured as penultimate males, Fig. 1A, D, F). Most similar to Hoggicosa are the monotypic genus Knoelle and an undescribed Australian genus represented by the „Grey Wolf Spider‟ (see Framenau & Baehr, 2007). Knoelle also possess backwards bent macrosetae on the cymbium tip, but in a much larger patch than in Hoggicosa (see Framenau, 2006b, fig. 2). Specimens of the currently undescribed genus including the „Grey Wolf Spider‟ [Hickman, 1967: 17 misidentified as Dingosa simsoni (Simon, 1898)] are of similar size and shape as Hoggicosa species and build burrows with trapdoors, but they differ by the synapomorphies of Hoggicosa. Description: Large and robust wolf spiders, TL 11.6–21.6 in males and 14.4–25.6 in females. Prosoma longer than wide, length 6.4–11.4 in males and 6.9–10.7 in females. Dorsal shield of prosoma highest in cephalic region, sloping gently towards posterior end (Fig. 3A). Dorsal shield of prosoma uniform in colour or with faint radial pattern (sometimes artificially pronounced when preserved in ethanol), without median or marginal bands. AE row strongly procurved. PME greater than PLE and PME narrower than PLE row. Labium as long as wide or slightly longer than wide. Chelicerae with three promarginal teeth, the middle largest, and three retromarginal teeth of equal size. Male leg colouration uniform, females of some species with alternating light and dark metatarsus, also part of tibia in females. Legs III and IV with scopulae on tarsus and half of metatarsus, all of the metatarsus in females. Natural history: Hoggicosa are predominately found through semi-arid and arid regions of Australia. All species build burrows and many construct doors from sand, with H. snelli using pebbles or debris as a door (McKay, 1975). As the patch of macrosetae on the male cymbium is found only in adults, it is possible that these are used for courtship behaviour. We have made one observation of an attempted mating between a male and female of H. alfi. When approaching the female, the male used his palps to stroke the ground rapidly towards his body. This was carried out in repeated short bursts of less than a second, and created small „dugouts‟ in the soft sand. Hoggicosa are easily kept in the laboratory but can be difficult to rear past the penultimate stage. Our observations suggest that moulting can be triggered by hot and humid weather conditions. Distribution: Australia. 18 KEY TO MALES OF HOGGICOSA (Males of H. natashae are currently unknown) 1. Terminal apophysis curved apically, with tip of terminal apophysis located apically of its connection to the palea (Figs 8B, 13A)....................................................................2 Terminal apophysis not curved apically, pointing retrolaterally, with tip of terminal apophysis level with connection to palea (Figs 11A, 16A)...............................................8 2. Subterminal apophysis large and easily visible next to terminal apophysis in ventral view (Fig. 13B)..................................................................................................................3 Subterminal apophysis small and hard to see beneath terminal apophysis in ventral view (Figs 7A–D, 8B)................................................................................................................6 3. Tegular apophysis with straight ridge between ventral process and apical point (Fig. 6A–D)................................................................................................................................4 Tegular apophysis with curved ridge between ventral process and apical point (Fig. 5A– C).......................................................................................................................................5 4. Ventral abdomen cream with black epigastric stripe (Fig. 4F)..........................H. snelli Ventral abdomen black.......................................................................................H. bicolor 5. Ventral abdomen all black, tegular apophysis with rounded ventral process (Fig. 5G– H)...........................................................................................................................H. storri Ventral abdomen pale, although may have faint „V‟ pattern or spots in black (Fig. 4E), tegular apophysis with large flange on ventral process (Fig. 5C–D)...............H. brennani 6. Tegular apophysis with curved ridge between ventral process and apical point (Fig. 5A–E), ventral abdomen with black patch........................................................................7 Tegular apophysis with straight ridge between ventral process and apical point (Fig. 5F), ventral abdomen cream or with a few black dots.................................H. wolodymyri 7. Prominent black stripe (cardiac or chevron mark) on dorsal abdomen (Fig. 1C), pars pendula transparent and joined to embolus below embolus tip (Fig. 7C)..........H. forresti Tan or brown stripe on dorsal abdomen, pars pendula opaque and joined to embolus at tip (Fig. 7A)......................................................................................................H. castanea 19 8. Ventral process of tegular apophysis with bifurcate tip (Fig. 6G, H), terminal apophysis very short and robust (Fig. 16A).......................................................H. duracki Ventral process of tegular apophysis with rounded end (Fig. 6E), terminal apophysis elongate and pointed, pars pendula with oval dark patch (Fig. 11B)........................H. alfi KEY TO FEMALES OF HOGGICOSA Body and leg colouration can be important for distinguishing females, which have very similar epigyne morphology amongst species and are somewhat variable within species. 1. Ventral abdomen all black or with large patch of black extending to spinnerets on ventral surface (Fig. 4B)....................................................................................................2 Ventral abdomen all pale (cream, yellow, or light orange) or with some black lines or dots only (Fig. 4E–F).........................................................................................................7 2. Abdomen entirely black except for a pale (yellowish or cream) anterior median stripe on the dorsal surface, femur, and part of patella black, with remainder of leg pale (Fig. 1A)......................................................................................................................H. bicolor Dorsal abdomen pale with black transverse stripes, mottled brown or grey with transverse lines, or black with pale stripes down lateral sides; leg colouration grey, brown, or if alternating dark and pale segments the patella and tibia are of the same colour.................................................................................................................................3 3. Abdomen entirely black except for pale stripes along either side extending from spinnerets, patella and tibia black with rest of leg pale.........................................H. storri Dorsal abdomen pale with black transverse stripes, mottled brown or grey with transverse lines, or with black anterior median stripe; leg colouration grey, brown or if alternating dark and pale segments with pale tibia and patella……………………….....4 4. Dorsal abdomen with prominent black stripe (cardiac or chevron mark) (Fig. 1C) ........................................................................................................................... H. forresti Dorsal abdomen without black stripe................................................................................5 20 5. Dorsal abdomen pale with black transverse lines, epigynum with long anterior pockets (Figs 1F, 19A–B)................................................................................H. natashae Dorsal abdomen mottled brown or grey with transverse lines or black dots.....................6 6. Anterior pockets of epigynum much wider than posterior transverse part (Fig. 8C) ..........................................................................................................................H. castanea Anterior pockets of epigynum only slightly wider than posterior transverse part (Fig. 11C) ..........................................................................................................................H. alfi 7. Dorsal abdomen cream or with faint black dots only ...................................................8 Dorsal abdomen with median anterior stripe and transverse markings (Fig. 4D, H, I) ....9 8. Ventral abdomen with black line posterior to epigastric furrow (Fig. 4F)........H. snelli Ventral abdomen without black epigastric line..................................................H. duracki 9. Legs pale orange or yellow, epigynum small with anterior pockets only slightly wider than posterior transverse part (Fig. 24C).....................................................H. wolodymyri Legs brown or grey, epigynum large with anterior pockets much wider than posterior transverse part (Fig. 15C).................................................................................H. brennani 21 HOGGICOSA CASTANEA (HOGG, 1905) COMB. NOV. (FIGS 1I, 3A, B, 4A–C, 5A, B, 7A, 8A–D, 9A, B, 10) Lycosa castanea Hogg, 1905: 577–579, fig. 83A–B; Rainbow, 1911: 266; Bonnet, 1957: 2637; McKay, 1973: 396, fig. 3J; McKay, 1985: 75; Platnick, 1993: 487. Lycosa errans Hogg, 1905: 579, fig. 84; Rainbow, 1911: 267; Roewer, 1955: 247; Bonnet, 1957: 2640; McKay, 1973: 379, 394–395, fig. 3I. syn. nov. Lycosa skeeti Pulleine, 1922: 83, pl. 5, fig. 1; Bonnet, 1957: 2664; McKay, 1973: 397– 398, fig. 3K–L. syn. nov. Lycosa perinflata Pulleine, 1922: 84, pl. 5, fig. 2; Bonnet, 1957: 2657; McKay, 1973: 395, fig. 3F–H. syn. nov. Allocosa castanea Roewer, 1955: 206. Types: Holotypes. Hoggicosa castanea, ♀ from Australia: no locality given, no date (SAM NN015). L. errans, ♀ from Australia: no locality given [Hogg, 1905: „(without locality) sent from Adelaide‟], no date (SAM NN016). L. skeeti, ♀ from South Australia: Wilson, Flinders Ranges, 31°60′S, 138°21′E, iv.1908 (SAM NN019); L. perinflata, ♀ from South Australia: Whyte Yarcowie, 33°13′S, 138°53′E, i.1908 (SAM NN017). Other material examined: 179 males, 134 females, and five juveniles from 232 records (Appendix 3). Diagnosis: Hoggicosa castanea is most similar to H. brennani and H. forresti. The subterminal apophysis of H. castanea is very small and reduced (Fig. 7A), whereas it is much longer in H. brennani and H. forresti (Figs 7C, 15B). The pars pendula in H. castanea is opaque and joins at the embolus tip (Fig. 7A), whereas in H. brennani and H. forresti it is transparent and joins below the embolus tip (Figs 7C, 15B). Hoggicosa forresti may be distinguished by the presence of a black stripe on the dorsal abdomen, which is absent in both H. castanea and H. brennani. The venter of H. castanea, which is black (Fig. 4B), may also be used to distinguish it from H. brennani, in which it is pale with dark patches (Fig. 4E). 22 Description: Male: Based on SAM NN19314, Gluepot Station, Gluepot Homestead, 33°44′51′S, 140°01′07′E, South Australia (SA). Dorsal shield of prosoma brown, with faint radial pattern, covered with black setae. Sternum and labium brown with scattered black setae. Chelicerae dark brown with white setae. Legs brown, but ventral side of femur paler. Opisthosoma dorsally greyish-black, with mottled pale and brown patches. Grey median longitudinal band in anterior half with partial border of black. Remainder of opisthosoma with faint transverse lines angled to posterior (Fig. 4A). Opisthosoma laterally cream with black setae. Venter cream with darker central area, which does not reach spinnerets, all covered in white setae. Terminal apophysis of pedipalps curved slightly apically, commonly with slight twist in tip (Figs 7A, 8B). Pars pendula obvious, opaque, and connected to embolus at embolus tip (Fig. 7A). Subterminal apophysis present, but reduced and difficult to see under the terminal apophysis. Tegular apophysis with rounded ventral process located centrally and projecting perpendicular. Prominent ridge curving from ventral process to apical point (Fig. 5A). Female: Based on SAM NN15185, Fisherman Point, Lincoln National Park, 34°45′S, 135°59′E, SA. Dorsal shield of prosoma as male but with black and white setae. Sternum, labium, chelicerae, and legs as male. Opisthosoma dorsally as male. Opisthosoma laterally cream with white setae and occasional black dots. Venter cream with a black patch covered in black setae. Epigyne with small anterior pockets, often located anterior to and far from posterior transverse part (Fig. 8C). Internal epigyne with wide spermathecal heads (Fig. 8D). Variation: Hoggicosa castanea displays a large range of body colouration, particularly between specimens from Western and South Australia. Specimens from Western Australia can display a much paler dorsal abdomen colouration (Fig. 4C) compared with that typical of H. castanea (Fig. 4A). Some specimens from the Pilbara region of Western Australia were found to have a reduced black pattern on the ventral abdomen. In addition, female specimens from southern Western Australia can also display leg markings similar to that of H. forresti. The tegular apophysis of H. castanea also differs slightly in the west of its range with a broader ventral process and longer apical point (Fig. 5A vs. 5B). Although displaying these differences amongst locations, the palea region and attachment of the pars pendula, which is the most powerful character in distinguishing species, was identical amongst all male specimens and we currently consider these conspecific. 23 Remarks: Examination of the holotypes of L. errans, L. perinflata, and L. skeeti showed that they conform to the variation in epigynes and colouration found in all currently available material of H. castanea. This confirms McKay‟s (1973) suggestion that L. errans and L. perinflata are junior synonyms of H. castanea. McKay (1973) also suggested that L. skeeti may be a subspecies of L. bicolor, but we cannot confirm this assumption. We give L. castanea precedence over L. errans as it was established in preceding pages of the same publication. Measurements: ♂ NN19324 (♀ holotype): TL, 17.8 (18.6); PL, 9.3 (9.3); PW, 7.6 (7.8). Eyes: AME, 0.50 (0.50); ALE, 0.27 (0.36); PME, 1.04 (1.00); PLE, 0.86 (0.91). Sternum (length/width): 4.1/3.6 (4.1/3.7). Labium (length/width): 1.4/1.4 (1.6/1.6). OL, 8.6 (9.3); OW, 5.0 (6.4). Legs, lengths of segments (femur + patella/tibia + metatarsus + tarsus = total length): pedipalp, 4.6 + 4.4 + – + 3.6 = 12.6; I, 9.3 + 11.1 + 8.7 + 4.7 = 33.8; II, 8.8 + 10.6 + 8.6 + 4.6 = 32.6; III, 8.1 + 9.3 + 8.7 + 4.4 = 30.5; IV, 10.1 + 11.4 + 11.7 + 4.8 = 38.0 (pedipalp, 4.3 + 4.6 + – + 3.1 = 12.0; I, 8.6 + 10.3 + 6.8 + 4.0 = 29.7; II, 8.4 + 9.7 + 7.0 + 3.8 = 28.9; III, 7.7 + 8.7 + 7.3 + 3.8 = 27.5; IV, 9.7 + 11.0 + 10.1 + 4.3 = 35.1). ♂ (♀) (range, mean ± SD): TL, 14.3–20.7, 18.0 ± 1.8; PL, 8.0–10.7, 9.6 ± 0.8; PW, 6.3– 8.6, 7.7 ± 0.7; N = 10 (TL, 17.9–25.3, 20.7 ± 2.0; PL, 10.0–11.3, 10.6 ± 0.4; PW, 8.4– 9.4, 8.9 ± 0.4; N = 11). Natural history: Hoggicosa castanea has been collected from areas with white sand and limestone, red sandy loam, clay, or coastal dunes and rocky hills. This species has been found in association with Acacia, Melaleuca, and Black Box (Eucalyptus largiflorens) woodlands, as well as Callitris, Mulga (Acacia anuera), Mallee (Eucalyptus), and Spinifex (Triodia) habitats. Adult females have been collected all year round with adult males present from November to May. They build burrows with trapdoors, which can be very well camouflaged (Fig. 9A, B). Distribution: New South Wales, Northern Territory, South Australia, Victoria, Queensland, and Western Australia (Fig. 10). 24 HOGGICOSA ALFI SP. NOV. (FIGS 1G, H, 6E, F, 11A–E, 12) Types: Holotype. ♂ from Western Australia: Lorna Glen Station, 26°12′04′S, 121°18′14′E, 31.x.–7.xi.2002, M.A. Cowan et al. (WAM T77405). Paratypes. 1 ♀ (WAM T77406) and 4 ♂s (WAM T53919), same data as holotype. Other material examined: 397 males, 53 females, and 20 juveniles from 191 records (Appendix 3). Etymology: The specific epithet is a patronym in honour of the senior author‟s recently deceased grandfather, Alf Cain, in appreciation for teaching me how to fish and the importance of a firm handshake. Diagnosis: Males of H. alfi can be distinguished from all other Hoggicosa by the presence of a dark oval patch on the pars pendula and an elongated terminal apophysis which points retrolaterally with only a slight apical bend (Fig. 11A, B). Females can be distinguished from all other Hoggicosa by the unique shape of the epigynum, which has broad anterior pockets and a comparatively wide posterior transverse part (Fig. 11C). Description: Male: Based on holotype. Dorsal shield of prosoma orange-brown, darker in eye quadrangle, with a faint radial pattern; covered with short black setae. Sternum and labium dark brown with scattered black setae. Chelicerae dark brown with white setae. Legs orange-brown. Opisthosoma dorsally mottled grey. Brown median longitudinal band at anterior end with faint transverse lines covering rest of opisthosoma (Fig. 1G). Cover of grey setae with longer black bristles scattered throughout. Opisthosoma laterally cream, venter black. Terminal apophysis of pedipalp pointing retrolaterally (Fig. 11A). Pars pendula connected to embolus near embolus tip, with unique dark oval patch (Fig. 11B). Subterminal apophysis small and only partly visible under terminal apophysis (Fig. 11B). Tegular apophysis with large rounded ventral process located towards apical point, with continuous ridge curving to the apical point (Fig. 6E). 25 Female: Based on paratype. Dorsal shield of prosoma similar to male but with darker radial pattern and cover of fine white setae. Sternum, labium, and chelicerae as male. Legs orange-brown, with the prolateral surface of the femur (especially leg I) darker. Ventral surface of patella darker than femur, less so for leg I. Opisthosoma dorsally pale (yellowish-cream) with scattered black dots and a faint anterior median band (Fig. 1H). Covered with grey setae, black setae forming dots from which a black bristle extends. Opisthosoma laterally cream with some black patches, venter black. Epigyne with broad anterior pockets, the base of which the median septum enters almost at right angles (Fig. 11C). Posterior transverse part large and long. Internal epigyne with spermathecae wider than anterior pockets (Fig. 11D). Variation: The tegular apophysis and terminal apophysis of males are somewhat variable within this species. The terminal apophysis of some males points more retrolaterally (little curve apically). The ridge between the ventral process of the tegular apophysis and apical point can also point retrolaterally with little curve (see Fig. 6E, F). The size of the female epigynum can vary (Fig. 11C, E). The opisthosoma of some females may be mottled and darker than described above. Measurements: ♂ holotype (♀ paratype): TL, 16.8 (21.3); PL, 9.7 (10.1); PW, 7.1 (7.3). Eyes: AME, 0.5 (0.54); ALE, 0.27 (0.36); PME, 0.95 (1.23); PLE, 0.82 (1.14). Sternum (length/width): 4.3/3.4 (4.1/3.4). Labium (length/width): 1.2/1.1 (1.6/1.4). OL, 7.1 (11.1); OW, 4.3 (8.3). Legs, lengths of segments (femur + patella/tibia + metatarsus + tarsus = total length): pedipalp, 4.4 + 3.8 + – + 3.1 = 11.3; I, 8.6 + 9.7 + 8.0 + 4.4 = 30.7; II, 8.0 + 9.7 + 7.8 + 4.4 = 29.9; III, 7.8 + 8.6 +7.8 + 4.3 = 28.5; IV, 9.3 + 10.7 + 10.0 + 4.4 = 34.4 (pedipalp, 4.6 + 4.3 + – + 3.0 = 11.9; I, 7.8 + 9.3 + 6.0 + 3.1 = 26.2; II, 7.6 + 9.0 + 6.0 + 3.3 = 25.9; III, 7.1 + 8.6 + 6.4 + 3.6 = 25.7; IV, 8.4 + 10.3 + 8.6 + 3.6 = 30.9). ♂ (♀) (range, mean ± SD): TL, 14.3–20.0, 17.4 ± 1.4; PL, 7.9–10.1, 9.2 ± 0.7; PW, 6.0– 7.9, 7.0 ± 0.5; N = 25 (TL, 15.7–22.4, 18.1 ± 1.9; PL, 7.6–11.0, 9.2 ± 1.0; PW, 5.7–7.9, 6.9 ± 0.7; N = 13). Natural history: Hoggicosa alfi has been collected from yellow sand plains, red dunes, interdunes, claypan edges, and stony tablelands. Specimens have been recorded in association with Callitris woodland, Mallee (Eucalyptus), Grevillea, and Banksia with Verticordia, Mulga (Acacia aneura), and Spinifex (Triodia) vegetation. Adult males 26 have been collected from September to December with adult females caught through August to May. This species excavates burrows, but does not seal them with doors. Distribution: New South Wales, Queensland, South Australia, and Western Australia (Fig. 12). 27 HOGGICOSA BICOLOR (HOGG, 1905) COMB. NOV. (FIGS 1A, B, 2A, 13A–D, 14) Lycosa bicolor Hogg, 1905: 580–582, fig. 85A–B; Rainbow, 1911: 266; Strand, 1913: 618; Bonnet, 1957: 2636; McKay, 1973: 378, 381–385, figs 1A–E, 2A–C; Main, 1976: 149, 231; McKay, 1985: 75; Platnick, 1993: 486. Allocosa bicolor Roewer, 1955: 205. Types: Lectotype (designated by McKay, 1973). ♀ from South Australia: no locality given, no date (SAM NN012). Paralectotypes. South Australia: 1 ♀, no locality given, no date (SAM NN011); 1 juvenile, no locality given, no date (SAM NN013). Other material examined: 335 males, 95 females, and 35 juveniles from 283 records (Appendix 3). Diagnosis: The male palp of H. bicolor is most similar to H. snelli, but these two species can be easy distinguished by abdomen colouration. Male H. bicolor have a grey opisthosoma and black venter, whereas male H. snelli have a cream opisthosoma and venter with a black epigastric stripe (Fig. 4F). Females and juveniles can be readily distinguished from all other Hoggicosa species by the striking colouration of the legs and abdomen (Fig. 1A). Description: Male: Based on WAM T62336, Mt Vernon Station, 24°30′S, 118°30′E, Western Australia (WA). Dorsal shield of prosoma brown, covered in short black and white setae. Sternum and labium brown with scattered black setae. Chelicerae dark brown with white setae. Legs brown. Opisthosoma grey with cover of grey and black setae (Fig. 1B). Opisthosoma laterally with faint longitudinal lines and black and white setae. Venter black with black setae. Terminal apophysis of pedipalp large and strongly curved apically (Fig. 13A). Pars pendula thin, transparent, and connected to embolus near embolus base (Fig. 13B). Subterminal apophysis large and easily visible next to terminal apophysis. Tegular apophysis with small, pointed ventral process, located close to apical point. Straight ridge between ventral process and apical point (Fig. 6A). 28 Female: Based on WAM T62336, data as above. Dorsal shield of prosoma reddishbrown, darker in eye quadrangle, cover of fine white setae. Sternum, labium, and chelicerae as male. Femur and first part of patella of all legs black–dark brown, with remainder of leg cream (can have almost yellow tinge when alive) (Fig. 1A). Opisthosoma dorsally black covered in fine black setae, with prominent cream anterior median lanceolate stripe (Fig. 1A). Opisthosoma laterally and ventrally black with black setae. Epigynum simple, with small anterior pockets located close to median septum (Fig. 13C). Internal epigyne with large spermathecal heads, which are very close to the anterior pockets (Fig. 13D). Variation: The size and shape of the pale lanceolate stripe on the opisthosoma of females can vary amongst individuals from a small streak at the anterior end to a large band nearly reaching the spinnerets and covering most of the dorsal surface (see McKay, 1973, fig. 1B–E). Females and juveniles may sometimes have cream markings on the black dorsal femur (Fig. 1A). Remarks: As the lectotype female is in poor condition, a representative male and female have been used for the redescription of H. bicolor. Measurements: ♂ (♀): TL, 18.6 (25.4); PL, 10.0 (11.8); PW, 7.6 (8.6). Eyes: AME, 0.54 (0.59); ALE, 0.27 (0.36); PME, 1.36 (1.41); PLE, 1.09 (1.27). Sternum (length/width): 4.6/3.3 (4.8/3.6). Labium (length/width): 1.4/1.3 (1.8 /1.8). OL, 8.6 (13.6); OW, 5.0 (8.8). Legs, lengths of segments (femur + patella/tibia + metatarsus + tarsus = total length): pedipalp, 5.1 + 5.3 + – + 3.6 = 14.0; I, 8.6 + 10.7 + 8.6 + 4.6 = 32.5; II, 8.6 + 10.6 + 8.1 + 4.6 = 31.9; III, 7.8 + 9.3 + 7.8 + 4.6 = 29.5; IV, 10.0 + 11.4 + 10.3 + 5.0 = 36.7 (pedipalp, 5.4 + 5.7 + – + 3.6 = 14.7; I, 9.6 + 11.6 + 7.4 + 4.3 = 32.9; II, 9.3 + 11.4 + 7.6 + 4.3 = 32.6; III, 8.6 + 9.8 + 7.8 + 4.1 = 30.3; IV, 10.4 + 12.8 + 10.7 + 4.8 = 38.7). ♂ (♀) (range, mean ± SD): TL, 13.6–18.6, 16.2 ± 1.4; PL, 7.4–10.0, 8.8 ± 0.7; PW, 5.4– 7.1, 6.5 ± 0.5; N = 23 (TL, 15.0–25.0, 20.2 ± 3.0; PL, 7.9–12.0, 10.0 ± 1.4; PW, 5.9– 9.1, 7.6 ± 1.0; N = 13). Natural history: Hoggicosa bicolor has been collected from areas with sandy plains, red sand, claypans, and stony soil. Found in locations with Mulga (A. aneura) and A. estrophiolata, Ironwood (A. estrophiolata) woodland, Eucalyptus socialis, chenopod 29 scrubland (Atriplex and Mairean), and Spinifex (Triodia irritans). Adult females have been collected all year round with adult males recorded from August to April. This species has been dug up from burrows with and without doors. Distribution: New South Wales, Northern Territory, Queensland, South Australia, and Western Australia (Fig. 14). 30 HOGGICOSA BRENNANI SP. NOV. (FIGS 4D, E, 5C, D, 15A–D, 18) Types: Holotype. ♂ from South Australia: 1.5 km south-west Middle dam, Taylorville Station, 33°53′10′S, 140°18′32′E, 8–13.xii.2000, Royal Geographic Society of South Australia (RGS)/Bookmark Survey, TV04 (SAM NN17017). Paratype. ♀ from South Australia: Casuarina Dam, Taylorville Station, 33°53′10′S, 140°18′32′E, 10.x.2000, RGS/Bookmark Survey, TV-camp, burrow with trapdoor (SAM NN17029). Other material examined: 53 males, 27 females, and four juveniles from 64 records (Appendix 3). Etymology: The specific epithet is a patronym in honour of Karl E. C. Brennan in recognition of his work on the ecology of Australian spiders. The senior author also thanks him for his support and friendship. Diagnosis: The pedipalps of H. brennani most closely resemble those of H. castanea and H. forresti. The pars pendula, which is transparent in H. brennani and joins below the embolus tip (Fig. 15B), may be used to distinguish it from H. castanea, in which it is opaque and joins at the embolus tip. In addition the subterminal apophysis of H. brennani is much longer than that of H. castanea (Figs 7A, 8B). The ventral process on the tegular apophysis of H. brennani has a flange on the prolateral side which is absent in H. forresti (Fig. 5C, D vs. 5E). Male and female H. brennani have a pale venter with dark patterning, whereas H. castanea and H. forresti have a black venter (Fig. 4B vs. 4D). Description: Male: Based on holotype. Dorsal shield of prosoma orange-brown in colour, darker in eye quadrangle, with black and white setae. Sternum pale orange, labium brown, both with scattered black setae. Chelicerae dark brown with white setae. Legs brown. Opisthosoma dorsally mottled. Brown median longitudinal stripe at anterior end with partial edging of black. Faint transverse lines (grey/black) over rest of opisthosoma (Fig. 4D). Cover of black, grey, and white setae. Opisthosoma laterally cream with scattered black patches. Venter cream with faint V-shape made of black patches of black setae (Fig. 4E). Terminal apophysis of pedipalp curved apically (Fig. 31 15A). Pars pendula transparent and connected to embolus just below embolus tip (Fig. 15B). Subterminal apophysis next to terminal apophysis (Fig. 15B). Tegular apophysis with a large ventral process located centrally and pointing away from tip of tegular apophysis. Ventral process with flange on prolateral side, which has a characteristic notch when viewed from anterior of pedipalp (Fig. 5C, D). Ridge from ventral process to apical point present and curved (Fig. 5C). Female: Based on paratype. Dorsal shield of prosoma as male. Sternum, labium, chelicerae, and legs as male. Opisthosoma dorsally and laterally as male (Fig. 4D, F). Venter cream with some scattered black dots that have a long black setae extending. Epigyne with small anterior pockets greater in width than posterior transverse part (Fig. 15C). Internal epigyne with clearly defined channel from anterior pockets to spermatheca (Fig. 15D). Variation: The abdomen colouration of H. brennani can vary. In some specimens the dorsal markings may be much paler and the ventral abdomen nearly completely cream. Likewise, some specimens display a more pronounced ventral marking with a greater number of black dots and enhanced black lines. Measurements: ♂ holotype (♀ paratype): TL, 19.8 (18.4); PL, 9.8 (9.6); PW, 7.9 (7.6). Eyes: AME, 0.46 (0.50); ALE, 0.31 (0.42); PME, 0.96 (1.15); PLE, 0.77 (1.00). Sternum (length/width): 4.5/3.9 (4.1/3.6). Labium (length/width): 1.6/1.4 (1.6/1.5). OL, 10.0 (8.8); OW, 6.2 (6.1). Legs, lengths of segments (femur + patella/tibia + metatarsus + tarsus = total length): pedipalp, 5.1 + 5.2 + – + 4.0 = 14.3; I, 9.9 + 12.0 + 9.2 + 5.2 = 36.3; II, 9.6 + 11.6 + 9.0 + 5.1 = 35.3; III, 8.4 + 10.0 + 9.2 + 4.7 = 32.3; IV, 10.0 + 12.1 + 11.2 + 5.4 = 38.7 (pedipalp, 4.9 + 5.0 + – + 3.6 = 13.5; I, 8.8 + 9.9 + 6.2 + 3.8 = 28.7; II, 8.1 + 9.4 + 6.4 + 3.8 = 27.7; III, 7.2 + 8.5 + 7.4 + 3.6 = 26.7; IV, 9.0 + 10.6 + 9.4 + 4.4 = 33.4). ♂ (♀) (range, mean ± SD): TL, 14.3–20.0, 17.1 ± 2.3; PL, 7.5–11.4, 9.0 ± 1.3; PW, 6.1– 9.0, 7.1 ± 1.0; N = 8 (TL, 18.1–25.6, 20.4 ± 3.0; PL, 8.8–11.9, 9.8 ± 1.3; PW, 6.9–9.4, 7.8 ± 1.0; N = 5). 32 Natural history: Hoggicosa brennani has been recorded from sand and dune locations with records of Mallee (Eucalyptus) vegetation with mixed understorey and Spinifex (Triodia). Adult females have been collected all year round with adult males found from October to March. This species builds burrows with trapdoors. Distribution: New South Wales, Queensland, and South Australia (Fig. 18). 33 HOGGICOSA DURACKI (MCKAY, 1975) COMB. NOV. (FIGS 6G, H, 7B, 16A–E, 18) Lycosa duracki McKay, 1975: 313–316, fig. 2A–E; Brignoli, 1983: 450; McKay, 1985: 76. Types: Holotype. ♀ from Western Australia: Old Argyle Downs Station (today submerged under Argyle Dam), Ord River, 16°12′S, 128°48′E, 23.x.1971, R. J. McKay, W. H. Butler, with young spiderlings (WAM 74/494). Paratypes. 1 ♂ and 2 ♀s from same location as holotype: 5.x.1971 (WAM 74/495-7). Other material examined: Six males and three females from five records (Appendix 3). Diagnosis: Males can be distinguished from the similarly coloured H. snelli by a very short terminal apophysis (Fig. 16A) and a large tegular apophysis, which has a ventral process with a bifurcate tip (Fig. 6G, H). Both sexes can be distinguished from H. snelli by the plain cream venter which lacks the black stripe of H. snelli (Fig. 4F). Description: Male: Based on paratype. Dorsal shield of prosoma pale orange, covered in fine black and white setae. Sternum pale orange, labium orange-brown, both with scattered black setae. Chelicerae dark brown with white setae. Legs pale orange-yellow. Opisthosoma dorsally cream, cover of fine black setae with longer black bristles scattered throughout. Opisthosoma laterally cream, venter cream with white setae. Terminal apophysis of pedipalp very short, robust, and pointing retrolaterally (Fig. 16A, B). Pars pendula thick and opaque, connected to embolus at embolus tip (Fig. 7B). Large tegular apophysis with a long ventral process located basally and pointing strongly away from tip of tegular apophysis. Ventral process with a bifurcate tip and no continuous ridge to the apical point (Fig. 6G, H). Female: Based on holotype. Dorsal shield of prosoma pale orange-yellow with fine white setae. Sternum, labium, and chelicerae as male. Legs pale yellow. Opisthosoma dorsally cream, covered with white setae and scattered longer black bristles. Opisthosoma laterally cream, venter cream with two faint dark spots at anterior end. Epigyne with small anterior pockets, close to the median septum (Fig. 16E). 34 Remarks: The epigyne of one of the female Paratypes (WAM 74/496, Fig. 16C, D) is vastly different to that of the holotype (Fig. 16E). Unfortunately, the epigyne of the second female paratype (WAM 74/497) is missing, but from McKay‟s (1975: fig. 2E) figure of the ventral view it appears to match that of the holotype. This difference may indicate that the epigynes of H. duracki are highly variable. However, the elongated structure of the ventral process on the tegular apophysis of the male specimens appears unlikely to „match‟ the holotype epigyne (see Zyuzin, 1993). The dimensions of the tegular apophysis fit better with that of the paratype epigyne figured here (Fig. 16C). Therefore, the females of H. duracki may represent two species, but given the current lack of material, in particular a morphologically different male, we currently consider them as conspecific. Measurements: ♂ paratype (♀ holotype): TL, 18.8 (19.4); PL, 9.4 (10.6); PW, 8.0 (8.0). Eyes: AME, 0.50 (0.62); ALE, 0.35 (0.38); PME, 1.15 (1.35); PLE, 0.81 (1.23). Sternum (length/width): 4.4/4.0 (4.9/3.8). Labium (length/width): 1.5/1.3 (1.5/1.5). OL, 9.4 (8.8); OW, 5.7 (6.2). Legs, lengths of segments (femur + patella/tibia + metatarsus + tarsus = total length): pedipalp, 4.4 + 4.3 + – + 3.8 = 12.5; I, 9.4 + 11.4 + 9.4 + 4.7 = 34.9; II, 9.1 + 10.8 + 9.4 + 4.7 = 34.0; III, 8.6 + 9.8 + 9.3 + 4.4 = 32.1; IV, 10.1 + 11.8 + 11.4 + 4.8 = 38.1 (pedipalp, 5.0 + 5.0 + – + 3.6 = 13.6; I, 8.2 + 10.2 + 6.2 + 3.4 = 28.0; II, 7.9 + 9.6 + 6.2 + 3.6 = 27.3; III, 7.5 + 8.8 + 6.2 + 3.8 = 26.3; IV, 8.8 + 10.6 + 8.1 + 4.4 = 31.9). ♂ (♀) (range, mean ± SD): TL, 15.7–18.8, 16.8 ± 1.3; PL, 7.9–9.4, 8.7 ± 0.7; PW, 6.1– 8.0, 7.2 ± 0.8; N = 4 (TL, 19.4–19.8, 19.5 ± 0.2; PL, 9.5–10.6, 10.0 ± 0.6; PW, 7.4–8.1, 7.8 ± 0.4; N = 3). Natural history: Hoggicosa duracki have been collected from bare gravel slopes and ridges without vegetation in heavy clay-gravel. Burrows were 15–20 cm deep and up to 14 mm diameter with or without hinged doors (McKay, 1973). Mature males and females have been collected in October and November. The holotype female was collected with young in October. Distribution: Northern Western Australia (Fig. 18). 35 HOGGICOSA FORRESTI (MCKAY, 1973) COMB. NOV. (FIGS 1C, 5E, 17A–D, 18) Lycosa forresti McKay, 1973: 385–389, figs 1F–G, 2D–G; Main, 1976: 138–141, 149, 231, fig. 37B, plate B7; Brignoli, 1983: 450; McKay, 1985: 77. Types: Holotype. ♀ from Western Australia: 8 miles west of Moorine Rock, 8.i.1970, W. H. Butler (WAM 70/44). Paratypes. Western Australia: 1 ♀, Buntine Reserve, c. 3 miles east of Buntine Railway Station, 29°59′S, 116°42′E (WAM 72/635); 2 ♀, 1 juvenile (juv.), Buntine Reserve, c. 3 miles east of Buntine Railway Station, 29°59′S, 116°42′E (WAM 72/639-41); 1 ♀, Hyden, 32°27′S, 118°52′E (WAM 69/801); 1 juv., Lake Moore, near south end, 30°20′S, 117°58′E (WAM 71/198); 1 ♀, Nevoria mine, 10 miles east-south-east Marvel Loch, 31°30′S, 119°34′E (WAM 70/30); 1 ♀, Mt Magnet, 323 mile peg, 28°03′S, 117°50′E (WAM 69/467); 1 ♀, Rudall River, 22°28′S, 122°29′E (WAM 71/1151); 1 ♀, Southern Cross, 31°14′S, 119°19′E (WAM 72/634); 1 ♀, Southern Cross, 6 miles east, 31°16′S, 119°25′E (WAM 69/40); 1 juv., Tammin, 31°38′S, 117°29′E (WAM 69/38); 1 juv., Wongan Hills, 106.6 miles north-east, (WAM 69/792); 1 ♀, Wongan Hills, Ballidu Road, 135 mile peg, 30°54′S, 116°43′E (WAM 69/827); 1 juv., Wongan Hills - Ballidu Road, 135 mile peg, 30°54′S, 116°43′E (WAM 69/828); 1 juv., Wubin, 30°07′S, 116°38′E (WAM 69/41); 1 juv., Wubin, 20 miles north-east, 29°54′S, 116°52′E (WAM 69/43); 1 ♀, Wubin, 10 miles north-east, 30°00′S, 116°31′E (WAM 69/456); 1 ♀, Yandil Station, 26°22′S, 119°49′E (WAM 38/916); 1 ♀, 6 juv., Yellowdine, 38 miles south, 31°51′S, 119°39′E (WAM 71/69-75). Misidentification, these are H. alfi: 1 ♀, Mt Magnet, 323 mile peg, 28°03′S, 117°50′E (WAM 69/791); 1 ♀, Paynes Find area, 323 mile peg, 29°15′S, 117°41′E (WAM 70/186). Misidentification, these are H. castanea: 1 ♀, Morawa, 29°13′S, 116°00′E (WAM 70/172); 1 ♀, Mt Magnet, 323 mile peg, 28°03′S, 117°50′E (WAM 69/46); 1 ♀, Kulin, 32°40′S, 118°9′E (WAM T53819; 33/1607); 1 ♀, Coonana, 12 miles north-west, 30°54′S, 123°01′E (WAM 69/35); 1 ♀, Laverton, Police Station, 28°38′S, 122°24′E (WAM 26/716, published as WAM 26/717 in McKay, 1973); 1 ♀, Marloo Station, 28°19′S, 116°11′E (WAM 69/42). Currently missing: 1 ♀, Carrabin (WAM 69/37); 1 juv., no locality (WAM 71/76); 1 ♂, no locality (WAM 69/465). 36 Other material examined: 64 males, 48 females, and 19 juveniles from 105 records (Appendix 3). Diagnosis: Most similar to H. forresti are H. brennani and H. castanea. Males and females of H. forresti may be distinguished from both by the presence of a prominent black lanceolate stripe on the anterior of the dorsal abdomen (Fig. 1C). In addition, H. brennani has a pale venter with dark patterning compared with the black venter of H. forresti and H. castanea. McKay (1973) indicated the leg colouration of forresti, with a pale diamond on the femur, as a diagnostic feature, but females of H. castanea can also display this leg coloration, but lack a dark lanceolate stripe. Males of H. forresti may be distinguished from H. castanea by the pars pendula, which is thin and transparent and joins below the embolus tip (Fig. 7C), whereas in H. castanea it is thick and opaque and joins at the tip of the embolus (Fig. 7A). The tegular apophysis may be used to distinguish H. brennani males, which have a flange on the ventral process (Fig. 5C, D) that is lacking in H. forresti (Fig. 5E). Description: Male: Based on WAM T47762, North Baandee Nature Reserve, 31°38′S, 117°43′E, WA. Dorsal shield of prosoma brown, faint radial pattern, covered with black and white setae. Sternum light brown, labium brown, with scattered black setae. Chelicerae dark brown with white setae. Legs brown with black setae, pale diamond shape at base of femurs. Also pale with white setae on retrolateral side of ventral surface of legs I and II. Opisthosoma dorsally mottled grey. Black median lanceolate stripe at anterior end with black setae (Fig. 1C). Cover of black and white setae. Opisthosoma laterally cream with white setae. Venter with black triangular patch extending to spinnerets. Terminal apophysis of pedipalps strongly curved apically (Fig. 17A, B). Pars pendula transparent and connected to embolus just below embolus tip (Fig. 7C). Subterminal apophysis present, but small and difficult to see beneath terminal apophysis (Fig. 7C). Tegular apophysis with rounded ventral process projecting perpendicular. Prominent curved ridge between ventral process and apical point (Fig. 5E). Female: Based on WAM T47762, data as above. Dorsal shield of prosoma pale orange with white setae covering. Sternum, labium, and chelicerae as male. Legs dark brown with black setae, same pale diamond shape on femur as male. Ventral side of legs on 37 femur and patella brown, remainder pale. Opisthosoma dorsally as male with faint transverse rows from end of black stripe to spinnerets. Opisthosoma laterally cream with some black patches. Venter as male. Epigyne with broad anterior pockets (Fig. 17C). Internal epigyne with large ovoid anterior pockets and „s‟ shaped sclerotized median septum channels (Fig. 17D). Variation: The carapace of males can be darker with a more obvious radial pattern; some display a less obvious leg pattern or lack it altogether with grey/brown legs. The dorsal opisthosoma can vary but always has a dark lanceolate stripe. The dark lanceolate stripe is sometimes surrounded by cream or with black extensions in the middle (bulges). The length between the process and the tip of the tegular apophysis can vary, but the pars pendula and subterminal apophysis are always the same. Remarks: McKay (1973) noted atypical specimens from Forrest, Rawlinna, and Fitzgerald River in his description. Our examination of these specimens identified them as H. castanea. Measurements: ♂ (♀) both T47762: TL, 18.1 (20.3); PL, 9.6 (11.0); PW, 8.0 (8.6). Eyes: AME, 0.54 (0.54); ALE, 0.32 (0.36); PME, 0.95 (1.32); PLE, 0.91 (1.14). Sternum (length/width): 4.1/3.6 (4.8/4.0). Labium (length/width): 1.45/1.36 (1.59/1.59). OL, 8.6 (9.3); OW, 4.3 (6.0). Legs, lengths of segments (femur + patella/tibia + metatarsus + tarsus = total length): pedipalp, 4.6 + 4.3 + – + 3.6 = 12.5; I, 9.7 + 11.4 + 9.3 + 5.0 = 35.4; II, 9.3 + 10.8 + 9.3 + 4.8 = 34.2; III, 8.6 + 10.0 + 9.6 + 4.8 = 33.0; IV, 10.7 + 12.1 + 12.1 + 5.6 = 40.5 (pedipalp, 5.0 + 5.0 + – + 3.6 = 13.6; I, 9.3 + 11.4 + 7.1 + 4.3 = 32.1; II, 8.8 + 10.7 + 7.1 + 4.3 = 30.9; III, 7.8 + 9.7 + 8.3 + 4.3 = 30.1; IV, 10.0 + 12.1 + 11.4 + 5.0 = 38.5). ♂ (♀) (range, mean ± SD): TL, 13.6–20.0, 16.8 ± 1.7; PL, 7.9–10.3, 9.0 ± 0.7; PW, 6.3– 8.3, 7.3 ± 0.6; N = 31 (TL, 17.1–22.4, 20.4 ± 1.3; PL, 9.3–11.4, 10.5 ± 0.6; PW, 7.1– 9.4, 8.6 ± 0.6; N = 18). Natural history: Hoggicosa forresti has been collected from gritty, stony, and fine clayey loams as well as sandplains. Found in woodlands of Gimlet (Eucalyptus salubris), Salmon Gum (E. salmonophloia), and E. dundasii as well as Mallee (Eucalyptus) and Spinifex (Triodia). Adult females were collected all year round and 38 adult males from November to May. This species excavates burrows armed with a trapdoor (see Main, 1976, fig. 37B). Distribution: Southern Western Australia and South Australia (Fig. 18). The record of a specimen from Cottesloe, Perth (WAM 22/5) is considered dubious and may be mislabelled. 39 HOGGICOSA NATASHAE SP. NOV. (Figs 1F, 19A–D, 20, 22) Types: Holotype. ♀ from South Australia: Lake Gilles, 32°41′20′S, 136°55′20′E, 9.xii.1994, P. Hudson (SAM NN14785). Paratypes. South Australia: 1 ♀ from Lake Gilles, 32°41′20′S, 136°55′20′E, 15.v.1993, P. Hudson (SAM NN14786); 1 ♀ from Lake Gilles, 32°43′S, 136°47′E, i.1996, P. Hudson (SAM NN13801). Other material examined: Seven females and one juvenile from six records (Appendix 3). Etymology: The specific epithet is a matronym in honour of the senior author‟s mother, Natasha Langlands, in appreciation for all her love and support. Diagnosis: This species can be distinguished from all other Hoggicosa by its striking dorsal abdominal pattern, which consists of black transverse markings on a pale cream abdomen (Fig. 1F). In addition, the anterior pockets of the epigynum are much larger than in any other species (Fig. 19A). Description: Male: Currently unknown. Female: Based on holotype. Dorsal shield of prosoma orange-brown, darker in eye quadrangle, faint radial pattern and cover of white setae. Sternum and labium brown with scattered black setae. Chelicerae dark brown with white setae. Legs grey. Opisthosoma dorsally cream with black transverse lines (Fig. 1F). Opisthosoma laterally and ventrally cream. Epigynum with broad and very elongate anterior pockets (Fig. 19A). Internal epigyne with spermatheca slightly wider than anterior pockets (Fig. 19B). Remarks: A juvenile female displays the same dorsal abdominal pattern. 40 Measurements: ♀ holotype: TL, 19.0; PL, 9.0; PW, 6.8. Eyes: AME, 0.50; ALE, 0.36; PME, 1.04; PLE, 1.00. Sternum (length/width): 3.8/3.1. Labium (length/width): 1.4/1.1. OL, 10.0; OW, 7.1. Legs, lengths of segments (femur + patella/tibia + metatarsus + tarsus = total length): pedipalp, 4.3 + 4.0 + – + 2.7 = 11.0; I, 7.3 + 8.6 + 5.7 + 2.8 = 24.4; II, 7.0 + 8.3 + 5.4 + 2.8 = 23.5; III, 6.3 + 7.4 + 6.0 + 2.8 = 22.5; IV, 7.7 + 9.1 + 8.1 + 3.3 = 28.2. ♀ (range, mean ± SD): TL, 16.7–23.6, 20.4 ± 3.3; PL, 8.9–11.4, 10.1 ± 1.2; PW, 6.4– 8.6, 7.4 ± 0.9; N = 5. Natural history: Several specimens have been collected in low depressions covered with vegetation of Chenopods and Samphire. Mature females collected from December to May. The holotype was dug from a burrow with a thick bathtub plug-like door (Fig. 20). Distribution: New South Wales, Queensland, and South Australia (Fig. 22). 41 HOGGICOSA SNELLI (MCKAY, 1975) COMB. NOV. (FIGS 4F, G, 6B–D, 21A–E, 22) Lycosa snelli McKay, 1975: 313–316, fig. 1A–G; Main, 1976: 141, fig. 32D; Brignoli, 1983: 450; McKay, 1985: 83. Types: Holotype. ♀ from Western Australia: Towera Station, north of Lyndon River, 23°11′S, 115°07′E, i.1952, A. Snell (WAM 69/797). Paratypes. Western Australia: 2 juv., Barradale, 18 km south, 22°50′S, 114°57′E (QM W4021); 2♂, 1 ♀, Barrow Island, 20°48′S, 115°24′E (WAM 74/498-9, 71/1716); 1 ♀, Carnarvon, 14.5 km north, on NW Highway, 24°50′S, 113°47′E (WAM 69/1035); 2 juv., Lyndon Station, 23°38′S, 115°15′E (WAM 69/798-9); 1 ♀, 2 juv., Lyndon Station, via Carnarvon, 23°38′S, 115°15′E (WAM 69/803-5); 12 juv., Manberry, 7 miles from, towards Wandagee, 23°56′S, 114°10′E (WAM 37/117-28, published as 72/117-28 in McKay (1973); 1 juv., Mardie Station, 21°13′S, 115°58′E (WAM 71/1718); 1 juv., Marilla Station, 22°58′S, 114°28′E (QM W4022); 1 ♀, 1 juv., North Western Highway, 760 mile peg, near Marrilla Station Turnoff, 23°05′S, 114°32′E (WAM 70/163, 70/164); 1 ♀, Yannarie River, 23°15′S, 115°12′E (WAM71/1717); 1 ♀, 3 juv., Yannarie River, near Barradale, 22°50′S, 114°57′E (QM W4023). Other material examined: Forty-five males, ten females, and six juveniles from 25 records (Appendix 3). Diagnosis: Males and females can be easily distinguished from all other Hoggicosa by the presence of a black line posterior to the epigastric furrow on the ventral opisthosoma (Fig. 4F). The male palp is most similar to that of H. bicolor but can be distinguished by the ventral process of the tegular apophysis, which is much larger and connected to the anterior edge (Fig. 6B vs. 6A). Description: Male: Based on WAM T65133, Woodleigh Station, 26°11′45′S, 114°25′24′E, WA. Dorsal shield of prosoma orange-brown, darker in eye quadrangle, with a faint radial pattern; covered with black and white setae. Sternum pale orange and labium brown with scattered white setae. Chelicerae dark brown with white setae. Legs orange-brown, with tibia, metatarsus, and tarsus somewhat darker, especially legs I and 42 II. Opisthosoma dorsally cream with two small black patches on anterior half. Cover of white setae with longer black setae scattered. Opisthosoma laterally cream. Venter cream with characteristic black stripe below epigastric furrow, covered in white setae (Fig. 4F). Terminal apophysis of pedipalp large, strongly curved apically (Fig. 21A). Pars pendula transparent and connected to embolus near embolus base (Fig. 21B). Subterminal apophysis large, easily visible next to the terminal apophysis. Tegular apophysis with angular ventral process located apically with straight ridge to the apical point (Fig. 6B). Female: Based on WAM T47612, Shothole Canyon, Cape Range National Park, 22°02′41′S, 114°20′14′E, WA. Dorsal shield of prosoma orange, black around eye quadrangle, no radial pattern with cover of white-grey setae. Sternum, labium, and chelicerae as male. Legs yellowish-orange, with patella, tibia, metatarsus, and tarsus somewhat more orange. Opisthosoma entirely cream with white setae, except for characteristic bar of black setae to posterior of epigastric furrow (Fig. 4F). Epigyne with anterior pockets not much greater in width than posterior transverse part (Fig. 21C). Internal epigyne with spermatheca base as long as wide and nearly as wide as anterior pockets (Fig. 21D). Variation: McKay (1975) described the males of H. snelli from Barrow Island, off the north-west coast of Western Australia. As there appear to be slight differences between the tegular apophysis of mainland and island specimens (Fig. 6B vs. 6C), both variations are figured here. In addition, the palp of a male found in the Kimberley region of Western Australia also shows variation in the tegular apophysis (Fig. 6D) and palea (Fig. 21E). These specimens are similar in all other morphological aspects, including the characteristic epigastric stripe, and we consider them conspecific. A female from Munda Station was found to have an enlarged epigastric stripe (Fig. 4G). Measurements: ♂ WAM T65133 (♀ WAM T47612): TL, 19.9 (17.8); PL, 10.5 (10.0); PW, 7.5 (6.6). Eyes: AME, 0.54 (0.50); ALE, 0.31 (0.27); PME, 1.35 (1.18); PLE, 1.23 (1.09). Sternum (length/width): 5.1/3.8 (4.3/3.1). Labium (length/width): 1.6/1.3 (1.6/1.4). OL, 9.4 (7.8); OW, 5.4 (5.0). Legs, lengths of segments (femur + patella/tibia + metatarsus + tarsus = total length): pedipalp, 5.2 + 5.6 + – + 3.8 = 14.6; I, 9.4 + 11.0 + 8.2 + 4.4 = 33.0; II, 8.8 + 10.4 + 8.1 + 4.1 = 31.4; III, 7.6 + 9.0 + 7.9 + 4.2 = 28.7; IV, 10.0 + 11.9 + 10.1 + 4.4 = 36.4 (pedipalp, 4.3 + 4.3 + – + 2.8 = 11.4; I, 7.3 + 8.8 + 5.3 + 43 3.1 = 24.5; II, 6.6 + 8.6 + 5.4 + 2.8 = 23.4; III, 6.0 + 7.4 + 5.7 + 2.8 = 21.9; IV, 7.4 + 10.0 + 7.7 + 3.4 = 28.5). ♂ (♀) (range, mean ± SD): TL, 12.5–21.6, 18.0 ± 1.8; PL, 7.5–11.3, 9.9 ± 0.8; PW, 5.5– 8.1, 7.0 ± 0.7; N = 25 (TL, 18.6–24.1, 20.7 ± 1.7; PL, 10.0–11.6, 10.8 ± 0.6; PW, 6.7– 8.5, 7.6 ± 0.6; N = 9). Natural history: Hoggicosa snelli have been found on bare gravel slopes or red clayey loams with one record from a red dune. Specimens have been recorded in association with Mulga (Acacia aneura), Snakewood (A. xiphophylla), and Spinifex (Triodia) vegetation or bare areas. Mature females were found all year round, but are particularly common from January to April. Males have been collected from August to February. Hoggicosa snelli differs in its burrowing behaviour in that it uses a pebble or debris to block its burrow entrance. McKay (1975) described that each morning the pebble is replaced in the same position, which leads to the build up of a visible circular ring of silk on the pebble. Distribution: Northern Western Australia (Fig. 22). 44 HOGGICOSA STORRI (MCKAY, 1973) COMB. NOV. (FIGS 1D, E, 5G, H, 23A–D, 25) Lycosa storri McKay, 1973: 389–394, figs 1H–J, 3A–E; Main, 1976: 48, 141, 149, 231, pl. B8; Brignoli, 1983: 450; McKay, 1985: 83. Types: Holotype: ♀ from Western Australia: Yellowdine, 38 miles south, 31°51′S, 119°39′E, 6.xi.1970, W. H. Butler (WAM 70/240). Epigynum missing. Paratypes: Western Australia: 1 juv., Albion Downs, 24 miles south-west, 27°17′S, 120°01′E (WAM 71/875); 1 juv., Billeranga, 29°19′S, 115°52′E (WAM 68/501); 1 juv., Grants Patch, Broad Arrow near Kalgoorlie, 30°27′S, 121°20′E (WAM 70/22); 2 juv., Burnabinmah Station, 28°47′S, 117°22′E (WAM 68/823, 68/828); 1 ♀, Clinker Hill, 30°53′S, 121°46′E (WAM 68/504); 1 ♀, Corrigin, 32°20′S, 118°52′E (WAM 71/874); 1 juv., Dulbelling, 32°02′S, 117°15′E (WAM 68/825); 2 juv., Hyden, The Humps, 32°19′S, 118°58′E (WAM 69/884, 71/456); 1 ♀, Karonie, 4 miles northeast, 30°56′S, 122°35′E (WAM 68/505); 1 juv., Kellerberrin, 31°38′S, 117°43′E (WAM 38/1298); 1 ♀, 1 juv., Koorda, 30°50′S, 117°29′E (WAM 70/245, 39/2169); 2 ♀, 2 juv., Lake Moore, near south end, 30°12′S, 117°25′E (WAM 71/174, 71/175-6, 71/197); 1 juv., Leonara, 15 miles east, 28°53′S, 121°35′E (WAM 70/206); 1 ♀, Marloo Station, 28°19′S, 116°11′E (WAM 69/835); 1 ♀, 1 juv., Merredin, 31°29′S, 118°16′E (WAM 69/888, 68/502); 1 juv., Moorine Rock, 31°18′S, 119°08′E (WAM 68/500); 1 ♀, 2 juv., Mt Gibson, 29°36′S, 117°24′E (WAM 71/877, 71/876); 1 juv., Mullewa, 1.5 miles east, 28°32′S, 115°31′E (WAM 68/829); 1 ♂, 2 ♀ Muralgarra, 28°32′S, 117°03′E (WAM 39/2564, 39/2565, 39/2566); 1 ♀, 31 juv., Narembeen Camp Site, 32°04′S, 118°23′E (WAM 69/962-94); 1 ♀, Noongar, 31°20′S, 118°58′E (WAM 39/2472); 1 juv., Nukarni, 31°18′S, 118°12′E (WAM 47/963); 5 juv., Paynes Find, 29°15′S, 117°41′E (WAM 70/53, 70/202-3, 70/54, 69/454); 1 juv., Quairading, 32°01′S, 117°24′E (WAM 69/885); 1 juv., Randells, 13 miles west on railway, 31°00′S, 121°59′E (WAM 68/827); 1 juv., Walebing, 3 miles north, 30°39′S, 116°13′E (WAM 68/498); 1 juv., Walyahmoning Rock, 1 mile south-west, 30°38′S, 118°45′E (WAM 70/57, published as WAM 70/51 in McKay (1973); 1 ♀, Warburton Ranges, 26°06′S, 126°39′E (WAM 71/878); 1 juv., Wialki at Arnolds Water Reserve, 30°25′S, 118°03′E (WAM 68/826); 1 ♀, Williams, 33°02′S, 116°53′E (WAM 68/824); 1 juv., Ravensthorpe, 33°35′S, 120°03′E (WAM 69/886); 1 ♀, Cocklebiddy, 70 miles north, 32°03′S, 124°54′E (WAM 69/890). 45 Other material examined: Ninety-four males, 23 females, and 29 juveniles from 152 records (Appendix 3). Diagnosis: Females and immature males can be readily distinguished from all other Hoggicosa species by the distinct leg coloration and abdominal pattern (Fig. 1D). This pattern consists of light femora, tarsi, and metatarsi, with black patellae and tibiae, with a pale stripe along either side of the abdomen from the spinnerets. The male palp is most similar to that of H. brennani, but can be distinguished by the shape of the ventral process on the tegular apophysis. In H. storri the ventral process lacks the flange found in H. brennani (Fig. 5C, D vs. 5G) and is located closer to the apical tip. Description: Male: Based on male from type locality, WAM T53406, Yellowdine, 31°51′S, 119°39′E, WA. Dorsal shield of prosoma orange-brown, darker in eye quadrangle, covered with white setae. Sternum and labium brown with scattered black setae. Chelicerae dark brown with white setae. Legs all greyish-brown, except for paler ventral femur. Projections on apical end of coxae I (see remarks). Opisthosoma all greyblack with black setae except for creamish-yellow streak running from spinnerets two-thirds along either side. Terminal apophysis of pedipalp large, strongly curved apically (Fig. 23A). Pars pendula transparent and connected just below embolus tip (Fig. 23B). Subterminal apophysis large and easily visible next to terminal apophysis. Tegular apophysis with angular ventral process located centrally or near apex. Curved ridge connecting ventral process and apical point (Fig. 5G). Female: Based on WAM 68/503, Wubin, 30°07′S, 116°38′E, WA. Dorsal shield of prosoma orange-brown with cover of white setae. Sternum, labium, and chelicerae as male. Legs; femur, metatarsus, and tarsus yellowish-cream with tibia and patella black (Fig. 1D). Small projections on apical end of coxae I (see remarks). Opisthosoma black, with pale side streak as male. Epigyne with small anterior pockets, much greater in width than posterior transverse part (Fig. 23C). Internal epigyne with strongly sclerotised anterior pockets and head of spermatheca elongate (Fig. 23D). 46 Variation: In some males a small black longitudinal stripe is present at the anterior end of the dorsal abdomen. The length of the ridge between the ventral process and apical point of the tegular apophysis can vary (Fig. 5G vs. 5H). Some females have a faint pale longitudinal stripe at the anterior end of the dorsal abdomen. Remarks: The epigynum of the holotype is missing and so a representative female is used here for redescription. Several of the male specimens, including those from the type locality, were found to have prominent protuberances extending from the tip of coxae I and II near the sternum. Following further examination, some females were found to have similar, but reduced, bulges. No other morphological differences were found between those with and without coxal points and as the distributions overlap we currently consider them conspecific. Measurements: ♂ WAM T53406 (♀ WAM 68/503): TL, 17.6 (17.0); PL, 9.7 (9.1); PW, 7.3 (7.3). Eyes: AME, 0.45 (0.50); ALE, 0.23 (0.32); PME, 1.09 (1.18); PLE, 0.73 (1.04). Sternum (length/width): 3.6/3.4 (4.0/3.3). Labium (length/width): 1.0/1.0 (1.4/1.4). OL, 7.8 (7.8); OW, 5.7 (5.8). Legs, lengths of segments (femur + patella/tibia + metatarsus + tarsus = total length): pedipalp, 4.3 + 4.0 + – + 3.4 = 11.7; I, 8.6 + 9.8 + 8.6 + 5.0 = 32.0; II, 8.3 + 9.6 + 8.8 + 4.4 = 31.1; III, 7.1 + 7.8 + 7.8 + 4.3 = 27.0; IV, 9.3 + 10.4 + 10.0 + 4.7 = 34.4 (pedipalp, 4.0 + 4.3 + – + 2.8 = 11.1; I, 7.4 + 9.0 + 6.0 + 3.1 = 25.5; II, 7.1 + 8.6 + 6.0 + 3.1 = 24.8; III, 6.4 + 7.4 + 6.4 + 3.3 = 23.5; IV, 7.8 + 9.3 + 8.6 + 3.6 = 29.3). ♂ (♀) (range, mean ± SD): TL, 12.9–20.1, 17.2 ± 1.6; PL, 7.1–10.3, 9.4 ± 0.6; PW, 6.4– 8.1, 7.2 ± 0.4; N = 35 (TL, 17.9–23.0, 19.9 ± 1.7; PL, 8.7–10.3, 9.6 ± 0.5; PW, 6.6–8.1, 7.3 ± 0.5; N = 14). 47 Natural history: Hoggicosa storri have been collected from clay, gritty loam, rocky, red, and yellow soils. Specimens have been recorded in association with vegetation of Mallee (Eucalyptus) and Mulga (Acacia aneura) and woodlands of Wandoo (Eucalyptus wandoo), Salmon Gum (E. salmonophloia), York Gum (E. loxophleba), Gimlet (E. salubris), and E. striaticalyx, as well as open shrubland. Adult females have been collected all year round, whereas adult males have been predominately collected from October to March. Hoggicosa storri excavates burrows without doors. Distribution: Western Australia (Fig. 25). 48 HOGGICOSA WOLODYMYRI SP. NOV. (FIGS 4H, I, 5F, 7D, 24A–D, 25) Types: Holotype. ♂ from South Australia: Taylorville Station, 34°06′S, 139°58′E, 2– 4.x.1999, Strathalbyn Field Nats. (SAM NN16408). Paratype. ♀ with same data as holotype. (SAM NN16409). Other material examined: One-hundred and twenty-three males, 27 females, and four juveniles from 87 records (Appendix 3). Etymology: The specific epithet is a patronym in honour of the senior author‟s late grandfather Wolodymyr Kowal, who after immigrating to Australia fell in love with the outback landscapes where this species is found. Diagnosis: Hoggicosa wolodymyri can be distinguished from all other Hoggicosa by its small body size and orange-yellow colour. The male palp is most similar to that of H. bicolor, but can be distinguished by the tegular apophysis, which has a ridge between the ventral process and the tip much longer than that of H. bicolor (compare Fig. 5F vs. 6A). The female epigyne is similar in shape to H. bicolor but is smaller in size and females can be easily distinguished on body colour. Description: Male: Based on holotype. Dorsal shield of prosoma orange-brown, darker in eye quadrangle, with faint radial pattern; covered with black setae. Sternum orangeyellow, labium dark orange-brown, both with scattered black setae. Chelicerae dark brown with white setae. Legs orange-yellow. Opisthosoma dorsally orange-yellow with mottled black and grey patches; grey-black setae. Dark orange median longitudinal band at anterior end (Fig. 4H). Opisthosoma laterally and ventrally pale orange-yellow, some black dots on venter. Terminal apophysis of pedipalp large and strongly curved apically (Fig. 24A, B). Pars pendula transparent and joins embolus at embolus tip (Figs 7D, 24B). Subterminal apophysis present, but located under terminal apophysis and difficult to see (Fig. 7D). Tegular apophysis with small process, pointed ventrally and located away from apical point. Straight ridge between ventral process and apical point (Fig. 5F). 49 Female: Based on paratype. Dorsal shield of prosoma pale orange, darker in eye quadrangle, with faint radial pattern; covered with black setae. Sternum, labium, chelicerae, and legs as male. Opisthosoma dorsally and laterally as male (Fig. 4I). Venter without black patches. Epigyne with small and simple anterior pockets, only slightly greater in width than posterior transverse part (Fig. 24C). Internal epigyne with thin sclerotized channels of median septum clearly visible as well as thin and elongate spermatheca stalks (Fig. 24D). Variation: Male specimens from WA are slightly smaller and pale yellow in colour, with less colouration (dark patches) on the abdomen. Some males lack black patches on the ventral opisthosoma. Measurements: ♂ holotype (♀ paratype): TL, 15.5 (14.2); PL, 8.0 (6.8); PW, 6.0 (5.4). Eyes: AME, 0.38 (0.38); ALE, 0.23 (0.27); PME, 0.81 (0.88); PLE, 0.77 (0.73). Sternum (length/width): 3.4/2.7 (2.9/2.5). Labium (length/width): 0.9/1.1 (1.0/1.1). OL, 7.5 (7.4); OW, 4.6 (5.0). Legs, lengths of segments (femur + patella/tibia + metatarsus + tarsus = total length): pedipalp, 4.1 + 3.8 + – + – = 7.9; I, 9.4 + 10.0 + 8.5 + 5.0 = 32.9; II, 8.8 + 10.0 + 8.2 + 4.6 = 31.6; III, 8.2 + 9.4 + 8.8 + 4.6 = 31.0; IV, 10.2 + 11.2 + 11.5 + 5.4 = 38.3 (pedipalp, 3.1 + 3.4 + – + 2.6 = 9.1; I, 6.6 + 7.5 + 4.8 + 3.0 = 21.9; II, 6.7 + 7.1 + 4.8 + 2.8 = 21.4; III, 5.9 + 6.5 + 5.0 + 2.8 = 20.2; IV, 7.4 + 8.4 + 6.9 + 3.1 = 25.8). ♂ (♀) (range, mean ± SD): TL, 11.6–14.8, 13.3 ± 1.0; PL, 6.4–7.8, 6.9 ± 0.4; PW, 5.0– 6.0, 5.5 ± 0.3; N = 14 (TL, 14.4–16.9, 15.5 ± 1.0; PL, 6.9–8.5, 7.4 ± 0.7; PW, 5.3–6.4, 5.8 ± 0.5; N = 6). Natural history: Specimens have been collected from sand dunes, sand plains, interdune swales, and clayey soils associated with open Mallee (Eucalyptus) with mixed understorey or Mulga (Acacia aneura) and Spinifex (Triodia) vegetation. Males and females have been collected predominately from September to December, although a couple of males have been found in March and June. Records indicate H. wolodymyri constructs a doorless burrow. 50 Distribution: New South Wales, Northern Territory, South Australia, and Western Australia (Fig. 25). ACKNOWLEDGEMENTS We thank the following people at the WAM for useful comments and discussions: Mark Harvey, Julianne Waldock, Jung-Sun Yoo (now National Institute for Biological Resources, South Korea), and Karen Edward (now University of Western Australia). We extend thanks to Erich Volschenk for assistance with phylogenetic analyses and Mike Rix for help with SEMs. We greatly appreciate loans of material from David Hirst (SAM), Graham Milledge (AM), and Robert Raven and Owen Seaman (QM). Specimens were kindly sent to us by Maggie Hodge (Geolycosa) and Paolo Tongiorgi (Lycosa tarantula), both initially for the study by Murphy et al., 2006. Scanning electron micrographs were carried out using facilities at the Centre for Microscopy, Characterization and Analysis, The University of Western Australia (UWA), which are supported by University, State, and Federal Government funding. Financial support to V. W. Framenau was initially (2002–2005) provided by an Australian Biological Resources Study (ABRS) grant to Mark Harvey (WAM) and Andy Austin (The University of Adelaide) for a revision of the Australian Lycosidae and later (2005– 2008) by an ABRS grant to Volker Framenau and Nikolaj Scharff (University of Copenhagen, Denmark) for a revision of the Australian orb-weaving spiders of the subfamily Araneinae. We thank David Hirst (Fig. 1F), Peter Hudson (Fig. 20) and Mark Harvey (Fig. 9) for permission to use their photographs. We thank Karl Brennan (Western Australian Department of Environment and Conservation), Bob Black (UWA), and Barbara York Main (UWA) for comments on draft manuscripts. 51 REFERENCES Álvares ÉSS. 2006. A Subfamilia Lycosinae no Brasil (Araneae, Lycosidae), com notas sobre especies correntes em paises limitrofes. Unpublished Masters Thesis, Instituto de Biociências da Universidade de São Paulo. Banks N. 1895. Some Missouri spiders. Entomological News 6: 204–207. Bonnet P. 1957. Bibliographia Araneorum: analyse Méthodique de toute la Littérature Aranéologique jusqu’en 1939. Tome II (3me partie: G-M). Douladoure: Toulouse, 1927–3026. Bremer K. 1994. Branch support and tree stability. Cladistics 10: 295–304. Brignoli PM. 1983. A catalogue of the Araneae described between 1940 and 1981. Manchester: Manchester University Press in association with The British Arachnological Society. Byrne M, Yeates DK, Joseph L, Kearney M, Bowler J, Williams MAJ, Cooper S, Donnellan SC, Keogh JS, Leys R, Melville J, Murphy DJ, Porch N, Wyrwoll KH. 2008. Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17: 4398–4417. Casanueva ME. 1980. Los licosidos de Chile. Estudio biologic y taxonomico por los metodos de sistematica alfa y taxonomica numerica (Araneae: Lycosidae). Gayana (Zoology) 42: 5–76. Dondale CD. 1986. The subfamilies of wolf spiders (Araneae: Lycosidae). Actas X Congreso Internacional Aracnologia 1: 327–332. Dondale CD, Redner JH. 1983. Revision of the wolf spiders of the genus Arctosa C. L. Koch in North and Central America (Araneae: Lycosidae). Journal of Arachnology 11: 1–30. Fabricius JC. 1777. Genera insectorum eorumque characters naturales, secundum numerum, figuram, situm et proportionem omnium partium oris; adjecta mantissa specierum nuper detectarum: Chilonii. Framenau VW. 2005. Gender specific differences in activity and home range reflect morphological dimorphism in wolf spiders (Araneae, Lycosidae). Journal of Arachnology 33: 334–346. Framenau VW. 2006a. Mainosa, a new genus for the Australian „Shuttlecock wolf spider‟ (Araneae, Lycosidae). Journal of Arachnology 34: 206–213. Framenau VW. 2006b. Knoelle, a new monotypic wolf spider genus from Australia (Araneae: Lycosidae). Zootaxa 1281: 55–67. 52 Framenau VW. 2007. The wolf spiders of Australia (Araneae, Lycosidae). Checklist, taxonomy and identification. Available at: http://www.lycosidae.info/identification/australia (accessed 25 February 2009). Framenau VW, Baehr BC. 2007. Revision of the Australian wolf spider genus Dingosa Roewer, 1955 (Araneae, Lycosidae). Journal of Natural History 41: 1603– 1629. Framenau VW, Gotch TB, Austin AD. 2006. The wolf spiders of artesian springs in arid South Australia, with a revalidation of Tetralycosa (Araneae, Lycosidae). Journal of Arachnology 34: 1–36. Framenau VW, Hebets EA. 2007. A review of leg ornamentation in male wolf spiders, with the description of a new species from Australia, Artoria schizocoides (Araneae, Lycosidae). Journal of Arachnology 35: 89–101. Framenau VW, Vink CJ. 2001. Revision of the wolf spider genus Venatrix Roewer (Araneae: Lycosidae). Invertebrate Systematics 15: 927–970. Framenau VW, Yoo J-S. 2006. Systematics of the new Australian wolf spider genus Tuberculosa (Araneae: Lycosidae). Invertebrate Systematics 20: 185–202. Fuhn IE, Niculescu-Burlacu F. 1971. Fauna Republicii Socialiste România. Arachnida. Volumul V. Fascicula 3. Fam. Lycosidae. Bucuresti: Editura Academiei Republicii Socialiste România. Goloboff PA, Farris JS. 2001. Methods for quick consensus estimation. Cladistics 17: S26–S34. Goloboff PA, Farris J, Nixon K. 2003. T.N.T.: tree analysis using new technology. Program and documentation available from the authors, and at: http://www.zmuc.dk/public/ Phylogeny/TNT/ (accessed 25 February 2009). Gotch TB, Adams M, Murphy NP, Austin AD. 2008. A molecular systematic overview of wolf spiders associated with Great Artesian Basin springs in South Australia: evolutionary affinities and an assessment of metapopulation structure in two species. Invertebrate Systematics 22: 151–165. Greenstone MH. 1982. Ballooning frequency and habitat predictability in two wolf spider species (Lycosidae: Pardosa). Florida Entomologist 65: 83–90. Griswold CD, Ramirez MJ, Coddington JA, Platnick NI. 2005. Atlas of phylogenetic data for entelegyne spiders (Araneae: Araneomorphae: Entelegynae) with comments on their phylogeny. Proceedings of the California Academy of Sciences, Fourth Series 56, Supplement II: 1–324. 53 Guy Y. 1966. Contribution à l‟étude des araignées de la famille des Lycosidae et de la sous-famille des Lycosinae avec étude spéciale des espèces du Maroc. Traveaux de l’Institut Scientifique Chérifien, Série Zoologie 33: 1–174. Heatwole H. 1987. Major components and distributions of the terrestrial fauna. In: Dyne GR, Walton DW, eds. Fauna of Australia, Vol. 1A. Canberra: Australian Government Publishing Service, 101–135. Hickman VV. 1967. Some common spiders of Tasmania. Hobart: Tasmanian Museum and Art Gallery. Hogg HR. 1900. A contribution to our knowledge of the spiders of Victoria: including some new species and genera. Proceedings Royal Society of Victoria 13: 68–123. Hogg HR. 1905. On some South Australian spiders of the family Lycosidae. Proceedings of the Zoological Society London 1905: 569–590. Koch CL. 1836. Die Arachniden. Dritter Band: Nürnberg. 1–104. Koch L. 1865. Beschreibungen neuer Arachniden und Myriapoden. Verhandllungen der kaiserlich-königlichen zoologisch-botanischen Gesellschaft in Wien 15: 857– 892. Koch L. 1877. Die Arachniden Australiens nach der Natur beschrieben und abgebildet. Theil 1, Erste Lieferung. Bauer & Raspe: Nürnberg: 889–968. Koch L. 1878. Die Arachniden Australiens nach der Natur beschrieben und abgebildet. Theil 1, Zweite Lieferung. Bauer & Raspe: Nürnberg: 969–1044. Latreille PA. 1817. Articles sur les arraignées. Nouveau Dictionnaire D’Histoire Naturelle: N. Ed., Paris. Linnaeus C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus differentiis, synonymis, locis. Editio decima, reformata: Holmiae. Main BY. 1976. Spiders. Sydney: Collins. McKay RJ. 1973. The wolf spiders of Australia (Araneae: Lycosidae): 1. The bicolor group. Memoirs of the Queensland Museum 16: 375–398. McKay RJ. 1975. The wolf spiders of Australia (Araneae: Lycosidae): 5. Two new species of the bicolor group. Memoirs of the Queensland Museum 17: 313–318. McKay RJ. 1985. Lycosidae. In: Walton DW, ed. Zoological catalogue of Australia, Vol. 3. Arachnida, Mygalomorphae, Araneomorphae in part, Pseudoscorpionida, Amblypygida, Palpigradi. Canberra: Australian Government Publishing Service, 73–88. 54 Mello-Leitão Cfd. 1941. Las aranas de la provincia de Santa Fe colectadas por el Profesor Birabén. Revista del Museo de la Plata (N.S., Zool.) 2: 199–225. Moya-Laraño J, Taylor PW, Fernandez-Montraveta C. 2003. Body patterns as potential amplifiers of size and condition in a territorial spider. Biological Journal of the Linnean Society 78: 355–364. Murphy NP, Framenau VW, Donnellan SC, Harvey MS, Park Y-C, Austin AD. 2006. Phylogenetic reconstruction of the wolf spiders (Araneae: Lycosidae) using sequences from the 12S rRNA, 28S rRNA, and NADH1 genes: implications for classification, biogeography, and the evolution of web building behavior. Molecular Phylogenetics and Evolution 38: 583–602. Nixon KC. 2002. WinClada: data management analysis and tree editing, 1.00.08 edn. Ithaca, NY: Published by the author. Page RDM. 2001. Nexus data editor. Version 0.5.0. Available at: http://taxonomy.zoology.gla.ac.uk/rod/NDE/nde.html (accessed 25 February 2009). Platnick NI. 1993. Advances in spider taxonomy 1988–1991. With synonymies and transfers 1940–1980. New York: New York Entomological Society in association with The American Museum of Natural History. Platnick NI. 2009. The world spider catalog. version 9.5. American Museum of Natural History. Available at: http://research.amnh.org/entomology/spiders/catalog/index.html (accessed 25 February 2009). Prenter J, Elwood RW, Montgomery WI. 1998. No association between sexual size dimorphism and life histories in spiders. Proceedings of the Royal Society of London. Series B. Biological Sciences 265: 57–62. Prenter J, Elwood RW, Montgomery WI. 1999. Sexual size dimorphism and reproductive investment by female spiders: a comparative analysis. Evolution 53: 1987–1994. Prenter J, Montgomery WI, Elwood RW. 1997. Sexual dimorphism in northern temperate spiders: implications for the differential mortality model. Journal of Zoology, London 243: 341–349. Pulleine RH. 1922. Two new species of Lycosa from South Australia. Transactions of the Royal Society of South Australia 46: 83–84. Rainbow WJ. 1911. A census of Australian Araneidae. Records of the Australian Museum 9: 107–391. 55 Roewer CF. 1955 [imprint date 1954]. Katalog der Araneen von 1758 bis 1940. Institut Royal de Sciences Naturelles de Belgique: Bruxelles 2a: 1–1751. Roewer CF. 1959 [imprint date 1958]. Araneae Lycosaeformia II. (Lycosidae). Exploration du Parc National de l’Upemba – Mission G. F. de Witte 55: 1–518. Roewer CF. 1960 [imprint date 1959]. Araneae Lycosaeformia II (Lycosidae) (Fortsetzung und Schluss). Exploration du Parc National de l’Upemba – Mission G. F. de Witte 55: 519–1040. Scharff N, Coddington JA. 1997. A phylogenetic analysis of the orb-weaving spider family Araneidae (Arachnida, Araneae). Zoological Journal of the Linnean Society 120: 355–434. Simon E. 1898. Descriptions d‟arachnides nouveaux des familles des Agelenidae, Pisauridae, Lycosidae et Oxyopidae. Annales de la Société Entomologique de Belgique 42: 5–34. Simon E. 1909. Araneae, 2me partie. In: Michaelsen W and hartmeyer H, eds. Die Fauna Südwest-Australiens. Ergebnisse der Hamburger südwest-australischen Forschungsreise 1905. Jena: Gustav Fischer. 152–212. Strand E. 1913. Über einige australische Spinnen des Senckenbergischen Museums. Zoologische Jahrücher für Systematik 35: 599–624. Stratton GE. 2005. Evolution of ornamentation and courtship behavior in Schizocosa: insights from a phylogeny based on morphology (Araneae, Lycosidae). Journal of Arachnology 33: 347–376. Thackway R, Cresswell ID. 1997. A bioregional framework for planning the national system of protected areas in Australia. Natural Areas Journal 17: 241–247. Thorell T. 1870. Araneae nonnullae Novae Hollandiae, descriptae. Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar 27: 367–389. Townley MA, Tillinghast EK. 2003. On the use of ampullate gland silks by wolf spiders (Araneae, Lycosidae) for attaching the egg sac to the spinnerets and a proposal for defining nubbins and tartipores. Journal of Arachnology 31: 209– 245. Vink CJ. 2001. A revision of the genus Allotrochosina Roewer (Araneae: Lycosidae). Invertebrate Taxonomy 15: 461–466. Vink CJ. 2002. Lycosidae (Arachnida: Araneae). Fauna of New Zealand 44. Lincoln: Manaaki Whenua Press. Vink CJ, Paterson AM. 2003. Combined molecular and morphological phylogenetic analyses of the New Zealand wolf spider genus Anoteropsis (Araneae: Lycosidae). Molecular Phylogenetics and Evolution 28: 576–587. 56 Walker SE, Rypstra AL. 2001. Sexual dimorphism in functional response and trophic morphology in Rabidosa rabida (Araneae: Lycosidae). American Midland Naturalist 146: 161–170. Walker SE, Rypstra AL. 2002. Sexual dimorphism in trophic morphology and feeding behavior of wolf spiders (Araneae: Lycosidae) as a result of differences in reproductive roles. Canadian Journal of Zoology 80: 679–688. Wallace HK. 1942. A revision of the burrowing spiders of the genus Geolycosa (Araneae, Lycosidae). The American Midland Naturalist 27: 1–62. Yoo J-S, Framenau VW. 2006. Systematics and biogeography of the sheet-web building wolf spider genus Venonia (Araneae: Lycosidae). Invertebrate Systematics 20: 675–712. Zyuzin AA. 1993. Studies on the wolf spiders (Araneae: Lycosidae). I. A new genus and new species from Kazakhstan, with comments on the Lycosinae. Memoirs of the Queensland Museum 33: 693–700. Zyuzin AA, Logunov DV. 2000. New and little-known species of the Lycosidae from Azerbaijan, the Caucasus (Araneae, Lycosidae). Bulletin of the British Arachnological Society 11: 305–319. 57 58 Table 1. Morphological character matrix. Character and character state descriptions in Appendix B. Character 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Arctosa cinerea 0 1 0 0 2 1 0 0 1 0 0 0 1 1 1 1 0 0 Dingosa serrata 1 2 1 0 2 2 0 0 0 0 0 1 0 D. simsoni 1 2 1 0 2 2 0 0 0 0 0 1 0 Geolycosa hubbelli 1 0 1 0 1 2 1 3 0 0 1 1 1 0 0 0 G. missouriensis 1 0 1 0 0 0 1 3 0 0 1 1 1 0 0 0 ‘Grey Wolf Spider' 1 0 1 0 2 2 0 0 1 1 0 0 0 1 1 0 1 1 1 Hoggicosa alfi 1 1 2 0 2 1 0 3 1 1 1 0 1 0 1 1 0 1 0 H. bicolor 1 0 2 1 1 1 1 3 1 1 1 1 0 1 1 0 0 1 1 H. brennani 1 0 2 0 2 1 0 1 1 1 1 0 0 0 1 0 0 1 1 H. castanea 1 1 2 1 2 1 0 3 1 1 1 0 0 0 1 0 0 1 0 H. duracki 1 0 2 0 1 0 0 0 1 1 1 0 1 0 1 1 1 1 0 H. forresti 1 1 2 1 2 2 0 3 1 1 1 0 1 0 1 0 0 1 0 H. snelli 1 0 2 0 0 0 0 1 1 1 1 1 1 1 1 0 0 1 1 H. storri 1 0 2 1 0 0 0 3 1 1 1 1 1 0 1 0 0 1 1 H. wolodymyri 1 1 2 0 2 2 0 0 1 1 1 0 0 1 1 0 0 1 0 Hogna crispipes 0 1 2 0 2 1 0 0 1 0 2 0 1 1 1 0 0 1 1 H. immansueta 0 1 1 0 2 2 0 1 1 0 0 1 0 0 2 0 1 1 1 H. kuyani 0 1 2 0 2 2 0 0 1 0 2 0 1 1 1 0 0 1 1 Knoelle clara 0 1 2 0 2 2 0 1 1 2 1 0 1 2 1 1 1 1 Lycosa godeffroyi 0 2 2 0 2 2 0 3 1 0 0 1 1 0 1 0 1 1 0 L. leuckarti 0 2 2 0 2 2 0 2 1 0 0 0 1 1 1 0 1 1 0 L. praegrandis 1 1 2 0 2 2 0 1 1 0 0 0 1 ? 1 0 0 ? ? L. tarantula 1 1 2 0 2 2 0 1 1 0 0 0 1 ? 1 0 0 ? ? Mainosa longipes 1 1 1 0 2 1 1 3 0 1 0 1 1 0 0 1 1 Venator spenceri 0 1 1 0 2 2 0 1 1 0 0 1 0 0 2 0 1 1 1 Symbols: (-) = not applicable, (?) = missing data. 20 0 0 0 0 0 0 1 0 0 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 21 1 0 0 0 0 0 1 0 1 2 2 1 0 1 2 0 0 0 0 0 1 1 1 0 0 22 0 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 0 0 1 1 23 1 0 1 0 0 1 1 1 1 1 1 1 1 1 1 0 0 0 1 1 1 0 0 0 0 24 0 0 0 0 0 1 1 1 1 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 25 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 0 0 0 0 1 1 1 1 1 0 26 0 0 0 0 0 1 0 1 1 1 1 1 1 0 0 0 0 0 0 0 - 27 0 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 - Table 2. Distribution of Hoggicosa in Australia with biogeographic regions within states (after Thackway & Cresswell, 1997). Species Australian Distribution Hoggicosa alfi sp. nov. New South Wales, Queensland (ML), South Australia (CHC, EYB, GAW, MDD, SSD, STP), Western Australia (AW, CAR, COO, CR, GAS, GS, GSD, GVD, LSD, MUR, NUL, PIL, TAN, YAL) New South Wales, Northern Territory (DMR, MAC), Queensland (MGD, MII, ML), South Australia (EYB, MDD, SSD, STP), Western Australia (AW, CAR, COO, CR, FIN, GAS, GAW, GD, GS, GVD, LSD, MAL, MUR, PIL, TAN, YAL) New South Wales (CP, DRP, MDD, NSS), Queensland (BBS, NAN), South Australia (EYB, KAN, NCP, STP) H. bicolor (Hogg, 1905) H. brennani sp. nov. H. castanea (Hogg, 1905) H. duracki (McKay, 1975) New South Wales (BHC, CP), Northern Territory (MAC), Queensland (ML), South Australia (CHC, CR, EYB, FLB, GAW, MDD, SSD, STP), Victoria (VVP), Western Australia (AW, CAR, COO, ESP, GAS, GS, GSD, GVD, MAL, MUR, NSS, NUL, PIL, SWA, WAR, YAL) Western Australia (PIL, VB) H. forresti (McKay, 1973) Western Australia (AW, COO, GAS, GVD, HAM, MAL, MUR, NUL, SWA), South Australia (EYB, FLB, MDD) H. natashae sp. nov. * New South Wales (BHC), Queensland (CHC), South Australia (GAW, SSD) H. storri (McKay, 1973) Western Australia (AW, COO, CR, ESP, GAS, GS, JF, LSD, MAL, MUR, NUL, SWA, YAL) H. snelli (McKay, 1975) Northern Western Australia (CAR, GAS, GSD, NK, PIL, TAN) H. wolodymyri sp. nov. New South Wales (CP), Northern Territory (GSD), South Australia (CR, EYB, FIN, FLB, GAW, MDD, NUL, RIV, STP), Western Australia (GVD, MUR) Abbreviations of biogeographic regions: AW = Avon Wheatbelt, BBS = Brigalow Belt South, BHC = Broken Hill Complex, CAR = Carnarvon, CHC = Channel Country, COO = Coolgardie, CP = Cobar Peneplain, CR = Central Ranges, DMR = Davenport Murchison Ranges, DRP = Darling Riverine Plains, ESP = Esperance Plains, EYB = Eyre Yorke Block, FIN = Finke, FLB = Flinders Lofty Block, GAS = Gascoyne, GAW = Gawler, GD = Gibson Desert, GS = Geraldton Sandplains, GSD = Great Sandy Desert, GVD = Great Victoria Desert, HAM = Hampton, JF = Jarrah Forest, KAN = Kanmantoo, LSD = Little Sandy Desert, MAC = MacDonnell Ranges, MAL = Mallee, MDD = Murray Darling Depression, MGD = Mitchell Grass Downs, MII = Mount Isa Inlier, ML = Mulga Lands, MUR = Murchison, NAN = Nandewar, NCP = Naracoorte Coastal Plain, NK = Northern Kimberley, NSS = New South Wales South Western Slopes, NUL = Nullarbor, PIL = Pilbara, RIV = Riverina, SSD = Simpson Strzelecki Dunefields, STP = Stony Plains, SWA = Swan Coastal Plain, TAN = Tanami, VB = Victoria Bonaparte, VVC = Victorian Volcanic Plains, WAR = Warren, YAL = Yalgoo. (Interim Biogeographic Regionalisation for Australia, version 6.1, after Thackway & Cresswell, 1997). * = only females known. 59 Figure 1. Hoggicosa species, habitus photos (A–H) and male diagnostic character (I). A, Hoggicosa bicolor (Hogg) penultimate ♂ from Western Australia (WA), not registered. B, H. bicolor (Hogg) adult ♂ from Lorna Glen Station, WA, WAM T70329. C, Hoggicosa forresti (McKay, 1973) adult ♂ from Lorna Glen Station, WA, WAM T65610. D, Hoggicosa storri (McKay) penultimate ♂ from WA, not registered. E, H. storri (McKay) adult ♂ from Lorna Glen Station, WA, WAM T70277. F, Hoggicosa natashae sp. nov. holotype ♀ from Lake Gilles, SA, SAM NN14785, Photo courtesy of D. Hirst. G, Hoggicosa alfi sp. nov. ♂ from type locality, Lorna Glen Station, WA, not registered. H, H. alfi sp. nov. ♀ from type locality, WAM T70334. I, Hoggicosa castanea (Hogg) lateral view of male cymbium tip with diagnostic macrosetae bent dorsally, from Gluepot Station, SA, SAM NN19314. Scale bars = 5 mm. 60 Figure 2. Strict consensus tree showing the relationships amongst Hoggicosa species and other Lycosinae (length = 104, consistency index = 35, retention index = 53). Characters are indicated by squares: character number above and character state below square with shaded squares for nonhomoplasious characters. Bremer support and relative Bremer support (0–100) values for each branch are indicated. Inset of colour dimorphism (character 4, bold) under fast optimization (ACCTRAN) and slow optimization (DELTRAN). 61 Figure 3. Hoggicosa castanea (Hogg) carapace. A, lateral view. B, frontal view. Scale bars = 1 mm. 62 Figure 4. Hoggicosa species, abdomen (A–G) and habitus (H–I). A–C, ♀ Hoggicosa castanea (Hogg). A, dorsal; B, ventral, from Fisherman Point, Lincoln National Park, South Australia (SA), SAM NN15185; C, dorsal, from north of Lake Goorly, Western Australia (WA), WAM T47784. D–E, Hoggicosa brennani sp. nov. ♂ holotype from Taylorville Station, SA, SAM NN17017. D, dorsal; E, ventral. F–G, Hoggicosa snelli (McKay), ventral. ♂ from Kennedy Range National Park, WA, WAM T65149. G, ♀ from Munda Station, WA, WAM T53870. H–I, Hoggicosa wolodymyri sp. nov. from Taylorville Station, SA. H, ♂ holotype, SAM NN16408. I, ♀ paratype, SAM NN16409. Scale bars = 5 mm. 63 Figure 5. ♂ Hoggicosa species, male palp, tegular apophyses. A–B, Hoggicosa castanea (Hogg). A, north of Bower, South Australia (SA), WAM T64050; B, palpal variation from east of Mount Barren, Western Australia (WA), WAM T53507. C–D, Hoggicosa brennani sp. nov. holotype from Taylorville Station, SA, SAM NN17017; D, tegular apophysis ventral spur enlarged. E, Hoggicosa forresti (McKay) from North Baandee Nature Reserve, WA, WAM T47762. F, Hoggicosa wolodymyri sp. nov. from Queen Victoria Springs Nature Reserve, WA, WAM T52527. G–H, Hoggicosa storri (McKay). G, south of the type locality Yellowdine, WA, WAM T53406; H, palpal variation from Yuinmery, WA, WAM T62781. All scale bars = 0.2 mm except for D = 0.1 mm 64 Figure 6. ♂ Hoggicosa species, male palp, tegular apophyses. A, Hoggicosa bicolor (Hogg) from Mt Vernon Station, Western Australia (WA), WAM T62336. B–D, Hoggicosa snelli (McKay). B, mainland male from Woodleigh Station, WA, WAM T65133; C, palpal variation from Barrow Island, WA, WAM T57697; D, palpal variation from Drysdale River Station, Kimberley, WA, WAM T56397. E–F, Hoggicosa alfi sp. nov. E, paratype from Lorna Glen Station, WA, WAM T53919; F, palpal variation from Nanga Station, WA, WAM T65565. G–H, Hoggicosa duracki (McKay) from Hope Downs Station, WA, WAM T65119. H, tegular apophysis ventral spur enlarged. Scale bars = 0.2 mm. 65 Figure 7. ♂ Hoggicosa species, male palp, palea region. A, Hoggicosa castanea (Hogg) from East Mt Barren, Western Australia (WA), WAM T53507. B, Hoggicosa duracki (McKay) from Hope Downs Station, WA, WAM T65119. C, Hoggicosa forresti (McKay) from Helena-Aurora Ranges, WA, WAM T47763. D, Hoggicosa wolodymyri sp. nov. from Queen Victoria Springs Nature Reserve, WA, WAM T52527. Scale bars = 0.1 mm. Note: twisting of terminal and subterminal apophysis is a result of scanning electron microscopy drying process. 66 Figure 8. Hoggicosa castanea (Hogg). A–B, palp, ♂ from Gluepot Station, South Australia (SA), SAM NN19314. A, ventral; B, palea. C–D, epigynum, ♀ from Fisherman Point, Lincoln National Park, SA, SAM NN15185. C, ventral; D, dorsal. Scale bars = 0.5 mm. 67 Figure 9. Hoggicosa castanea burrow from Stirling Range caravan park, Western Australia, WAM T58378. A, door closed. B, door open. Photos courtesy of M. Harvey. Scale bars = 10 mm. Figure 10. Distribution records of Hoggicosa castanea (Hogg). 68 Figure 11. Hoggicosa alfi sp. nov. A–B, palp, ♂ paratype from Lorna Glen Station, Western Australia (WA), WAM T53919. A, ventral; B, palea. C–D, epigynum, ♀ paratype, from Lorna Glen Station, WA, WAM T77406. C, ventral; D, dorsal. E, epigynum, dorsal, ♀ variation from Nanga Station, WA, WAM T62772. Scale bars = 0.5 mm. 69 Figure 12. Distribution records of Hoggicosa alfi sp. nov. 70 Figure 13. Hoggicosa bicolor (Hogg) from Mt Vernon Station, Western Australia, WAM T62336. A–B, ♂, palp. A, ventral; B, palea. C–D, ♀, epigynum. C, ventral; D, dorsal. Scale bars = 0.5 mm. 71 Figure 14. Distribution records of Hoggicosa bicolor (Hogg). 72 Figure 15. Hoggicosa brennani sp. nov. from Taylorville Station, South Australia. A– B, ♂ holotype, palp, SAM NN17017. A, ventral; B, palea. C–D, ♀ paratype, epigynum, SAM NN17029. C, ventral; D, dorsal. Scale bars = 0.5 mm. 73 Figure 16. Hoggicosa duracki (McKay). A–D, paratypes from Argyle Downs Homestead, Western Australia (WA), WAM 74/495-6. A–B, ♂ palp. A, ventral; B, palea. C–D, ♀, epigynum. C, ventral; D, dorsal. E, epigynum, ventral, ♀ holotype from Argyle Downs, WA, WAM 74/494. Scale bars = 0.5 mm. 74 Figure 17. Hoggicosa forresti (McKay) from North Baandee Nature Reserve, Western Australia, WAM T47762. A–B, ♂, palp. A, ventral; B, palea; C–D, ♀, epigynum. C, ventral; D, dorsal. Scale bars = 0.5 mm. 75 Figure 18. Distribution records of Hoggicosa brennani sp. nov. (black circles), Hoggicosa duracki (McKay) (grey circles), and Hoggicosa forresti (McKay) (white circles). 76 Figure 19. Hoggicosa natashae sp. nov. A–B, epigynum, ♀ holotype from Lake Gilles, South Australia (SA), SAM NN14785. A, ventral; B, dorsal. C–D, epigynum variation, ♀ from Cooper Creek, SA, SAM NN020. C, ventral; D, dorsal. Figure 20. Hoggicosa natashae sp. nov. burrow entrance of holotype from Lake Gilles, South Australia, SAM NN14785. Photo courtesy of P. Hudson. Diameter of coin = 28 mm. 77 Figure 21. Hoggicosa snelli (McKay). A–B, palp, ♂ from Woodleigh Station, Western Australia (WA), WAM T65133. A, ventral; B, palea. C–D, epigynum, ♀ from Shothole Canyon, Cape Range National Park, WA, WAM T47612. C, ventral; D, dorsal. E, palp, ♂ palea variation from Drysdale River Station, Kimberley, WA, WAM T56397. Scale bars = 0.5 mm. 78 Figure 22. Distribution records of Hoggicosa natashae sp. nov. (grey circles) and Hoggicosa snelli (McKay) (white circles). 79 Figure 23. Hoggicosa storri (McKay). A–B, palp, ♂ from Stoneville, Western Australia (WA), WAM T47794. A, ventral; B, palea. C–D, epigynum, ♀ from Wubin, WA, WAM 68/503. C, ventral; D, dorsal. Scale bars = 0.5 mm. 80 Figure 24. Hoggicosa wolodymyri sp. nov. from Taylorville Station, South Australia. A–B, ♂ holotype, palp, SAM NN16408. A, ventral; B, palea. C–D, ♀ paratype, epigynum, SAM NN16409. C, ventral; D, dorsal. Scale bars = 0.5 mm. 81 Figure 25. Distribution records of Hoggicosa storri (McKay) (white circles) and Hoggicosa wolodymyri sp. nov. (grey circles). 82 H. wolodymyri sp. nov. H. storri (McKay, 1973) H. duracki (McKay, 1975) H. forresti (McKay, 1973) H. snelli (McKay, 1975) H. castanea (Hogg, 1905) H. bicolor (Hogg, 1905) H. brennani sp. nov. Hoggicosa alfi sp. nov. "Grey Wolf Spider" G. missouriensis (Banks, 1895) Geolycosa hubbelli Wallace, 1942 D. simsoni (Simon, 1898) Dingosa serrata (L. Koch, 1877) Species Arctosa cinerea (Fabricius, 1777) ♂ ♀ ♂ ♀ ♂ ♀ ♂ ♀ ♂ ♀ ♂ ♀ ♂ ♀ ♂♀ ♂ ♀ ♂ ♀ ♂♀ ♂♀ ♂ ♀ ♂ ♀ ♂ ♀ 83 Location Isar River near Wallgau, Germany Lech River near Forchach, Austria Yanchep, Western Australia Inglewood, Perth, Western Australia Yokrakine Rock, Western Australia Boorabin, Western Australia Archbold Biological Station, Florida, United States of America * (Wallace, 1942) Holmes County State Park, Mississippi, United States of America Holmes County State Park, Mississippi, United States of America Broken Hill, New South Wales Runnymede Flora Reserve, Victoria Lorna Glen Station, Western Australia Lorna Glen Station, Western Australia Mt Vernon Station, Western Australia Taylorville Station, South Australia Taylorville Station, South Australia Gluepot Station, South Australia Fisherman Point, Lincoln National Park, South Australia Argyle Downs Homestead, W North Baandee Nature Reserve, Western Australia Woodleigh Station, Western Australia Shothole Canyon, Cape Range National Park, Western Australia Stoneville, Western Australia Wubin, Western Australia Taylorville Station, South Australia Taylorville Station, South Australia WAM WAM WAM WAM WAM WAM WAM SAM SAM SAM SAM WAM WAM WAM WAM WAM WAM SAM SAM Deposition WAM WAM WAM WAM WAM WAM WAM Species used for phylogenetic analysis with specimen location, place deposited and registration number. * = characters scored from secondary sources. APPENDIX 1 T56118 T56117 T62654 T56072 T53919 T77406 T62336 NN17017 NN17029 NN19314 NN15185 74/495-7 T47762 T65133 T47612 T47794 68/503 NN16408 NN16409 Registration T56247 T56246 T58321 T68496 71.1422 T51253 T56572 V. spenceri Hogg, 1900 Mainosa longipes (L. Koch, 1878) L. tarantula (Linnaeus, 1758) L. praegrandis C. L. Koch, 1836 L. leuckarti (Thorell, 1870) Lycosa godeffroyi L. Koch, 1865 Hogna immansueta (Simon, 1909) H. kuyani Framenau, Gotch & Austin, 2007 Knoelle clara (L. Koch, 1877) Hogna crispipes L. Koch, 1877 ♂ ♀ ♂♀ ♂ ♀ ♂ ♀ ♂ ♀ ♂ ♀ ♂ ♀ ♂ ♀ ♂ ♀ ♂ ♀ 84 Deepdale, Western Australia South Lake, Western Australia Red Bluff, Red Bluff Caravan Park, Western Australia `Sieda`, East of Grass Patch,Western Australia Laverton, Western Australia Drysdale River Station, Western Australia Kapalga, Northern Territory Maylands, Perth, Western Australia Eneabba, Western Australia Burekup, Western Australia Gardner Reserve Road, Western Australia * (Zyuzin & Logunov, 2000) Amissa, Greece * (Álvares, 2006) San Rossore, Pisa, Italy Francois Peron National Park, Western Australia Nerren Nerren Station, Western Australia Kanyaka Creek, Wilson, South Australia Adelaide, South Australia WAM WAM WAM WAM WAM WAM WAM WAM WAM WAM WAM WAM WAM WAM WAM WAM WAM T56055 T48038 94/1940 T51488 T64071 90/566 T65662 T75884 T58331 T53586 T55296 T56241 T58372 T65006 T65005 T53852 T53675 APPENDIX 2 Character descriptions 1. Carapace, profile in lateral view: cephalic region not elevated, profile horizontal (0) (Framenau et al., 2006: fig. 23); cephalic region elevated, profile strongly sloping away from eyes (1) (Fig. 3A). 2. Carapace, radial colour pattern, female: absent (0) (Fig. 1A); weak or indistinct (1) (Fig. 1H); strong pattern of black and white lines „Union-Jack-colour pattern‟ (2). The „Union-Jackcolour pattern‟ is seen in Lycosa leuckartii (Main, 1976: figs 26, 27). 3. Anterior eye row, curvature in frontal view: straight (0); slightly procurved (1) (Framenau, 2006a: fig. 4); strongly procurved (2) (Fig. 3B). Eye row curvature is determined by drawing a line through the middle of the anterior median eyes. If the line passed through the middle of the anterior lateral eyes it was scored as straight. If the line passed through the upper half of the anterior lateral eyes it was considered slightly procurved and strongly procurved if the line did not pass through the anterior lateral eyes. 4. Leg colouration, alternating dark and light segments, in females but not adult males: absent (0) (Fig. 1G, H); present (1) (Fig. 1A, B). 5. Abdomen, dorsal colour, female: unicoloured (0) (Fig. 1D); dual coloured (1) (Fig. 1F); multicoloured (2) (Fig. 4A). 6. Abdomen, cardiac (heart) or chevron mark, female: absent (0) (Fig. 1D); present, light colour compared to surrounding (1) (Fig. 1A); present, dark colour compared to surrounding (2) (Fig. 1C). If multiple colours surrounded the cardiac mark, the predominate colour was used. 7. Abdomen, lateral colour pattern, female: pale (0) (fig. 4F); black (1) (Framenau, 2006a, fig. 1). 8. Abdomen, ventral colour pattern, female: uniform light (0); light with dark pattern (1) (Fig. 1E, F); dark with light pattern (2); uniform dark (3) (Fig. 4B). If the black pattern reached all the way to the spinnerets and covered most of the ventral abdomen it was considered uniform dark. 9. Cymbium tip, macrosetae: absent (0) (Framenau, 2006a: fig. 6); present (1) (Fig. 1I). 10. Cymbium tip, number of macrosetae: One to ten macrosetae (0) (Framenau et al., 2006: fig. 20); 10–30 macrosetae (1) (Fig. 1I); > fig. 2). 85 11. Cymbium tip, macrosetae direction: straight (0) (Álvares, 2006: fig. 13); curved dorsally (1) (Framenau et al., 2006: fig. 21); curved ventrally (2) (Fig. 1I). 12. Tegular apophysis, position of ventral process: distance between ventral process tip and tegular apophysis tip greater than half distance of tegular apophysis (0) (Fig. 5A); distance between ventral process tip and tegular apophysis tip less than half distance of tegular apophysis (1) (Fig. 6A). The distance of the tegular apophysis was taken as the length of the line from the apical point through the ventral process to the edge of the strongly sclerotized tegular apophysis (Fig. 5A). 13. Tegular apophysis, connection of ventral process to apical edge: absent (0) (Fig. 5A); present (1) (Fig. 6B). The apical edge of the tegular apophysis is defined in relation to the palp. 14. Tegular apophysis, connection of ventral process to apical point, ridge shape: curved (0) (Fig. 5A); straight (1) (Fig. 6A). 15. Tegular apophysis, number of ventral processes: none (0) (Framenau & Baehr, 2007: fig. 2C); one (1) (Fig. 1A); two (2) (Framenau, 2006b: fig. 6). 16. Terminal apophysis, direction: curved apically (0) (Fig. 8B); straight, pointing retrolaterally (1) (Fig. 11B). 17. Terminal apophysis, shape of tip: pointed, sharp tip (0) (Fig. 8B); truncated, broad tip (1) (Fig. 16B). 18. Subterminal apophysis: absent (0) (Framenau & Baehr, 2007: fig. 6A); present (1) (Fig. 11B). 19. Subterminal apophysis, visibility in ventral view: Beneath terminal apophysis and difficult to see (0) (Figs 7A, 8B); Next to terminal apophysis and easily visible (1) (Fig. 13B). 20. Pars pendula, thickness: thin, transparent (0) (Fig. 13B); thick and obvious, opaque (1) (Fig. 8B). 21. Pars pendula, connection to embolus: Joins well below embolus tip (0) (Fig. 13B); Joins just below embolus tip (1) (Fig. 11B); along whole embolus (2) (Fig. 7A, B). If the pars pendula was only found at the base of the embolus (Fig. 21B) or the length of embolus without the pars pendula was close to the length of the terminal apophysis (Fig. 13B), it was scored as 0. If the pars pendula joined at the embolus tip it was scored as 2 and those that had a pars pendula intermediate to these cases were scored as 1. 22. Epigyne, inverted T-shaped median septum: absent (0) (Zyuzin & Logunov, 2000: fig. 2); present (1) (Fig. 8C). 86 23. Epigyne, median septum, widening anteriorly: absent (0) (Zyuzin & Logunov, 2000: fig. 2); present (1) (Fig. 8C). 24. Epigyne, distance between anterior pockets: equal or shorter than width of posterior transverse part (0) (Framenau, 2006b: fig. 9); greater than width of posterior transverse part (1) (Fig. 8C). 25. Burrow, female and immatures: absent (0); present (1). 26. Burrow, trapdoor: absent (0) (Framenau, 2006a: fig. 2); present (door or rock) (1) (Fig. 9A, B). 27. Burrow, palisade: absent (0) (Fig. 9B); present (1) (Framenau, 2006a: fig. 2). 87 APPENDIX 3 List of material examined by species then state. Hoggicosa castanea NSW: 2 ♂, Birdwood Station, Lower Murray-Darling Region, 33º29'54''S, 142º44'30''E (AM KS71096); 1 ♀, Broken Hill, 31º57'S, 141º27'E (SAM NN17226); 1 ♀, Broken Hill, Springs Creek, Barrier Hwy, 120km E, 31º43'S, 142º41'E (SAM NN17227); 1 ♂, Glen Tilt Station, Lower Murray-Darling region, 31º41'59''S, 143º35'32''E (AM KS66996); Gundabooka National Park, Yanda Creek, near Ben Lomond,, 30º31'145''S, 145º48'E (SAM NN18280); 1 ♀, Gundabooka National Park, Yanda Creek, near Ben Lomond,, 30º31'S, 145º48'E (SAM NN18283); 10 ♂, 1 ♀, no exact locality (AM KS91620, KS91594, KS92508, KS91602, KS91605); 1 ♂, Nymagee, 6 mi S, 32º04'S, 146º19'E (AM KS5728); 45 ♂, Orange Grove Station, Lower Murray-Darling region, 33º36'15''S, 143º34'39''E (AM KS66841); 1 ♂, Prill Park Station, Lower Murray-Darling region, 34º40'20''S, 142º41'33''E (AM KS66966); 1 ♀, Rabbit Camp (no exact location), no exact locality (WAM 69/36); 1 ♂, 2 ♀, Round Hill, Euabalong, 32º57'S, 146º9'E (AM KS86505, KS82567, KS69276); 3 ♂, Round Plain Station, Lower Murray-Darling region, 33º43'18''S, 143º37'40''E (AM KS66838); 1 ♂, Woolcunda Station, Lower Murray-Darling region, 32º51'25''S, 141º21'05''E (AM KS67437); NT: Hermannsburg, 23º56'S, 132º46'E (SAM NN16555); Qld: 1 ♂, `Gumbardo`, 26º04'38''S, 144º45'20''E (QM S63293); 1 ♀, Ambathala, 1mile E of No 3 bore on old road, 25º58'S, 145º20'E (QM S70660); SA: 1 ♂, no exact locality (QM S22456); 1 ♀, 31º53'45''S, 137º32'43''E (SAM NN13782); 1 ♀, ALGE campsite, no exact locality (SAM no reg.); 1 ♀, Amyton Cementary, 32º37'S, 138º20'E (SAM NN14720); 1 ♀, Bairds Bay, 4.1km ESE, 33º9'30''S, 134º24'22''E (SAM no reg.); 1 ♀, Billeroo Well, S of Lake Frome, 31º12'S, 139º55'E (SAM NN13776); 3 ♂, Bower, N of, 34º00'31''S, 139º21'24''E (WAM T64050); 1 ♀, Brookfield Conservation Park, 34º20'S, 139º31'E (SAM NN13806); 1 ♀, Cadell, 34º02'S, 139º46'E (SAM NN13808); 1 ♀, Calperum Station, Frogamerry, 33º55'04''S, 140º24'42''E (SAM NN13807); 1 ♀, Chambers Gorge turnoff, 8km W, 30º58'S, 139º16'E (SAM NN13759); 2 ♀, Claire, N of, 33º50'S, 138º36'E (WAM 69/802, 69/800); 1 ♀, Coffin Bay, 34º40'S, 135º19'E (SAM NN15186); 1 ♀, Coffin Bay National Park, Yangie Bay camping area, 34º38'S, 135º22'E (SAM NN15188); 1 ♀, Dalhousie Springs, 1km N of spring, 26º28'S, 135º29'E (NMV K8162); 1 ♂, Danggali Conservation Park, Tomahawk Dam, 3km N, 33º19'39''S, 140º42'50''E (SAM NN17074); 1 ♀, Emu-Cook Junction, 26mi S, no exact locality (WAM 69/807); 1 ♀, Erudina Woolshed, near, 31º29'S, 139º23'E (SAM NN13802); 2 ♀, Euro Bluff, NW of Pt Augusta, 32º21'S, 139º21'E (SAM NN13781, NN13765); 1 ♀, Eyre Peninsula, 32º32'18''S, 136º34'31''E (SAM NN13770); 2 ♂, Eyre Peninsula (no exact location), 33º39'S, 137º11'E (SAM NN13773-4); 1 ♀, Flinders Ranges, between Hawker and Wilpena, 31º50'S, 138º35'E (WAM 69/921); 1 ♀, Gawler Ranges, no exact locality, 32º44'S, 136º47'E (SAM NN13764); 1 ♂, Gluepot Station, Gluepot Homestead, 11.5km W, 33º45'08''S, 139º59'51''E (SAM NN19439); 2 ♂, Gluepot Station, Gluepot Homestead, 4.7km N, 33º43'23''S, 140º07'26''E (SAM NN18801, NN18800); 1 ♀, Gluepot Station, Gluepot Homestead, 7.2km WNW, 33º44'29''S, 140º02'54''E (SAM NN19498); 2 ♂, Gluepot Station, Gluepot Homestead, 7km N, 33º42'01''S, 140º07'24''E (SAM NN18881-2); 1 ♂, Gluepot Station, Gluepot Homestead, 9.6km W-WNW, 33º44'51''S, 140º01'07''E (SAM NN19314); 1 ♀, Inila Rock Waters, 31º47'S, 133º46'E (SAM NN16236); 1 ♀, Innamincka, 27º44'S, 140º44'E (SAM NN436); 1 ♀, Kappawanta Basin, 33º40'S, 135º22'E (SAM NN15187); 1 ♀, 1 juv., Lake Callabonna, 29º40'S, 140º01'E (WAM 71/243-4); 3 ♀, Lake Gilles, 32º43'S, 136º47'E (SAM NN13787, NN13792, NN13791); 1 ♀, Lake Pitikanta, 28º21'S, 138º18'E (SAM NN15193); 1 ♂, Lake Torrens to Andamooka, no exact locality (QM S21351); 1 ♀, Lincoln National Park, Fisherman Point, 34º45'S, 135º59'E (SAM NN15185); 2 ♀, Lincoln National Park, near Spalding Cave, 34º46'S, 135º58'E (SAM NN15183-4); 1 ♀, Lucky Bay, 33º42'S, 137º02'E (SAM NN13786); 2 ♀, Middleback, 32º56'S, 137º23'E (SAM NN17004, NN16996); 1 ♀, Minnipa, Agricultural Research Centre, 3km NNW, 32º51'S, 135º9'E (SAM NN13798); 1 ♀, Mt Sturt, 3km S, 32º45'S, 135º24'E (SAM NN13761); 2 ♀, Mt Whyalla, 32º49'S, 137º26'E (SAM NN13793, NN13794); 1 ♀, Muckera Rockhole, 30º02'S, 130º03'E (SAM NN17006); 1 ♂, 1 ♀, 1 juv., Murninnie Beach, 33º20'S, 137º25'E (SAM NN13797, NN13795); 1 ♀, Musgrave Park, No.25 Bore, Mt. Caroline, 25miles N, 26º20'S, 130º49'E (SAM NN15243); 3 ♀, no locality given (SAM NN021); 1 ♀, North Well, nr Kingoonya, 30º50'S, 135º18'E (SAM NN13777); 1 ♀, Orroroo, 32º44'S, 138º36'E (SAM NN13805); 1 ♀, OTC Station, E of, N of Ceduna, 32º07'S, 133º40'E (SAM NN13760); 1 ♂, Peake Station, Edith Spring, 4.8km NE, 28º26'10''S, 136º07'41''E (SAM NN15781); 1 ♂, Peake Station, Four Hills Trig, 6.8km NNW, 28º27'04''S, 136º27'46''E (SAM NN15787); 1 ♂, Peake Station, Mussel WH, 1.5km NE, 28º27'12''S, 136º23'26''E (SAM NN15802); 1 ♀, Pinkawillinie Conservation Park, 33º03'S, 135º50'E (SAM NN17061); 1 ♀, Pinkawillinie Conservation Park, 33º07'S, 136º00'E (SAM NN13799); 1 ♀, Pt Gawler, 34º38'S, 138º28'E (SAM NN13785); 1 ♂, 2 ♀, Redcliffe plant site, 33º04'S, 137º34'E (SAM NN13784, NN13788, NN13790); 2 ♂, 1 ♀, Roxby 88 Downs, 30º33'S, 136º54'E (SAM NN16172, NN13803, NN13804); 1 ♂, Roxby Downs, Olympic Dam site, 30º27'S, 136º54'E (SAM NN13758); 1 ♀, 1 juv., Salt Creek nr Iron Knob, 32º46'S, 137º05'E (SAM NN13762); 2 ♀, Scrubby Peak, 32º31'S, 135º19'E (SAM NN13800, NN13767); 1 ♀, She-Oak Log, 34º30'S, 138º49'E (SAM NN13789); 1 ♀, Simpson Desert, 26º07'S, 138º37'E (NMV K8177); 1 ♀, South Gap Station, nr edge Lake Torrens, 31º36'S, 137º32'E (SAM NN13757); 1 ♂, South west of SA, no exact locality (SAM NN13779); 2 ♀, Spider Lake, 35º13'S, 136º55'E (SAM NN15191-2); 1 ♂, Survey Locks Well, via Woomera, 31º9'S, 136º48'E (SAM NN13783); 1 ♀, Tarcoola Turnoff, Glendambo area, 30º57'S, 135º43'E (SAM NN17010); Tregalana Station, Whyalla, 20km N, 32º50'S, 137º37'E (SAM no reg.); 1 ♀, Tregalana Station, Whyalla, 20km N, 32º52'S, 137º33'E (SAM NN13780); 2 ♂, Uno Range, 32º43'S, 136º45'E (SAM NN13771-2); 3 ♀, Wauraltee, 34º35'S, 137º33'E (SAM NN026, NN027, NN028); 3 ♀, Whyalla Airport, 33º03'S, 137º30'E (SAM NN13796, NN13778, NN13775); WhyteYarcowie, 33º13'S, 138º53'E (SAM NN14721); 1 ♂, Willow Springs Station, Willow Springs Homestead, 13.5km NNE, 31º21'31''S, 138º50'17''E (SAM NN16196); 1 ♀, Woolshed Flat, 34º07'S, 138º38'E (SAM NN018); 1 ♀, Wynbring Rocks, 30º33'S, 133º32'E (SAM NN13754); 1 ♀, Yardea Station, Moonarie Homestead, 29km S, 32º22'S, 135º31'E (SAM NN13763); 1 ♀, Yumbarra Conservation Park, Yumbarra Rockhole Track, 31º46'S, 133º29'E (SAM NN13755); VIC: 1 ♂, Altona North, 37º52'S, 144º51'E (AM KS20520); 1 ♀, Little Desert, 36º33'S, 141º50'E (NMV K8253); WA: 1 ♀, Albion Downs, 24 miles SW, 27º29'S, 120º10'E (WAM 71/1686); 1 ♂, Benjiwarn, 27º47'55''S, 122º40'55''E (WAM T48023); 2 ♂, Bidgemia Station, Gasgoyne Jn, 25º12'34''S, 115º30'50''E (WAM T65128); 1 ♂, Boolathana Station, 24º24'47''S, 113º40'30''E (WAM T65121); 1 ♂, Boolathana Station, 24º24'48''S, 113º42'24''E (WAM T65122); 1 ♂, Boolathana Station, 24º24'38''S, 113º39'47''E (WAM T65123); 2 ♀, Boorabin, 31º14'S, 120º19'E (WAM T48021, T48024); 1 ♂, Boulder, 30º47'S, 121º29'E (AM KS86506); 2 ♀, Buningonia Spring, 31º26'20''S, 123º32'10''E (WAM T48022); 1 ♀, Burnerbinmah Station, Nangel Paddock, S of Wadda Wadda Well, 28º42'56''S, 117º12'17''E (WAM T52346); 2 ♂, Bush Bay, 25º07'30''S, 113º49'22''E (WAM T65129); 1 ♀, Carnarvon, 24º53'S, 113º40'E (WAM T63319); 1 ♂, Commonwealth Rd, West (SE. of Kulin), 32º44'13''S, 118º16'16''E (WAM T62796); 1 ♂, Durokoppin Nature Reserve, 31º30'S, 117º44'E (WAM T62789); 1 ♂, East Mt Barren, 33º55'S, 120º01'E (WAM T53507); 1 ♂, Edel Land, 26º31'44''S, 113º29'56''E (WAM T47780); 1 ♂, Eneabba, R.G.C. Mineral Sands, 29º50'S, 115º15'E (WAM T62793); 2 ♀, 1 juv., Fitzgerald River Inlet, 34º08'S, 119º24'E (WAM 71/769-770, 70/187); 1 ♂, 1 ♀, Fitzgerald River Reserve, 34º04'S, 119º25'E (WAM 71/199-200); 1 ♂, Fitzgerald River, near; 29km S of Highway 1, 34º08'S, 119º24'E (WAM T55335); 2 ♀, Forrest, 30º44'S, 127º41'E (WAM 69/45169/837); 1 ♂, Francois Peron National Park, 25º58'34''S, 113º34'15''E (WAM T65569); 1 ♂, Francois Peron National Park, 25º52'31''S, 113º32'59''E (WAM T65556); 1 ♀, Golden Ridge, 30º51'S, 121º39'E (WAM 28/347); 1 ♀, Great Victoria Desert, Googs Track, E of, SE of Mt Finke,, 31º08'S, 134º10'E (WAM T63318); 2 ♀, 1 juv., Greenhead, Airstrip 2mi E, no exact locality (QM S70664-5); 1 ♀, Group 147, via Northcliffe, 34º37'S, 116º08'E (WAM 37/2822); 1 ♂, Hamelin Homestead, 26º25'S, 114º11'E (WAM T53475); 1 ♂, Howatharra Nature Reserve, 28º32'51''S, 114º39'41''E (WAM T62783); 1 ♂, Irrunytju Rockhole, 26º07'S, 128º58'E (WAM T53379); 1 ♂, Jibbarding Nature Reserve, 30º00'32''S, 116º49'19''E (WAM T62771); 1 ♂, Kalgoorlie, 50km NE, Black Swan Nickel Mine, 30º23'07''S, 121º38'59''E (WAM T53250); 1 ♂, Kalgoorlie, 50km NE, Black Swan Nickel Mine, 30º23'56''S, 121º41'06''E (WAM T56961); 1 ♂, Kalgoorlie, 50km NE, Black Swan Nickel Mine, 30º24'02''S, 121º40'59''E (WAM T56941); 1 ♂, Kalgoorlie, 5mi N, 30º40'S, 121º27'E (WAM T53818); 1 ♂, Kambalda, ca. 15km SW, 31º12'58''S, 121º27'23''E (WAM T56553); 1 ♂, Koolanooka Dam Road, SE of Morawa, 29º14'40''S, 116º06'06''E (WAM T62777); 1 ♀, Lake Colville, 29º30'S, 126º40'E (WAM 89/293-5); 1 ♀, Lake King, 33º05'S, 119º36'E (WAM T53734); 1 ♂, Lake Lefroy, road to NE reference site, 31º14'S, 121º50'E (WAM T41651); 1 ♂, Lake Lefroy, SW, 31º17'01''S, 121º38'13''E (WAM T53840); 1 ♀, Lake Lefroy, SW, 31º17'03''S, 121º38'15''E (WAM T45937); 2 ♂, Lake Moore, 35-40 km WNW of Beacon, 30º20'01''S, 117º29'14''E (WAM T51462); 2 ♂, Lorna Glen Station, 26º17'50''S, 121º33'08''E (WAM T64816, T66451); 3 ♂, Lorna Glen Station, 26º16'14''S, 121º29'52''E (WAM T66443); 2 ♂, Lorna Glen Station, 26º18'13''S, 121º33'46''E (WAM T66449); 1 ♂, Lorna Glen Station, 26º18'11''S, 121º28'31''E (WAM T66847); 4 ♂, Lorna Glen Station, 26º04'30''S, 121º27'08''E (WAM T66473, T64843); 1 ♂, Lorna Glen Station, 26º16'26''S, 121º33'34''E (WAM T66453); 1 ♂, Lorna Glen Station, 26º12'10''S, 121º22'32''E (WAM T64792); 1 ♂, Lorna Glen Station, 26º01'56''S, 121º33'36''E (WAM T64829); 1 ♂, Mardathuna station, 24º30'41''S, 114º38'14''E (WAM T65125); 1 ♀, McDermid Rock, 32º01'S, 120º44'E (WAM T47785); 1 ♀, Mesa A, 32.7km WSW of Pannnawonica, 21º42'56''S, 116º01'03''E (WAM T77612); 1 ♂, Minniberri, Norrish's property, 3 km W. of Kodj Kodjin on Minniberri Rd,, 31º15'S, 117º52'E (WAM T62794); 2 ♂, Moore Rd, south (SW. of Mullewa), 28º43'20''S, 115º19'46''E (WAM T62782); 1 ♂, Morawa-Perenjori road, 29º13'56''S, 116º04'05''E (WAM T65115); 1 ♀, Mt Gibson, 29º35'20''S, 117º12'05''E (WAM T70324); 1 ♂, 1 ♀, Mt Gibson, 29º36'S, 117º24'E (QM S70658-9); 2 ♀, Mt Holland, 20km N, 31º56'S, 119º37'E (WAM T53733, T53694); 1 ♀, Munda Station, 20º30'21''S, 118º03'36''E (WAM T47610); 1 ♂, Murchison settlement, camp just N, 26º52'35''S, 115º57'9''E (WAM T51477); 1 ♂, Nanga Station, 26º29'23''S, 114º03'24''E (WAM T65557); 89 3 ♂, Nanga Station, 26º28'40''S, 114º04'34''E (WAM T65120); 1 ♂, Naretha, 31º00'S, 124º49'E (WAM T53626); 1 ♀, Newman, 23º22'S, 119º44'E (WAM 71/1735); 1 ♀, Nullarbor Plain, ca. 18km NE of Haig, 30º56'59''S, 126º15'50''E (WAM T56243); 1 ♂, Oakajee Nature Reserve, 28º34'11''S, 114º38'30''E (WAM T65117); 1 ♂, Ora Bunda, 30º32'30''S, 121º01'10''E (WAM T62765); 1 ♂, PWD Camp, Murchison River, 27º42'S, 114º9'E (WAM 69/782); 1 ♀, Queen Victoria Springs Nature Reserve, 30º14'S, 123º41'E (WAM T53064); 2 ♂, 1 ♀, Quobba Station, Cape Cuvier, 24º14'41''S, 113º33'08''E (WAM T65150, T63279); 2 ♂, Ravensthorpe, 20km S, 33º46'S, 120º03'E (WAM T62792); 1 ♀, Rawlinna, 70mile E, 30º20'S, 126º03'E (WAM 71/413); 1 ♀, Red Bluff, Red Bluff Caravan Park, 27º44'37''S, 114º08'49''E (WAM T58302); 1 ♂, Salt River, 45 km ENE of Quairading, 31º53'31''S, 117º49'46''E (WAM T51471); 1 ♀, Sanderson Road, N of Lake Goorly, 29º57'29''S, 117º01'05''E (WAM T47784); 1 ♀, State Forest, 32º57'04''S, 115º43'03''E (WAM T53815); 1 ♂, 1 ♀, Stirling Range caravan park, 34º18'55''S, 118º11'14''E (WAM T58378, T62795); 2 ♂, Tutanning, 32º32'S, 117º19'E (WAM T62790, T62791); 2 ♂, Urawa Nature Reserve, North, 28º24'02''S, 115º34'34''E (WAM T62327); 1 ♀, Wandana Reserve, 28º19'S, 115º9'E (WAM T47783); 1 ♂, Weelhamby Lake, 29º11'22''S, 116º28'25''E (WAM T62780); 1 ♂, Weelhamby Lake, West, 29º11'02''S, 116º27'32''E (WAM T48090); 1 ♀, Wiluna, 26º35'S, 120º14'E (WAM 71/1687); 4 ♂, Woodleigh Station, 26º11'31''S, 114º30'33''E (WAM T65124); 1 ♂, Yuinmary, 28º33'15''S, 119º05'30''E (WAM T48007); 6 ♂, Yuinmery, 28º31'55''S, 119º11'35''E (WAM T56593). Paratypes of H. forresti: 1 ♀, Morawa, 29º13'S, 116º00'E (WAM 70/172); 1 ♀, Mt Magnet, 323mile peg, 28º03'S, 117º50'E (WAM 69/46); 1 ♀, Kulin, 32º40'S, 118º9'E (WAM T53819; 33/1607); 1 ♀, Coonana, 12 miles NW, 30º54'S, 123º01'E (WAM 69/35); 1 ♀, Laverton, Police Station, 28º38'S, 122º24'E (WAM 26/716, published as WAM 26/717 in McKay, 1973); 1 ♀, Marloo Station, 28º19'S, 116º11'E (WAM 69/42). Hoggicosa alfi NSW: 1 ♂, Beleuah Station, 90 km NE of Bourke, 29º21'24''S, 146º13'13''E (AM KS37163); 5 ♂, Sturt National Park, 13.7 km E intersection Middle Road & Camerons Corner Road, 29º02'40''S, 141º18'26''E (AM KS71045); QLD: 1 ♀, Naretha, 26º35'S, 143º57'E (WAM T55624); SA: 1 ♀, Ampeinna Hills, 10.2 km E, 27º05'04''S, 131º13'45''E (SAM NN11165); 1 ♀, Ampeinna Hills, 10.5 km E, 27º05'13''S, 131º13'15''E (SAM NN11166); 1 ♀, Ampeinna Hills, 11.6 km E, 27º04'52''S, 131º14'03''E (SAM NN11167); 1 ♂, Anna Creek Station, Mungutana Dam, 7 km NNW, 29º16'13''S, 135º38'53''E (SAM NN15801); 1 ♀, Approdinna Attora Knolls, Lake Tamblyn camp, 26º02'30''S, 137º36'13''E (SAM NN15929); 1 ♀, Arckaringa Station, Nasa Bore, 4.6 km NE, 27º46'41''S, 135º12'42''E (SAM NN15826); 2 ♂, Arcoona Station, May Hill, 5.1 km W, 31º17'16''S, 136º34'59''E (SAM NN15777-8); 1 ♂, Calperum Station, Yabbie Tanks, 2.25 km NE, 33º36'S, 140º34'E (SAM NN16388); 4 ♂, Cheesman Peak, 12.2 km NW, 27º20'13''S, 130º14'15''E (SAM NN11099-102); 1 ♂, Cheesman Peak, 4.5 km NE, 27º22'26''S, 130º21'44''E (SAM NN11108); 1 ♂, Cheesman Peak, 4.7 km NNE, 27º22'01''S, 130º21'06''E (SAM NN11127); 2 ♂, Cheesman Peak, 9.3 km NNW, 27º19'46''S, 130º17'36''E (SAM NN11103-4); 12 ♂, Clifton Hills Outstation (E of), 26º30'S, 139º29'E (SAM NN16001-12); 9 ♂, Clifton Hills Outstation, 4.1 km NE, 26º30'40''S, 139º28'42''E (SAM NN15332-8, NN15388, NN15400); 11 ♂, Clifton Hills Outstation, 4.25 km NW, 26º31'07''S, 139º26'04''E (SAM NN15360-70); 7 ♂, Clifton Hills Outstation, 5.25 km E, 26º32'25''S, 139º30'17''E (SAM NN15339, NN15355-8, NN15398-9); 26 ♂, Clifton Hills Outstation, 5.7 km NE, 26º30'37''S, 139º29'33''E (SAM NN15340-54, NN15371-81); 6 ♂, 2 ♀, Clifton Hills Ruin, 26º31'S, 139º26'E (SAM NN16013-6, NN16021, NN16022, NN16024, NN16025); 1 ♂, Coomandook, 2 km S, 35º29'03''S, 139º41'10''E (SAM NN16332); 2 ♂, 1 ♀, 3 juv., Coongie, 27º12'S, 140º10'E (SAM NN16087-8, NN16150); 1 ♀, Cowell, 33º19'29''S, 137º05'36''E (SAM no reg.); 10 ♂, Dalarinna Hill, 8.5 km E, 29º43'13''S, 138º40'18''E (SAM NN15790-9); 1 ♂, Dalarinna Hill., 10 km SE, 29º45'27''S, 138º40'04''E (SAM NN15775-6); 2 ♀, Danggali Conservation Park, Tomahawk Dam, 3 km N, 33º19'39''S, 140º42'50''E (SAM NN17069, NN17075); 1 ♀, Emu, 51 km W, 28º32'S, 131º43'E (SAM NN16109); 1 ♂, Galga, 19 km N, 34º30'06''S, 139º55'18''E (SAM NN16306); 1 ♀, Gluepot Station, Gluepot Homestead, 8.5 km W-WNW, 33º44'49''S, 140º01'56''E (SAM NN18699); 1 ♀, 1 juv., Head of Bight, 31º29'S, 131º9'E (SAM NN16158); 1 ♂, Ketchalby Rockhole, 9.6 km S, 32º37'28''S, 135º01'56''E (SAM no reg.); 1 ♂, 1 juv., Koonchera Waterhole, 10.5 km SSE, 26º45'42''S, 139º31'48''E (SAM NN15397); 1 ♂, Koonchera Waterhole, 4 km SSE, 26º42'46''S, 139º30'42''E (SAM NN15389); 2 ♂, Kunytjanu, 25 km N, 26º29'16''S, 129º10'25''E (SAM NN11122-3); 4 ♂, Mabel Creek Homestead, 25 km SSW, 29º10'20''S, 134º14'30''E (SAM NN15253, NN15254-5, NN15258); 1 ♂, Mabel Creek Homestead, 28 km SSW, 29º11'40''S, 134º15'E (SAM NN15260); 1 ♂, Mabel Creek Homestead, 28 km SW, 29º10'S, 134º11'30''E (SAM NN15252); 2 ♂, Mabel Creek Homestead, 30 km SW, 29º9'40''S, 134º06'30''E (SAM NN15256-7); 1 ♂, Mabel Creek Station, Lake Phillipson, 10 km N, 29º21'30''S, 134º28'E (SAM NN15259); 1 ♂, Maralinga, 12 km SSW, 30º16'00''S, 131º33'10''E (SAM NN15249); 1 ♂, Memory Bore Camp, 26º42'S, 135º32'E (SAM NN15786); 2 ♂, Mootatunga, 4 km S, 34º59'16''S, 140º50'16''E (SAM NN16304-5); 3 ♂, Mt Hoare, no exact locality (SAM no reg.); 8 ♂, 3 ♀, Mt Hoare, 6 km W, 27º03'23''S, 129º38'20''E (SAM no reg.); 1 ♂, 1 juv., Mt Lindsay, 3.1 km WNW, 27º01'9''S, 129º51'01''E (SAM 90 NN11128); 1 ♂, 2 ♀, Mt Lindsay, 48.9 km SE, 27º18'50''S, 130º15'54''E (SAM NN11124-6); 2 ♂, Mt Lindsay, 6.6 km WNW, 27º03'39''S, 129º49'26''E (SAM NN11129-30); 2 ♂, Muckera Rockhole, 52 km N, 29º33'33''S, 130º08'19''E (SAM NN15248, NN16100); 1 ♀, Munyaroo Conservation Park, 17 km SSE Moonabbie, 33º23'44''S, 137º21'17''E (SAM no reg.); 1 ♂, New Alton Downs Homestead, 19.7 km SE, 26º35'27''S, 139º08'01''E (SAM NN15359); 1 ♂, 1 ♀, no exact locality (SAM NN1629, no reg.); 1 ♂, Pile Hill., 6.3 km SW, 29º08'11''S, 138º24'9''E (SAM NN15783); 3 ♂, Pinkawillinie, 33º03'20''S, 135º46'57''E (SAM no reg.); 2 ♂, Relief Bore, Tallaringa Conservation Park, 68.5 km W, 28º13'S, 133º22'E (SAM NN15246-7); 1 ♀, Serpentine Lakes, 28º30'S, 129º00'E (SAM NN16127); 1 ♀, Simpson Desert, Knolls Track, 26º07'S, 137º37'E (WAM T53488); 1 ♂, Simpson Desert, Purni Bore, Colson Track, 69.8 km E, 26º15'36''S, 136º47'39''E (SAM NN15932); 1 ♂, Simpson Desert, Purni Bore, French Line, 29.4 km ENE, 26º13'48''S, 136º23'00''E (SAM NN15931); 1 ♂, Simpson Desert, Purni Bore, Rig Rd, 30.3 km E, 26º15'41''S, 136º23'51''E (SAM NN15930); 1 ♂, Simpson Desert, Purni Bore, Rig Rd, 44.7 km E, 26º19'16''S, 136º32'30''E (SAM NN15927); 1 ♀, South Gap Station, Beda Hill (near), 31º51'S, 137º37'E (SAM NN16179); 1 ♂, Tallaringa Well, Tallaringa Conservation Park, 2.7 km W, 29º01'S, 133º16'E (SAM NN15245); 6 ♂, Taylorville Station, 15 Mile Dam, 34º02'12''S, 140º11'04''E (SAM NN17040-1, NN17013-6); 1 ♂, Taylorville Station, Casuarina Dam, 33º53'10''S, 140º18'32''E (SAM NN17038); 2 ♂, Taylorville Station, Middle Dam, 1.5 km SW, 33º54'37''S, 140º12'20''E (SAM NN17031-2); 1 ♂, Todmorden Homestead, 18.4 km NNE, 26º58'58''S, 134º48'36''E (SAM NN15926); 3 ♂, 2 ♀, 1 juv., Warburton River, Yelpawaralinna Waterhole, 27º07'30''S, 138º42'30''E (SAM NN15996, NN15997-8, NN15999, NN16049); 3 ♂, Warburton River, Yelpawaralinna Waterhole, 1.8 km ESE, 27º05'19''S, 138º41'01''E (SAM NN15394-6); 6 ♂, Warburton River, Yelpawaralinna Waterhole, 7.6 km NNW, 27º06'22''S, 138º41'49''E (SAM NN15382-7); 2 ♀, Wyola Lake, 29º9'S, 130º14'E (SAM NN16103, NN13672); WA: 1 ♂, 1 ♀, no exact locality (WAM T77405, T77406); 3 ♂, Badjaling, 1.5km N, 31º59'S, 117º30'E (WAM T53790); 7 ♂, Boolathana Station, 24º24'49''S, 113º44'41''E (WAM T65567); 1 ♀, Boorabie, Nullabor Plain, 29º03'S, 128º26'E (WAM T65116); 3 ♂, Bungalbin Hill, Aurora Range, 30º16'11''S, 119º43'30''E (WAM T62809); 1 ♂, Buningonia Spring, 3.5 km SW, 31º26'S, 123º32'E (WAM T51394); 1 ♀, Carnarvon, 50 miles N, 24º10'S, 113º40'E (WAM T53873); 1 ♀, East Nugadong Nature Res., 30º12'S, 116º57'E (WAM T63247); 1 ♀, Edel Land, 26º08'45''S, 113º26'15''E (WAM T62314); 1 ♂, Goongarrie Station, 30º06'04''S, 121º9'18''E (WAM T46063); 1 ♂, Goongarrie Station, 29º53'59''S, 121º10'35''E (WAM T46062); 1 ♂, Goongarrie Station, 29º58'04''S, 121º04'54''E (WAM T46058); 1 ♂, Goongarrie Station, 29º56'48''S, 121º07'00''E (WAM T46057); 1 ♂, Goongarrie Station, 29º50'13''S, 120º57'36''E (WAM T46061); 2 ♂, Kennedy Range National Park, 24º34'14''S, 114º57'12''E (WAM T65560); 2 ♂, 1 ♀, Kennedy Range National Park, 24º30'01''S, 115º01'07''E (WAM T65559); 1 ♀, 3 juv., Koorda, 30º49'S, 117º29'E (SAM NN16594); 1 ♀, Lake Mackay, 22º26'47''S, 128º27'33''E (WAM T84373); 3 ♂, Laverton, 39 km E, 28º28'S, 122º50'E (WAM T55586, T65571, T65572); 1 ♂, Little Sandy Desert, 18.2 km NNE. Of Kulonoski East Well, 24º31'45''S, 120º17'44''E (WAM T66896); 1 ♂, Lorna Glen Station, 26º13'35''S, 121º31'08''E (WAM T55131); 2 ♂, Lorna Glen Station, 26º15'55''S, 121º16'53''E (WAM T70326); 1 ♂, 1 ♀, Lorna Glen Station, 26º15'58''S, 121º06'40''E (WAM T70333, T70334); 11 ♂, Lorna Glen Station, 26º16'53''S, 121º21'27''E (WAM T53917, T64664); 9 ♂, 1 ♀, Lorna Glen Station, 26º12'04''S, 121º18'14''E (WAM T53919, T64621); 3 ♂, Lorna Glen Station, 26º14'07''S, 121º16'53''E (WAM T53918, T64926); 3 ♂, Lorna Glen Station, 26º17'06''S, 121º22'9''E (WAM T53921, T64924); 1 ♂, Lorna Glen Station, 26º09'28''S, 121º33'50''E (WAM T64729); 2 ♂, Lorna Glen Station, 26º00'05''S, 121º33'48''E (WAM T66403); 12 ♂, 1 ♀, 2 juv., Mardathuna Station, 24º25'43''S, 114º30'00''E (WAM T65568, T62808); 1 ♂, 1 juv., Mardathuna Station, 24º24'23''S, 114º26'42''E (WAM T65570); 1 ♀, Monkey Mia, Shark Bay, Faure Island, 15 km SE, 25º52'30''S, 113º53'12''E (SAM NN14132); 1 ♂, 1 ♀, Moore Rd, south (SW. of Mullewa), 28º43'20''S, 115º19'46''E (WAM T62807); 3 ♂, Morgan Range, 25º53'30''S, 128º25'38''E (WAM T79150); 1 ♀, 3 juv., Mt Elvire Station, 29º33'36''S, 119º36'08''E (WAM T63249); 9 ♂, 1 juv., Nanga Station, 26º32'47''S, 113º57'47''E (WAM T65566); 1 ♂, 1 ♀, Nanga Station, 26º32'47''S, 113º57'47''E (WAM T47778, T62772); 3 ♂, Nanga Station, 26º31'21''S, 114º00'08''E (WAM T65564); 14 ♂, Nanga station, 26º35'32''S, 113º53'22''E (WAM T65118); 2 ♂, Nanga Station, 26º29'23''S, 114º03'24''E (WAM T65565); 2 ♂, Nerren Nerren Station, 27º00'22''S, 114º32'29''E (WAM T65563); 4 ♂, Nerren Nerren Station, 27º03'28''S, 114º30'25''E (WAM T65561); 4 ♂, Nerren Nerren Station, 27º03'00''S, 114º34'32''E (WAM T65562); 1 ♂, Nerren Nerren Station, 27º03'24''S, 114º34'23''E (WAM T65558); 29 ♂, Point Salvation, 7 – 8 km WNW, 28º12'S, 123º36'E (WAM T62373 T62810 T62374 T65573 T65574 T65575 T65576 T65577 T65585); 1 ♀, Queen Victoria Spring Nature Reserve, Streich Mound, 30º28'S, 123º41'E (WAM T63281); 1 ♂, Queen Victoria Spring, 1 km NE of, 30º28'S, 123º41'E (WAM T63246); 13 ♂, Queen Victoria Springs Nature Reserve, 30º14'S, 123º41'E (WAM T49114, T49134, T52355, T52540, T52569, T52675, T52682, T52700, T53135); 1 ♀, Rudall River, 22º28'S, 122º29'E (WAM 71/1582); 1 ♂, Tanami, 90 km SE of Sturt Creek, 19º35'20''S, 128º52'13''E (WAM T70981); 10 ♂, Walter James Range, 24º37'45''S, 128º45'22''E (WAM T79143,T79144); 3 ♂, Walter James Range, 24º41'37''S, 128º45'47''E (WAM T79139); 2 ♂, Walter James Range, 24º46'57''S, 128º46'48''E (WAM T79140); 2 ♂, Walter James Range, 24º39'16''S, 91 128º45'21''E (WAM T79137); 5 ♂, Walter James Range, 24º42'27''S, 128º45'38''E (WAM T79142); 8 ♂, Walter James Range, 24º48'03''S, 128º47'50''E (WAM T79141); 5 ♂, Walter James Range, 24º33'35''S, 128º42'36''E (WAM T79138); 1 ♀, Wandana Reserve, 28º19'S, 115º9'E (WAM T62770); 1 ♀, 3 juv., Well 43, Canning Stock Route, 21º12'S, 125º59'E (WAM 73/140-3); 23 ♂, Yamarna Station, 28º13'30''S, 123º35'30''E (WAM T65586, T65587, T65588); 3 ♂, Yundamindra, 29º24'00''S, 122º28'05''E (WAM T47776); 1 ♂, Zuytdorp, 27º15'50''S, 114º04'14''E (WAM T63248). Paratypes of H. forresti: 1 ♀, Mt Magnet, 323 mile peg, 28º03'S, 117º50'E (WAM 69/791); 1 ♀, Paynes Find area, 323 mile peg, 29º15'S, 117º41'E (WAM 70/186). Hoggicosa bicolor NT: 1 ♂, 2 ♀, Alice Springs, 23º42'S, 133º52'E (SAM NN17243, AM KS71064, NTMAG, no reg.); 1 ♀, Alice Springs Desert Park, 23º42'S, 133º52'E (AM KS69273); 1 ♀, 1 juv., Alice Springs, 8 km W, 23º42'S, 133º53'E (AM KS40990); 1 ♀, Bitter Springs (near), 23º33'S, 134º27'E (SAM NN13671); 1 juv., Charlotte Waters, 25º55'S, 134º54'E (NMV K8201); 1 ♀, Hermannsburg, 23º56'S, 132º46'E (SAM NN17220); 1 ♀, Idacowra Station, nr Finke River, 23º55'S, 132º43'E (SAM NN17219); 1 ♂, Kings Creek Station, 24º26'21''S, 131º49'15''E (SAM NN16570); 1 juv., Palm Creek, 24º03'S, 132º40'E (NMV K8202); 1 ♀, Rabbit Flat Roadhouse, 24 km W, 20º11'S, 129º45'E (NTMAG no reg.); 1 ♀, Tennant Creek, 19º39'S, 134º11'E (SAM NN17221); 6 ♀, 10 juv., Tennant Creek, 19º39'S, 134º11'E (QM S21353); 2 ♀, Trephina Gorge turnoff, near Alice Springs, 23º32'S, 134º24'E (QM S21358); 1 ♂, Uluru, 25º20'S, 131º02'E (SAM NN16560); 2 ♀, 1 juv., Uluru, 25º21'S, 131º02'E (QM S21357); 2 ♀, Wauchope, 20º39'S, 134º13'E (QM S21364); 1 ♀, Yulara, 25º15'S, 122º58'E (AM KS69260); Qld: 1 ♀, Beaudesert, 21º32'S, 141º06'E (QM S70647); 1 ♂, Byrock, 24 km NW, 26º03'S, 143º08'E (AM KS82556); 2 ♂, 5 ♀, 5 juv., Camooweal, 19º56'S, 138º07'E (QM S21362); 1 ♂, 2 ♀, 3 juv., Lake Julius turnoff, 20 km E Mt Isa, 20º44'S, 139º29'E (QM S21363); SA: 1 ♂, Amata, 35 km SE, 26º16'37''S, 131º27'38''E (SAM NN11118); 1 ♀, Bookaloo, E of, 31º54'9''S, 137º28'06''E (SAM NN13684); 1 ♀, Candosa, no exact locality (SAM no reg.); 3 ♂, Cheesman Peak, 4.7 km NNE, 27º22'01''S, 130º21'06''E (SAM NN11105-7); 1 ♀, 2 juv., Coober Pedy, 10 miles E, 29º01'S, 134º55'E (WAM 70/214-6); 1 ♀, Cook, 145 km N, 29º29'S, 130º10'E (SAM NN13677); 1 ♂, Donald Well Hill, 0.7 km WNW, 26º12'17''S, 132º19'42''E (SAM NN11117); 1 ♂, East Arckaringa Homestead, 27º54'30''S, 134º50'20''E (SAM NN16151); 1 ♂, Everard Ranges, 27º10'S, 132º25'E (SAM NN21381); 1 ♂, Everard Ranges, 2.6 km WNW Etelerinna Hill, 27º08'S, 132º24'E (SAM no reg.); 1 ♀, Gawler Ranges, 32º43'39''S, 136º47'16''E (SAM NN13680); 1 ♂, Hambidge, 33º23'S, 135º57'E (SAM NN17001); 1 ♂, Hamilton Homestead, 13.4 km SSE, 26º49'20''S, 135º08'11''E (SAM NN15920); 1 ♀, Hamilton Station, Bitchera WH, 6.1 km WNW, 26º33'37''S, 134º28'25''E (SAM NN15622); 1 ♂, Hamilton Station, Bitchera WH, 10.9 km WNW, 26º31'25''S, 134º26'31''E (SAM NN15529); 1 ♂, Indulkana Creek, 26º55'S, 133º47'E (SAM NN21382); 1 ♂, Ketchalby Rockhole, 9.6 km S, 32º37'28''S, 135º01'56''E (SAM no reg.); 3 ♂, Kunytjanu, 26 km S, 26º24'18''S, 129º07'47''E (SAM NN11119-21); 2 ♂, Kunytjanu, 8 km NW, 26º36'55''S, 129º18'51''E (SAM NN11109-10); 1 ♀, Lake Gairdner (near), 32º17'S, 135º55'51''E (SAM NN13679); 1 ♂, Lambina Homestead, 12.6 km NNE, 26º48'01''S, 134º05'26''E (SAM NN15921); 1 ♂, Lambina Homestead, 47.1 km N, 26º29'14''S, 134º02'30''E (SAM NN15922); 1 ♂, Ludgate Well, 4 km WSW, 26º25'30''S, 134º51'15''E (SAM NN15523); 3 ♂, Mabel Creek Homestead, 28 km SW, 29º10'S, 134º11'E (SAM NN16036-8); 1 ♀, Makiri, 27º04'S, 131º09'E (SAM NN13675); 1 ♂, Marla, 34º20'S, 139º31'E (SAM NN13822); 1 ♂, Maryinna Hill, 11.5 km SSE, 27º03'34''S, 131º16'00''E (SAM NN21443); 1 ♂, Mimili, 26.3 km ENE, 26º54'47''S, 132º57'03''E (SAM NN11089); 1 ♂, Mimili, 26 km ENE, 26º54'50''S, 132º56'54''E (SAM NN11088); 1 ♂, 1 ♀, Mimili, 31.5 km ENE, 26º53'45''S, 132º59'58''E (SAM NN11060, NN11090); 1 ♂, Mt Lindsay, 27º01'30''S, 129º52'32''E (SAM NN11087); 1 ♀, Mt Lindsay (Wataru), 27º01'32''S, 129º52'36''E (SAM NN11061); 1 ♀, 1 juv., Mt Lindsay (Wataru), 27º01'30''S, 129º52'32''E (SAM NN11062); 8 ♂, 1 ♀, Mt Lindsay, 48.9 km SE, 27º18'50''S, 130º15'54''E (SAM NN11059, NN11091-3, NN11094-8); 1 ♂, Mt Sarah Homestead, 11.7 km SSW, 27º01'32''S, 135º13'10''E (SAM NN15923); 1 ♂, Ngarutjara Homeland, 10 km NNE Mt Woodroffe, 26º18'S, 131º44'E (SAM NN11086); 1 ♂, North Arckaringa Creek, 27º42'50''S, 134º49'10''E (SAM NN16073); 1 ♀, Pinkawillinie Conservation Park, 33º06'52''S, 135º59'50''E (SAM NN13681); 1 ♀, Pinkawillinie Conservation Park, 33º07'S, 136º00'E (SAM NN13678); 1 ♂, Relief Bore, Tallaringa Conservation Park, 65.6 km W, 28º12'S, 133º23'E (SAM NN14662); 2 ♂, Sentinal Hill, 1.4 km SW, 26º05'35''S, 132º26'37''E (SAM NN11113-4); 1 ♂, Sentinal Hill, 10.4 km SW, 26º08'19''S, 132º22'01''E (SAM NN11116); 1 ♂, Sentinal Hill, 10.5 km S, 26º10'34''S, 132º27'22''E (SAM NN11112); 1 ♂, Sentinal Hill, 14 km SE, 26º10'18''S, 132º32'52''E (SAM NN11115); 1 ♀, Sentinel Hill, nr & SW of, 26º05'S, 132º26'E (SAM NN11063); 1 ♀, Serpentine Lake, E side, 28º30'20''S, 129º02'30''E (SAM NN13674); 2 ♂, Simpson Desert, Purini Bore, Rig Rd, 43.7 km E, 26º19'17''S, 136º31'53''E (SAM NN15925, NN15928); 3 ♂, Tallaringa boundary at Mabel Creek Station, 28º59'S, 133º49'E (SAM NN16143-5); 1 ♀, Tallaringa Well, 50 km W, 28º55'S, 132º50'E (SAM NN13673); 1 ♂, Todmorden Homestead, 12.6 km ENE, 27º07'01''S, 134º52'43''E (SAM NN15924); 1 ♂, Todmorden Homestead, 17.4 km ESE, 27º11'04''S, 134º55'25''E (SAM NN15940); 1 ♀, 92 Uno Range, 32º43'S, 136º41'E (SAM NN13682); 1 ♀, Vokes Hill, 28º33'S, 130º34'E (SAM NN13676); 1 ♂, Womikata Bore, 6 km WSW, 26º06'33''S, 132º05'57''E (SAM NN11111); 1 ♀, Woomera, 31º9'S, 136º48'E (SAM NN13683); WA: 1 ♀, Amy Giles Rocks, 25º53'26''S, 128º31'35''E (WAM T79145); 1 ♂, Bencubbin Kellerberrin rd., 31º24'37''S, 117º45'06''E (WAM T63274); 1 ♂, Bidgemia Station, Gasgoyne Junction, 25º10'31''S, 115º29'17''E (WAM T65171); 4 ♂, Boolathana Station, 24º24'49''S, 113º44'41''E (WAM T65170, T65168); 2 ♂, Boolathana Station, 24º24'48''S, 113º42'24''E (WAM T65169); 2 ♂, Brockman, 16.5 km SW of Mount Brockman, 22º34'19''S, 117º11'34''E (WAM T78135); 1 ♂, Brockman, 19 km SW of Mount Brockman, 22º36'01''S, 117º11'21''E (WAM T78136); 1 ♂, Brockman, 3.5 km W. of Mount Brockman, 22º27'52''S, 117º16'07''E (WAM T78134); 1 ♀, 1 juv., Burnabinmah Station, 28º47'S, 117º22'E (WAM 71/880-1); 1 ♀, Canning Stock Route, Well 10-1, 24º51'S, 121º39'E (NMV K8169); 1 ♀, Canning Stock Route, Well 31, no exact locality (WAM T55303); 1 ♂, Cape Cuvier, Quobba Station, 24º13'24''S, 113º30'13''E (WAM T65555); 1 ♂, Chook Lake, 29º53'32''S, 123º35'44''E (SAM NN21380); 1 ♀, Coolcalalaya Station, vicinity of Murchison, 27º31'S, 115º03'E (WAM T53502); 2 ♀, Diamond Well Station, 26º27'39''S, 119º27'01''E (WAM T46097); 3 ♂, 2 juv., Durokoppin Nature Reserve, 31º30'S, 117º44'E (WAM T51355, T55579, T62484); 2 ♂, 1 juv., East Yorkrakine Reserve, 31º28'S, 117º41'E (WAM T55580, T63277); 3 ♂, Francois Peron National Park, 25º50'20''S, 113º36'23''E (WAM T63253); 1 ♀, Gibson Desert, Huntoil Road, 24º06'S, 125º51'E (WAM T47718); 1 ♂, Gill Pinnacle, Schwerin Mural Crescent, 24º53'S, 128º47'E (SAM NN16589); 2 ♂, Goongarrie Station, 29º58'12''S, 120º59'17''E (WAM T46069); 1 ♂, Goongarrie Station, 29º58'48''S, 121º07'00''E (WAM T46066); 1 ♂, Goongarrie Station, 29º55'16''S, 121º08'52''E (WAM T46072); 1 ♂, Goongarrie Station, 29º58'04''S, 121º04'54''E (WAM T46067); 2 ♂, Goongarrie Station, 29º55'24''S, 121º08'27''E (WAM T46071); 1 ♂, Goongarrie Station, 30º02'32''S, 120º52'53''E (WAM T46070); 1 ♂, Goongarrie Station, 29º58'06''S, 122º01'30''E (WAM T46068); 1 ♀, Grasspatch, 'Sieda', Fitzgerald Locality 41, 33º14'S, 121º43'E (WAM T47719); 1 ♀, Hamersley Range, 10 miles S, on Mt Tom Price Road, 22º21'S, 117º19'E (WAM 69/1045); 1 ♂, 1 ♀, Hope Downs Station, 22º30'S, 119º05'E (WAM T45560); 1 ♂, Hope Downs Station, 22º30'S, 119º14'E (WAM T45559); 1 ♀, Jiggalong Mission, vicinity of, 23º22'S, 120º47'E (WAM 71/1438); 4 ♂, Jundee, 11.7 km N of Jundee homestead, 26º14'57''S, 120º40'19''E (WAM T70263); 1 ♀, Jundee, 14.1 km ESE of Lake Violet Homestead, 26º35'59''S, 120º47'24''E (WAM T70262); 1 ♂, 1 ♀, Jundee, 8.5 km SSE of Jundee homestead, 26º25'16''S, 120º40'45''E (WAM T70264, T70265); 10 ♂, Laverton, 39 km E, 28º28'S, 122º50'E (WAM T65579, T62768, T62769, T55585, T55574, T55592, T65578); 1 ♂, Leonora, 370 km E, Warburton Road, 28º36'S, 124º47'E (WAM T63276); 1 ♂, Little Sandy Desert, 17.5 km SE of Burranbar Pool, 23º55'23''S, 120º31'51''E (WAM T47724); 1 ♂, Little Sandy Desert, 20.8 km NNE. Of Kulonoski East Well, 24º30'33''S, 120º18'31''E (WAM T66895); 1 ♂, Little Sandy Desert, Cooma Well, 24º04'46''S, 120º20'15''E (WAM T47723); 3 ♂, Lorna Glen Station, 26º11'32''S, 121º11'40''E (WAM T70327, T70328, T70329); 2 ♂, Lorna Glen Station, 26º11'59''S, 121º15'59''E (WAM T70276, T70280); 4 ♂, Lorna Glen Station, 26º12'04''S, 121º18'13''E (WAM T53920, T70278, T70279); 1 ♂, Lorna Glen Station, 26º15'58''S, 121º06'40''E (WAM T70332); 1 ♂, Lorna Glen Station, 26º13'35''S, 121º31'08''E (WAM T70330); 1 ♂, Lorna Glen Station, 26º12'04''S, 121º18'14''E (WAM T70273); 1 ♂, Lorna Glen Station, 26º15'55''S, 121º16'53''E (WAM T70325); 1 ♂, Lorna Glen Station, 26º17'50''S, 121º33'08''E (WAM T70275); 3 ♂, Lorna Glen Station, 26º16'53''S, 121º21'27''E (WAM T53911, T70274); 1 ♂, Lorna Glen Station, 26º14'07''S, 121º16'53''E (WAM T64925); 1 ♂, Lorna Glen Station, 26º16'57''S, 121º06'44''E (WAM T70331); 1 ♂, Lorna Glen Station, 26º04'30''S, 121º27'08''E (WAM T64768); 1 ♂, Lorna Glen Station, 26º04'09''S, 121º26'54''E (WAM T64761); 1 ♂, Lorna Glen Station, 26º07'48''S, 121º31'02''E (WAM T53913); 2 ♂, Mardathuna Station, 24º25'43''S, 114º30'00''E (WAM T65163); 3 ♂, 1 ♀, Mardathuna Station, 24º24'23''S, 114º26'42''E (WAM T47715, T65164); 1 ♂, Mardathuna Station, 24º26'36''S, 114º30'42''E (WAM T63272); 1 ♂, Mardathuna Station, 24º30'41''S, 114º38'14''E (WAM T65127); 6 ♂, Meedo Station, 25º40'49''S, 114º37'20''E (WAM T65158, T65161); 2 ♂, Meedo Station, 25º42'42''S, 114º35'58''E (WAM T65162); 1 ♀, Meedo Station, 25º37'23''S, 114º41'40''E (WAM T47717); 1 ♂, Meekatharra, 86 km N, 25º56'05''S, 118º50'36''E (WAM T52094); 1 ♂, Morgan Range, 25º55'58''S, 128º26'57''E (WAM T79147); 1 ♂, Morgan Range, 25º56'08''S, 128º26'16''E (WAM T79149); 1 ♂, Mt Brockman, 22º25'08''S, 117º24'32''E (WAM T63271); 1 ♂, Mt Brockman, 22º18'39''S, 117º15'35''E (WAM T63273); 1 ♀, Mt Brockman, Nammuldi Mine (Hamersley Iron), 22º24'41''S, 117º26'03''E (WAM T47720); 1 ♂, Mt Fraser, 25º38'S, 118º23'E (WAM T62334); 1 ♂, Mt Gibson Station, 29º47'9''S, 117º20'04''E (WAM T46044); 1 ♀, Mt Gibson turnoff, 2 miles E (Paynes Find), 29º37'S, 117º07'E (WAM 68/792); 3 ♂, Mt Keith mine, 13.8 km E. of Lake Way homestead, 26º57'43''S, 120º35'44''E (WAM T74638); 1 ♂, Mt Keith mine, 14.2 km ENE. of Mt Keith homestead, 27º14'35''S, 120º38'59''E (WAM T74650); 1 ♂, Mt Keith mine, 14.2 km E. of Mount Keith homestead, 27º38'56''S, 120º38'56''E (WAM T74629); 2 ♂, 1 ♀, Mt Keith mine, 14.3 km ENE. of Mount Keith homestead, 27º14'35''S, 120º38'59''E (WAM T74607, T74612, T74614); 16 ♂, Mt Keith mine, 14.4 km ESE. of Lake Way homestead, 26º58'53''S, 120º36'24''E (WAM T74280, T74630, T74633); 2 ♂, Mt Keith mine, 14.8 km E. of Lake Way homestead, 26º58'9''S, 120º36'53''E (WAM T74648); 12 ♂, Mt Keith mine, 15.5 km E. of Lake Way homestead, 26º57'41''S, 120º37'22''E (WAM T74634, T74642); 1 ♂, Mt Keith mine, 93 15.6 km E. of Mount Keith homestead, 26º57'41''S, 120º37'25''E (WAM T74279); 1 ♂, Mt Keith mine, 17.8 km ESE. of Lake Way homestead, 26º58'54''S, 120º38'33''E (WAM T74637); 4 ♂, 1 ♀, Mt Keith mine, 18.6 km SE. of Lake Way homestead, 27º03'29''S, 120º36'22''E (WAM T74631, T74651); 1 ♀, Mt Keith mine, 18.7 km SE. of Lake Way homestead, 27º03'29''S, 120º36'22''E (WAM T74613); 5 ♂, Mt Keith mine, 22.4 km E. of Mount Keith homestead, 27º14'19''S, 120º43'58''E (WAM T74283, T74290, T74641); 3 ♂, 1 ♀, Mt Keith mine, 22.5 km E. of Mount Keith homestead, 27º14'18''S, 120º44'01''E (WAM T74288, T74293, T74295, T74652); 5 ♂, Mt Keith mine, 22.5 km ESE. of Lake Way homestead, 26º58'53''S, 120º36'24''E (WAM T74645); 1 ♂, Mt Keith mine, 22.5 km SE. of Lake Way homestead, 27º06'17''S, 120º36'21''E (WAM T74610); 1 ♂, Mt Keith mine, 24.6 km E. of Mount Keith homestead, 27º15'11''S, 120º45'30''E (WAM T74289, T74628); 15 ♂, Mt Keith mine, 26.1 km ENE. of Mount Keith homestead, 27º13'22''S, 120º46'03''E (WAM T74287, T74291, T74292, T74294, T74602, T74609, T74611, T74636); 5 ♂, Mt Keith mine, 26.2 km ENE. of Mount Keith homestead, 27º13'21''S, 120º46'06''E (WAM T74282, T74284, T74286, T74605, T74606); 2 ♂, Mt Keith mine, 28.9 km ENE. of Mount Keith homestead, 27º11'22''S, 120º47'08''E (WAM T74604, T74608); 2 ♂, Mt Keith mine, 29 km ENE. of Mount Keith homestead, 27º11'22''S, 120º47'12''E (WAM T74285, T74603); 2 ♂, Mt Keith mine, 9.3 km ESE. of Lake Way homestead, 26º57'57''S, 120º33'31''E (WAM T74635, T74646); 2 ♂, Mt Keith mine, 9.5 km ESE. of Lake Way homestead, 26º58'25''S, 120º33'29''E (WAM T74277, T74278); 8 ♂, Mt Keith mine, 9.6 km ESE. of Lake Way homestead, 26º58'25''S, 120º33'29''E (WAM T74632, T74640); 2 ♂, Mt Keith mine, 9 km E. of Lake Way homestead, 26º56'22''S, 120º33'29''E (WAM T74649); 1 ♀, 2 juv., Mt Magnet, 323 mile peg, 28º03'S, 117º50'E (WAM 68/822, 69/843, 69/1037); 1 ♀, Mt Vernon, 24º13'S, 118º14'E (WAM T47710); 1 ♂, 1 ♀, Mt Vernon Station, 53.4 km SE, 24º30'S, 118º30'E (WAM T62336); 2 ♂, Nanga Station, 26º29'23''S, 114º03'24''E (WAM T63265, T65167); 4 ♂, 3 ♀, Nerren Nerren Station, 27º03'00''S, 114º34'32''E (WAM 94/1939, T51221, T51222, T63269, T63270, T63275, T65165); 4 ♂, Nerren Nerren Station, 27º03'24''S, 114º35'21''E (WAM T63268, T65166); 1 ♂, Nerren Nerren Station, 27º00'21''S, 114º32'29''E (WAM T51223); 1 ♂, Nerren Nerren Station, 27º00'22''S, 114º32'29''E (WAM T63255); 1 ♂, Nerren Nerren Station, 27º07'24''S, 114º35'21''E (WAM T51224); 1 ♂, Nerren Nerren Station, 27º03'28''S, 114º36'25''E (WAM T47725); 1 ♂, Nerren Nerren Station, 27º03'00''S, 114º34'32''E (WAM); Nerren Nerren Station, 27º03'28''S, 114º36'25''E (WAM 94/1942); 1 ♂, Nerren Nerren Station, 27º08'S, 114º38'E (WAM T62724); 2 ♂, Newman, 15 km N by W of, 23º13'S, 119º29'E (ANIC no reg.); 1 ♀, 3 juv., Paraburdoo, 100 miles SSW of edge of 4 East ore body, 24º52'S, 117º40'E (QM W7174); 1 ♂, 1 ♀, Point Salvation, 7 – 8 km WNW, 28º12'S, 123º36'E (WAM T47709, T65583); 1 ♀, Tallering Peak, 28º06'S, 115º38'E (WAM T54398); 1 ♀, 1 juv., Tanami, 90 km W of Tanami, 19º52'48''S, 128º46'12''E (WAM T70988, T70992); 2 ♀, Tuckanarra, 4.5 km N, 27º08'S, 118º08'E (WAM T47722); 1 ♂, Wanjarri, 53.6 km N of Leinster, 27º26'38''S, 120º47'56''E (WAM T76483); 1 ♀, Warburton Mission, 26º08'S, 126º35'E (WAM 69/836); 2 ♀, Warburton Ranges, 26º06'S, 126º39'E (WAM 68/511, 71/1434); 1 ♂, Western Australia, no exact locality (WAM T70178); 1 ♂, Wharburton, 26º08'S, 126º35'E (WAM 73/179); 7 ♂, 1 ♀, Woodleigh Station, 26º11'44''S, 114º32'14''E (WAM T47713, T65160); 9 ♂, Woodleigh Station, 26º11'45''S, 114º25'24''E (WAM T65151, T65152); 1 ♂, 1 ♀, Woodleigh Station, 26º13'01''S, 114º35'59''E (WAM T47714, T63254, T63266, T65155, T65156); 7 ♂, 1 ♀, Woodleigh Station, 26º12'30''S, 114º34'35''E (WAM T47716, T63267, T65157); 2 ♂, Woodleigh Station, 26º11'31''S, 114º30'33''E (WAM T65153, T65154); 9 ♂, 1 ♀, 1 juv., Woodline, 31º50'S, 122º19'E (WAM T47711, T51561); 1 ♀, Wubin, 30º07'S, 116º36'E (WAM 33/1613); 18 ♂, Yamarna Station, 28º13'30''S, 123º35'30''E (WAM T65580, T65581, T65582, T65584); 3 ♂, 1 ♀, Yamarna Station, B-area, 28º13'30''S, 123º35'30''E (WAM 99/188, T47765); 1 ♀, Yandi Station, 27º45'S, 114º48'E (WAM 38/915); 1 ♀, Yandicoogina, 22º47'S, 119º18'E (WAM T47721). Hoggicosa brennani NSW: 1 ♀, Bungeema, via Rand, 35º36'S, 146º35'E (AM KS86508); 1 ♀, Combara Siding, Coonamble Line, 31º08'S, 148º22'E (AM KS86512); 2 ♂, Condobolin, 17.6 km N, 32º57'S, 147º08'E (AM KS7873, KS7847); 1 ♀, Condobolin, 17 km N, 32º56'S, 147º06'E (AM KS5084); 2 ♂, Gulthul Station, 30 km N of Euston, 34º11'S, 142º57'E (AM KS57304); 4 ♂, Gulthul Station, near Euston, 34º17'S, 142º53'E (AM KS57290); 1 ♀, Hermidale, 31º33'S, 146º44'E (AM KS86511); 1 ♀, Lake Cargelligo 19.6 km S, 33º28'S, 146º20'E (AM KS50290); 1 ♂, Lake Cargelligo, 17.6 km S, 33º28'S, 146º20'E (AM KS50291); 4 ♂, Mallee Cliffs National Park, near Euston, 34º10'45''S, 142º40'15''E (AM KS57292); 1 ♂, Mallee Cliffs National Park, near Euston, 34º10'S, 142º40'E (AM KS54488); 2 ♀, Merrygoen, 31º50'S, 149º14'E (AM KS86509); 1 ♀, no exact locality (AM KS84844); 1 ♂, Nymagee, 9 km S, 32º04'S, 146º19'E (AM KS5726); 1 ♂, Rankins Springs, 33º51'S, 146º16'E (AM KS84047); 1 ♂, Round Hill Nature Reserve, 32º59'20''S, 146º05'06''E (QM S53118); 1 ♀, South Yathon National Park, 32º35'S, 145º24'E (NMV K8176); 1 ♂, 1 ♀, Yara, 32º51'54''S, 146º11'21''E (AM no reg.); 1 ♂, 1 ♀, Yathong Nature Reserve, 32º35'58''S, 145º32'28''E (AM KS79039, no reg.); Qld: 3 ♀, 3 juv., `Jaffra`, near Texas, 28º51'S, 151º10'E (AM KS8270 KS86507); 1 ♂, 1 ♀, Altonvale and Moombah Station, 40 km W Westmar, 94 27º53'S, 149º23'E (QM S70650); 1 ♂, Inglewood, 28º25'S, 151º04'E (QM S70651); 2 ♀, Morven, 26º25'S, 147º07'E (QM S52226); 1 ♀, Weengallon, 28º22'S, 149º03'E (QM S70648); SA: , no exact locality (SAM NN18149); 1 ♀, Dudley Conservation Park, Penneshaw PO, 14 km SW, 35º48'S, 137º52'E (SAM NN16463); 2 ♂, Calperum, 33º32'39''S, 140º20'38''E (SAM NN16416-7); 4 ♂, Calperum Station, Murphys Branchline Tank, 2.25 km SE, 33º37'S, 140º29'E (SAM NN16377-8, NN16379, NN16382); 2 ♂, Calperum Station, Yabbie Tanks, 4.25 km ESE, 33º36'S, 140º34'E (SAM NN16390-1); 1 ♂, Cape du Couedic, Kangaroo Island, 36º01'S, 136º44'E (SAM NN15264); 1 ♂, Cape Willoughby Lighthouse, Cape Hart Conservation Park, 4.2 km SW, 35º51'25''S, 138º05'15''E (SAM NN16464); 1 ♂, Coorong area, 3.3 km W Sth Taunta Homestead, 36º06'55''S, 139º50'55''E (SAM no reg.); 1 ♂, Coorong area, 4.5 km W Sth Taunta Homestead, 36º07'10''S, 139º50'07''E (SAM no reg.); 1 ♀, 1 juv., Dudley Conservation Park, 35º48'S, 137º52'E (SAM NN15190); 1 ♂, Dudley Conservation Park, Penneshaw PO, 16 km SE, 35º51'24''S, 137º58'20''E (SAM NN16465); 1 ♂, Edillilie area, 34º25'S, 135º43'E (SAM NN16245); 3 ♂, Flashjack Dam, 2.3 km N, 33º54'10''S, 140º31'10''E (SAM NN16265-7); 1 ♂, Flashjack Dam, 7 km SW, 33º56'10''S, 140º27'40''E (SAM NN16264); Frogamerry Dam, 4 km NE, 33º51'20''S, 140º32'10''E (SAM NN16268-9); 1 ♀, Gluepot Station, Gluepot Homestead, 1.9 km N-NNW, 33º44'51''S, 140º07'07''E (SAM NN19093); 1 ♀, Gluepot Station, Gluepot Homestead, 12.7 km W, 33º45'28''S, 139º59'07''E (SAM NN18703); 1 ♀, Gluepot Station, Gluepot Homestead, 9.6 km W-WNW, 33º44'51''S, 140º01'07''E (SAM NN19315); 1 ♂, Hideaway Hut, 10.5 km SW, 33º45'50''S, 140º25'50''E (SAM NN16263); 1 ♂, Kangaroo Flat, 2 km NW, 35º43'48''S, 139º42'28''E (SAM NN16323); 1 ♂, Karte, 2km SW, 35º04'26''S, 140º40'58''E (SAM NN16313); 1 ♂, Kiawarra, 1.7 km SSE, 35º55'42''S, 137º17'00''E (SAM NN16459); 1 ♂, Marree, 29º39'S, 138º04'E (QM S21352); 1 ♂, Monarto South, 5 km NE, 35º05'50''S, 139º9'41''E (SAM NN16311); 1 ♀, no exact locality (SAM no reg.); 1 ♂, Paringa, 11 km NW, 34º08'34''S, 140º54'21''E (SAM NN16312); 1 ♀, Ravine des Casoars, Cape Borda, 35º47'S, 136º34'E (SAM NN15189); 1 ♂, Seal Bay, 5 km N, 35º56'54''S, 137º19'04''E (SAM NN16462); 2 ♂, Taylorville Station, 20 Mile Dam, 3.5 km W, 34º02'12''S, 140º11'04''E (SAM NN17011-2); 2 ♀, Taylorville Station, Casuarina Dam, 33º53'10''S, 140º18'32''E (SAM NN17029, NN17049); 1 ♂, Taylorville Station, Middle Dam, 1.5 km SW, 33º53'10''S, 140º18'32''E (SAM NN17017); Taylorville Station, Turkey Nest Dam, 4 km SE, 33º56'26''S, 140º9'20''E (SAM NN17046); 1 ♂, The Needles, 1 km N, 35º49'44''S, 139º22'40''E (SAM NN16314); 2 ♂, Warrinya, 2.3 km SSW, 35º56'14''S, 137º16'51''E (SAM NN16460-1). Hoggicosa duracki WA: 3 ♂, Hope Downs Station, 20º54'S, 118º40'E (WAM T65119); 2 ♂, Karratha Station, 2 km NW, 20º52'34''S, 116º38'50''E (WAM no reg.). Hoggicosa forresti SA: 1 ♂, „Parcoola‟, SW of, 33º40'S, 139º59'E (WAM T53489); 1 ♂, Border Village, 31º38'S, 129º00'E (WAM T56655); 1 ♂, Border Village, 103 km E, 31º38'S, 129º57'E (WAM T62798); 1 ♂, Border Village, 8 km E, 31º38'S, 129º05'20''E (SAM NN13817); 1 ♂, Border Village, 8 km SE, 31º39'40''S, 129º05'E (SAM NN13816); 1 ♀, Brookfield Conservation Park, 34º22'S, 139º30'E (SAM NN13821); 2 ♀, Brookfield Conservation Park, 34º20'S, 139º31'E (SAM NN14133-4); 1 ♀, Clare, N. of (98 Miles N. of Adelaide), 33º50'S, 138º36'E (WAM 68/863); 1 ♀, Cook, 80 km NW, 29º58'S, 130º01'E (SAM NN13756); 1 ♀, Eyre Peninsula (no exact location), 33º39'S, 137º11'E (WAM 69/808); 1 ♂, Hambidge, 33º23'S, 135º57'E (SAM NN17007); 2 ♂, 3 ♀, Kimba, S, 33º11'S, 136º33'E (SAM NN13828-9, NN14135-7); 1 ♂, Kimba, S of, 33º11'S, 136º33'E (QM S70656); 1 ♀, Lake Gilles, 33º05'14''S, 136º36'00''E (SAM NN13818); 1 ♀, Muckera Rockhole, 30º02'S, 130º03'E (SAM NN16994); 1 ♂, 2 ♀, Poochera, 50 km E, 32º43'S, 135º20'E (SAM NN13825-7); 2 ♀, Weelbubly, Eucla area, 31º37'S, 128º35'E (SAM NN13823); Wirrulla, conservation park NE, 32º23'30''S, 134º48'45''E (SAM no reg.); 1 ♀, Yumbarra Conservation Park, Yumbarra Rockhole Track, 31º46'S, 133º29'E (SAM NN13819); WA: 3 ♀, Balladonia, 32º27'S, 123º51'E (SAM NN17240-2); 1 ♂, 2 juv., Bungalbin Hill, 30º18'S, 119º43'E (WAM T47781); 2 ♀, 2 juv., Buningonia Spring, 31º28'10''S, 123º30'00''E (WAM T47773, T47782); 1 ♂, Buningonia Spring, 31º26'S, 123º33'E (WAM T53690); 1 ♂, Buningonia Spring (Well), ca. 3 km NNE of, 31º26'S, 123º33'E (WAM T51400); 1 ♀, Burnabinmah Station, 28º46'35''S, 117º32'50''E (WAM T55281); 1 ♂, Chook Lake, 29º33'51''S, 123º36'20''E (SAM no reg.); 1 ♀, Cottesloe, 31º59'53''S, 115º45'34''E (WAM 22/5); 1 ♀, Cunderdin Road, 30º38'03''S, 118º29'03''E (WAM T47786); 1 ♀, Diemal, 1 km W, 29º40'S, 119º18'E (WAM T47770); 1 ♂, Dragon Rocks Nature Reserve, PingaringVarley Road, 32º45'35''S, 119º00'07''E (WAM T47792); 1 ♂, Durokoppin Field Station, 8 km E, Ryans Property, 31º24'S, 117º46'E (WAM T47399); 1 ♂, Durokoppin Reserve, 31º30'S, 117º44'E (WAM T62799); 1 ♂, Eucla Motel, 39.5 km WSW, 31º47'S, 128º29'E (WAM T55509); 1 ♂, Exclamation Lake area, 32º48'S, 121º24'E (WAM T45864); 1 ♂, Gardner Reserve Road, 31º46'45''S, 117º28'22''E (WAM T62801); 1 ♂, Goongarrie Station, 29º50'15''S, 120º57'36''E (WAM T46060); 1 ♂, Goongarrie Station, 29º58'06''S, 121º01'30''E (WAM T46059); 2 ♂, Grass Patch, 'Sieda', 33º13'56''S, 121º46'00''E (WAM 95 T53581, T70269); 2 ♂, Grass Patch, 'Sieda', Fitzgerald Locality 41, 33º14'S, 121º43'E (WAM T53486, T62466); 1 ♂, Grass Patch, 'Sieda', Fitzgerald Locality 41, 33º13'56''S, 121º46'00''E (WAM T62797); 3 ♂, Helena-Aurora Ranges, 30º24'S, 119º38'E (WAM T47763, T47764, T47767); 1 ♂, Kalgarrin National Park, S, 32º30'05''S, 118º33'39''E (WAM T48019); 1 ♀, Kalgoorlie, 30º45'S, 121º27'E (WAM T47768); 1 ♂, Kalgoorlie, 50 km NE, Black Swan Nickel Mine, 30º23'57''S, 121º40'42''E (WAM T56888); 1 ♂, Kellerberrin, 10 km NW, McClelands property, 31º34'S, 117º45'E (WAM T46094); 1 ♀, Lake Cronin, 32º24'S, 119º45'E (WAM T47772); 1 ♂, Lake Cronin, 32º22'S, 119º49'E (WAM T47777); 1 ♀, Lake Cronin, 32º23'00''S, 119º45'10''E (WAM T47779); 1 ♂, Lake Cronin, 32º22'15''S, 119º49'30''E (WAM T47771); 1 ♂, Lake Goorly, 29º49'50''S, 116º57'19''E (WAM T47791); 1 ♂, Lake Gulson Nature Reserve, 32º45'59''S, 119º24'12''E (WAM T48083); 1 ♂, Lake Magenta Nature Reserve, 33º40'37''S, 119º04'52''E (WAM T63278); 1 ♂, Lake Mollerin Station, 30º31'47''S, 117º34'45''E (WAM T47789); 4 ♂, 1 juv., Laverton, 39 km E, 28º28'S, 122º50'E (WAM T55571, T55587, T62380); 1 ♂, Lorna Glen Station, 26º15'24''S, 121º29'52''E (WAM T65610); 2 ♂, Minniberri, Norrish's property, 3 km W. of Kodj Kodjin on Minniberri Road, 31º15'S, 117º52'E (WAM T62800); 1 ♀, Moorine Rock, 8 miles W, 31º18'S, 119º00'E (WAM 70/44b); 1 ♀, Mt Kokeby, 32º13'S, 116º58'E (WAM T63280); 1 ♂, Mt Ragged, NE of, 33º13'17''S, 123º38'47''E (WAM T46098); 1 ♂, Mullamullang Cave area, 31º45'S, 127º14'E (AM exKS50234); 1 ♂, 1 ♀, Mundrabilla Homestead, Tampanna Cave, campsite nearby, ca. 30km N of Eyre Highway, 31º42'S, 127º40'E (WAM 91/919-20); 1 ♂, Mungarine Nature Reserve North, Beacon, 30º19'51''S, 117º45'12''E (WAM T47787); 1 ♂, Newdegate, 20 km NE, 32º50'S, 119º20'E (WAM T58343); 1 ♂, 1 ♀, North Baandee Nature Reserve, 31º38'S, 117º43'E (WAM T47762); 1 ♀, Nullarbor Camp 5, ca. 90 km N of Loongana, 30º07'18''S, 126º53'01''E (WAM T47766); 1 ♂, Point Salvation, 7 – 8 km WNW, 28º12'S, 123º36'E (WAM T62387); 1 ♂, Roe Plain, 32º04'23''S, 126º30'38''E (WAM T56395); 1 ♂, Shire Reserve, 11 km E of Latham, 29º44'10''S, 116º33'43''E (WAM T47790); 1 ♂, Tardun, 28º42'S, 115º42'E (WAM T62451); 1 ♂, Vermin Proof Fence West, E Beacon, 30º14'14''S, 118º18'9''E (WAM T47793); 1 ♂, 1 juv., Warrachuppin North Road, 31º00'06''S, 118º41'31''E (WAM T47788); 1 ♀, Westonia, 31º18'S, 118º41'E (WAM T70267); 1 ♀, Wialki, Arnold's farm, 30º29'S, 118º07'E (WAM 69/39); 3 ♂, 1 ♀, Woodline, 31º55'S, 122º24'E (QM S70646, S70655, S70657, WAM T47774); 1 ♀, Wubin, 30º06'S, 116º37'E (WAM T56591); 1 ♂, Yundamindra, 29º15'S, 122º06'E (WAM T47775); 1 ♂, Zanthus, 4 km W, 30º48'S, 124º00'E (WAM T47769). Hoggicosa natashae NSW: 1 ♀, Fowlers Gap Research Station, 75 km N of Broken Hill, 31º05'S, 141º44'E (AM KS64942); SA: 1 ♀, Cooper Creek, 28º23'S, 137º41'E (SAM NN020); 1 ♀, Lake Gilles, 32º43'S, 136º47'E (SAM NN13801); 2 ♀, Lake Gilles, 32º41'20''S, 136º55'20''E (SAM NN14785, NN14786); 2 ♀, 1 juv., Wilson, 4 km S, 28º08'S, 141º53'E (QM S21417). Hoggicosa snelli WA: 2 ♂, Barrow Island, 20º47'S, 115º24'E (WAM T57698, T57697); 1 ♂, Bidgemia Station, Gasgoyne Junction, 25º05'17''S, 115º22'48''E (WAM T65147); 2 ♂, Drysdale River Station, 15º42'S, 126º23'E (WAM T56397); 1 ♀, Gascoyne Junction, 25º03'S, 115º12'E (WAM T51556); 8 ♂, Kennedy Range National Park, 24º33'04''S, 114º57'32''E (WAM T62805, T65149); 4 ♂, Mardathuna Station, 24º26'36''S, 114º30'42''E (WAM T62804); 4 ♂, Mardathuna Station, 24º30'41''S, 114º38'14''E (WAM T65126, T65148); 4 juv., Marilla Station, 22º58'S, 114º28'E (QM W4022); 1 ♂, Meedo Station, 25º40'49''S, 114º37'20''E (WAM T65159); 2 ♂, Meedo Station, 25º39'13''S, 114º37'38''E (WAM T65130); 6 ♂, 1 ♀, Meedo Station, 25º37'23''S, 114º41'40''E (WAM T65132); 1 ♂, Meedo Station, 25º37'31''S, 114º42'19''E (WAM T65131); 2 ♀, 1 juv., Mt Gibson, camp near, 29º35'S, 117º11'E (WAM T53871); 1 ♀, Munda Station, 20º30'S, 118º03'E (WAM T53870); 3 ♀, 1 juv., Paraburdoo, 4 km W of '4 East' ord body, 23º12'S, 117º40'E (QM W7173); 1 ♀, Shothole Canyon, Cape Range National Park, 22º02'41''S, 114º20'14''E (WAM T47612); 1 ♂, Tanami, 90 km W of Tanami, 19º52'00''S, 128º50'46''E (WAM T70975); 1 ♂, Tanami, 92 km W of Tanami, 19º53'59''S, 128º49'37''E (WAM T70978); 1 ♂, Telfer, 21º43'S, 122º14'E (WAM T62806); 11 ♂, Woodleigh Station, 26º11'45''S, 114º25'24''E (WAM T65133, T65134, T65146); Hoggicosa storri WA: 1 ♂, Ajana Back Road, 27º59'57''S, 114º37'55''E (WAM T63252); 1 ♀, Boddington, SW of, Worsley Alumina, Overland Conveyor Belt (conveyor #1), line stand 580, 32º58'S, 116º25'E (WAM T71701); 1 ♂, Booanya, 32º45'S, 123º36'E (NMV K8251); 1 ♀, Bullfinch, 50 km N of, 30º20'S, 119º07'E (WAM T53616); 1 ♀, Bullock Holes Tribes Reserve, eastern boundary, 30º31'S, 121º51'E (WAM T62764); 1 ♂, Bungalbin Hill, 30º17'S, 119º43'E (WAM T48005); 1 ♀, Burnabinmah Station, 28º46'35''S, 117º32'50''E (WAM T55282); 6 ♂, 1 ♀, Caiguna, ca. 21 km E, ca. 1.1 km S Eyre Hwy, 96 32º10'52''S, 125º40'49''E (WAM T47733, T47734, T47737, T47736, T47735, T47738); 1 ♀, Coolgardie, 103 miles W, 30º57'S, 119º25'E (WAM 69/887); 1 ♀, Coolgardie, 20 km W, 30º57'S, 119º55'E (WAM T51235); 1 ♀, Diemal, 1 km W, 29º40'S, 119º18'E (WAM T47798); 1 ♂, Dongolocking Nature Reserve, 33º04'38''S, 117º41'44''E (WAM T62776); 2 ♂, Earoo Park, 8 km ENE, 29º31'S, 118º24'E (WAM T47796, T47794); 1 ♀, Eucla Motel, 39 km WSW, 31º04'S, 128º29'E (WAM T55505); 1 ♂, 1 juv., Goongarrie Station, 30º05'50''S, 121º10'24''E (WAM T46064);1 ♂, Goongarrie Station, 30º02'39''S, 120º55'16''E (WAM T48015); 1 ♂, Goongarrie Station, 30º01'54''S, 120º54'57''E (WAM T46065); 1 ♂, Grass Patch Wheat Bin, 33º14'S, 121º43'E (WAM T48014); 1 ♂, Grass Patch, 'Seida', 33º13'56''S, 121º46'00''E (WAM T70270); 2 ♂, 1 ♀, Grass Patch, 'Sieda', Fitzgerald Locality 41, 33º14'S, 121º43'E (WAM T48013, T62477, T47712); 1 ♀, Jibbering Well, 29º53'S, 116º49'E (WAM 71/873); 1 ♀, John Forrest National Park, 31º53'S, 116º05'E (WAM T48016); 1 ♂, Jundee, 8.5 km SSE of Jundee homestead, 26º25'16''S, 120º40'45''E (WAM T70266); 2 ♂, Kalgoorlie, 50 km NE, Black Swan Nickel Mine, 30º23'54''S, 121º40'53''E (WAM T53921); 2 ♂, 1 ♀, Kalgoorlie, 50 km NE, Black Swan Nickel Mine, 30º23'07''S, 121º38'59''E (WAM T53251, T53252); 2 ♂, 1 ♀, Kalgoorlie, 50 km NE, Black Swan Nickel Mine, 30º23'05''S, 121º38'41''E (WAM T53212, T53268); 1 ♂, Kalgoorlie, 50 km NE, Black Swan Nickel Mine, 30º23'56''S, 121º41'06''E (WAM T56962); 1 ♀, Kalgoorlie, 50 km NE, Black Swan Nickel Mine, 30º23'57''S, 121º40'42''E (WAM T56887); 1 ♂, 1 ♀, Kalgoorlie, 50 km NE, Black Swan Nickel Mine, 30º24'02''S, 121º40'59''E (WAM T56943, T56940); 1 ♂, Kalgoorlie, 50 km NE, Black Swan Nickel Mine, 30º23'25''S, 121º38'51''E (WAM T56831); 2 ♂, Kalgoorlie, 50 km NE, Black Swan Nickel Mine, 30º23'22''S, 121º39'03''E (WAM T53203); 3 ♂, Kalgoorlie, 50 km NE, Black Swan Nickel Mine, 30º23'29''S, 121º39'10''E (WAM T53319); 1 ♂, Kambalda, ca. 15 km SW, 31º12'58''S, 121º27'23''E (WAM T56551); 1 ♀, Koorda, 30º49'S, 117º29'E (SAM NN17314); 1 ♂, Kundip, Ravensthorpe, 33º40'56''S, 120º11'57''E (WAM T69836); 1 ♂, Kundip, Ravensthorpe, 33º40'28''S, 120º11'49''E (WAM T69835); 1 ♂, Kundip, Ravensthorpe, 33º40'04''S, 120º12'07''E (WAM T69834); 1 ♂, Kundip, Ravensthorpe, 33º40'50''S, 120º11'37''E (WAM T69831); 1 ♀, Lake Cronin, 33º22'57''S, 119º45'06''E (WAM T74411); 1 ♀, Lake Cronin, 32º23'S, 119º45'E (WAM T48003); 1 ♀, Lake Gilmore, 55 km S Norseman, 32º37'S, 121º36'E (SAM NN17313); 1 ♂, Lake Goorly, 29º49'50''S, 116º57'19''E (WAM T62786); 2 ♂, Lake Magenta Nature Reserve, 33º40'37''S, 119º04'52''E (WAM T62778); 1 ♂, Leinster, 75 km N of, 27º26'S, 120º22'E (WAM T66979); 1 ♂, Little Sandy Desert, 10.8 km NNE of Kulonoski East Well, 24º35'23''S, 120º15'41''E (WAM T62773); 1 ♂, 1 ♀, Lord Nelson, 10.15 km SSW of Black Hill, 28º9'26''S, 119º30'56''E (WAM T63356, T63347); 2 ♂, Lord Nelson, 10.3 km SSW of Black Hill, 28º9'23''S, 119º30'27''E (WAM T63351, T63353); 1 ♂, Lord Nelson, 10.4 km SSW of Black Hill, 28º9'43''S, 119º31'31''E (WAM T63349); 4 ♂, Lord Nelson, 11.4 km SSW of Black Hill, 28º10'06''S, 119º30'45''E (WAM T63352, T63350, T63348, T63355); 1 ♀, Lorna Glen Station, 26º09'55''S, 121º06'00''E (WAM T70281); 1 ♂, Lorna Glen Station, 26º11'59''S, 121º15'59''E (WAM T70277); 1 ♀, Lorna Glen Station, 26º01'56''S, 121º33'36''E (WAM T65374); 2 ♂, Lorna Glen Station, 26º02'39''S, 121º26'48''E (WAM T66467); 1 ♂, Lorna Glen Station, 26º06'46''S, 121º30'15''E (WAM T64773); 2 ♂, Lorna Glen Station, 26º17'50''S, 121º33'08''E (WAM T64720, T66400); 1 ♂, Lorna Glen Station, 26º16'27''S, 121º33'35''E (WAM T64857); 1 ♂, Lorna Glen Station, 26º02'39''S, 121º26'48''E (WAM T64754); 2 ♂, Lorna Glen Station, 26º18'13''S, 121º33'46''E (WAM T64927, T66848); 2 ♂, Lorna Glen Station, 26º15'46''S, 121º30'40''E (WAM T66398); 4 ♂, Lorna Glen Station, 26º12'10''S, 121º22'32''E (WAM T64629, T53915, T66978); 4 ♂, Lorna Glen Station, 26º18'11''S, 121º28'31''E (WAM T53916, T66442, T53912); 2 ♂, Lorna Glen Station, 26º07'49''S, 121º31'02''E (WAM T66410); 1 ♂, Lorna Glen Station, 26º00'05''S, 121º33'48''E (WAM T53914); 1 ♀, Maya, E, 29º52'S, 116º30'E (WAM T53617); 1 ♀, Merredin, 31º29'S, 118º17'E (WAM T44325); 1 ♀, Mollerin, 30º24'S, 117º31'E (WAM T47800); 1 ♀, Morgan Range, 25º55'52''S, 128º56'59''E (WAM T79146); 1 ♂, Mt Dean, N, 33º13'14''S, 123º38'48''E (WAM T62802); 1 ♂, Mt Gibson Station, 29º36'24''S, 117º24'38''E (WAM T46037); 1 ♂, Mt Gibson Station, 29º36'11''S, 117º24'20''E (WAM T46039); 1 ♂, Mt Gibson Station, 29º36'18''S, 117º24'30''E (WAM T46038); 2 ♂, Mt Gibson Station, 29º45'29''S, 117º19'07''E (WAM T46042); 1 ♂, Mt Gibson Station, 29º40'52''S, 117º28'02''E (WAM T48046); 4 ♂, 2 juv., Mt Gibson Station, 29º36'37''S, 117º24'36''E (WAM T46040); 1 ♂, Mt Gibson Station, 29º41'53''S, 117º24'21''E (WAM T46043); 1 ♂, Mt Jackson, 30º15'S, 119º15'E (WAM T48025); 1 ♂, Mt Keith mine, 10 km NE. of Mount Keith homestead, 27º12'29''S, 120º34'24''E (WAM T74660); 1 ♂, Mt Keith mine, 14.8 km E. of Lake Way homestead, 26º58'9''S, 120º36'53''E (WAM T74657); 1 ♂, Mt Keith mine, 14 km ESE. of Lake Way homestead, 26º58'9''S, 120º36'53''E (WAM T74654); 1 ♂, Mt Keith mine, 18.7 km E. of Mount Keith homestead, 27º14'54''S, 120º41'48''E (WAM T74655); 1 ♂, Mt Keith mine, 21.6 km E. of Lake Way homestead, 26º58'53''S, 120º40'57''E (WAM T74656); 4 ♂, Mt Keith mine, 22.5 km SE. of Lake Way Homestead, 27º06'17''S, 120º36'21''E (WAM T72734, T74658, T74661, T74659); 1 ♂, Mullewa Airstrip, 28º28'37''S, 115º30'20''E (WAM T63251); 1 ♀, Mundaring, 31º53'S, 116º10'E (WAM T48001); 1 ♀, Narrogin, Lyon Banks, 32º56'S, 117º11'E (WAM 36/5044); 1 ♀, Nimary, ca. 40 km NE Wiluna, 26º20'S, 120º38'E (WAM T48017); 1 ♂, no exact locality (WAM T63250); 1 ♂, 1 ♀, no locality on label, (WAM T48009); 1 ♀, no locality on label, (WAM 69/889); 1 ♀, Pickering Brook, 31º59'S, 116º11'E 97 (WAM 99/192); 1 ♀, Scaddan, 33º27'S, 121º44'E (WAM 30/921); 1 ♂, Stirling Range Caravan Park, 34º18'55''S, 118º11'14''E (WAM T62803); 1 ♀, Stoneville, 30º52'S, 116º10'E (WAM T47797); 1 ♀, Walk-Walkin, around, via Koorda, 30º49'S, 117º19'E (WAM 40/1100); 2 ♀, Walliston, 31º59'S, 116º04'E (WAM T48012, T47799); 1 ♀, Walyahmoning Rock, 2 miles SW, 30º39'S, 118º44'E (WAM 74/1467); 1 ♂, Wandana Reserve, 28º19'S, 115º9'E (WAM T47795); 1 ♂, Wanjarri, 50.3 km N of Leinster, 27º28'47''S, 120º49'27''E (WAM T76481); 1 ♀, Warburton Ranges Mission, 26º08'S, 126º35'E (WAM 74/1465); 1 ♀, Western Australia, no exact locality (NMV K8252); 1 ♀, Westonia, 15 km NW, 31º11'S, 118º46'E (WAM T66893); 1 ♂, 1 ♀, Wiluna, 26º34'13''S, 120º00'43''E (SAM NN16660-1); 1 ♀, 1 juv., Woodline, 31º57'S, 122º24'E (WAM T48002); 1 ♂, Wooraloo, no exact locality (WAM no reg.); 1 ♂, Worsley Alumina, Overland Conveyor Belt, start of Conveyor (tail end), SW of Boddington, 32º48'S, 116º28'E (WAM T58323); 1 ♀, Wubin, 32 miles NE, Great Northern Highway, 30º07'S, 116º38'E (WAM 68/503); 1 ♀, Wydgee Homestead, 17 km at 125deg, 28º56'S, 117º58'E (WAM T53749); 1 ♀, Yarloop, 32º58'S, 115º54'E (WAM 24/604); 1 ♂, Yellowdine, 38 miles S, 31º51'S, 119º39'E (WAM T53406); 1 ♂, Yuinmery, 28º31'55''S, 119º11'35''E (WAM T62775); 1 ♂, Yuinmery, 28º33'25''S, 119º05'30''E (WAM T48006); 1 ♂, Yuinmery, 28º32'00''S, 119º05'45''E (WAM T62781); 9 ♂, Yundamindra, 29º15'S, 122º06'E (WAM T48008, T56589, 90/568-9, T48010, T48004, T48011). Hoggicosa wolodymyri NSW: 1 ♀, 16km W of Euabalong West, 33º03'S, 146º24'E (AM KS44770); 1 ♂, Pulletop, 33º58'46''S, 146º03'28''E (QM S53673); 1 ♂, Round Hill, near Euabalong, 32º58'S, 146º9'E (AM KS8307); 6 ♂, Warrakoo Station, Lower Murray Darling region, 33º56'44''S, 141º08'13''E (AM KS72675); 1 ♂, Warrakoo Station, Lower Murray-Darling region, 33º51'16''S, 141º07'06''E (AM KS66843); 1 ♂, Yara, 32º56'48''S, 146º11'32''E (AM exVWF19129); NT: 1 ♂, Uluru National Park, ~6 km S of ranger station, 25º24'03''S, 130º59'18''E (AM KS63728); SA: 1 ♂, Adelaide & Pearson Island, between, no exact locality (SAM NN16231); 2 ♂, Balah, 7 km SE, 33º46'40''S, 139º49'00''E (SAM NN16282-3); 1 ♀, 1 juv., Bindyi Research Station, Koonamore, 32º07'S, 139º21'E (SAM NN15295); 2 ♂, 2 juv., Brooks Bore, 0.8 km W, 32º59'20''S, 140º54'30''E (SAM NN16285-6); 1 ♂, Bullock Dam, 7.6 km NNE, 33º21'20''S, 139º43'40''E (SAM NN16284); 2 ♂, Calperum Station, Murphys Branchline Tank, 2.25 km SE, 33º37'S, 140º29'E (SAM NN16380-1); 1 ♂, Calperum Station, Yabbie Tanks, 2.25 km NE, 33º36'S, 140º34'E (SAM NN16387); 3 ♂, Calperum Station, Yabbie Tanks, 4.25 km ESE, 33º36'S, 140º34'E (SAM NN16392-4); 2 ♂, Catalogue Dam, 2.7 km N, 33º46'20''S, 140º43'40''E (SAM NN16280-1); 1 ♂, Corrobinnie Hill, 1.2 km SSE, 32º59'33''S, 135º44'39''E (SAM no reg.); 1 ♀, Danggali Conservation Park, Tomahawk Dam, 8 km S, 33º24'46''S, 140º43'E (SAM NN17072); 1 ♂, Emu, 4 km NW, 28º38'S, 132º10'E (SAM NN15271); 2 ♂, Flashjack Dam, 2.3 km N, 33º54'10''S, 140º31'10''E (SAM NN16271-2); 5 ♂, Flashjack Dam, 7 km SW, 33º56'10''S, 140º27'40''E (SAM NN16275-9); 1 ♂, Frogamerry Dam, 4 km NE, 33º51'20''S, 140º32'10''E (SAM NN16270); 1 ♀, Gluepot Station, Gluepot Homestead, 1.5 km EENE, 33º45'50''S, 140º08'15''E (SAM NN19055); 1 ♀, Gluepot Station, Gluepot Homestead, 11.3 km W, 33º45'16''S, 139º59'58''E (SAM NN18702); 1 ♂, Gluepot Station, Gluepot Homestead, 4.7 km N, 33º43'23''S, 140º07'26''E (SAM NN18802); 1 ♂, Gluepot Station, Gluepot Homestead, 6.8 km N, 33º42'9''S, 140º07'16''E (SAM NN18748); 1 ♂, 2 ♀, Gluepot Station, Gluepot Homestead, 8.5 km WWNW, 33º44'49''S, 140º01'56''E (SAM NN18416, NN18698, NN18732); 1 ♀, Gluepot Station, Gypsum Dam, 33º46'19''S, 140º04'07''E (SAM NN18410); 1 ♂, Great Victoria Desert, 27º52'S, 130º22'E (SAM NN15270); 5 ♂, Hamilton Homestead, 13.2 km S, 26º50'03''S, 135º04'01''E (SAM NN15943-7); 1 ♂, Hamilton Homestead, 24.4 km SSE, 26º54'22''S, 135º11'46''E (SAM NN15948); 1 ♂, Hamilton Homestead, 6.8 km SE, 26º45'43''S, 135º07'08''E (SAM NN15949); 2 ♂, Hideaway Hut, 10.5 km SW, 33º45'50''S, 140º25'50''E (SAM NN16273-4); 1 ♂, Immarna Siding, 10.4 km S, 30º34'46''S, 132º12'27''E (SAM NN15289); 2 ♂, Ketchalby Rockhole, 3.4 km W, 32º32'37''S, 134º58'59''E (SAM no reg.); 2 ♂, Kunytjanu, 8 km NW, 26º36'45''S, 129º18'50''E (SAM NN11182-3); 1 ♀, Lake Gilles National Park, 33º03'S, 136º40'E (SAM NN15285); 1 ♂, Lambina Homestead, 43.7 km N, 26º31'00''S, 134º05'18''E (SAM NN15942); 1 ♂, Mabel Creek Homestead, 30 km SW, 29º10'30''S, 134º06'30''E (SAM NN15290); 1 ♂, Monash, 34º14'S, 140º33'E (SAM NN14776); 1 ♂, Mootatunga, 4 km S, 34º59'16''S, 140º50'16''E (SAM NN16322); 2 ♂, Mootatunga, 4 km S, 34º59'9''S, 140º50'9''E (SAM NN16320-1); 1 ♀, Mt Finke, 8 km E, 30º55'S, 134º00'E (SAM NN15280); 8 ♂, Mt Hoare, 3 km WSW, 27º03'51''S, 129º40'16''E (SAM no reg.); 2 ♀, Mt Hoare, 6 km W, 27º03'23''S, 129º38'20''E (SAM no reg.); 3 ♂, Muckera Rockhole, 30º02'S, 130º03'E (SAM NN16999, NN17000, NN17002); 1 ♂, North east of OTC Station, Ceduna, 31º52'S, 133º49'E (SAM NN15284); 1 ♀, Oakbore Station, 33º42'07''S, 140º35'06''E (SAM no reg.); 3 ♀, OTC Station, E of, Cenduna, 31º52'S, 133º49'E (SAM NN15281-3); 1 ♂, Paney Station shearer's quarters, 32º44'20''S, 135º42'00''E (SAM no reg.); 1 ♂, Paney Station shearer's quarters, 2.6 km SW, 32º39'46''S, 135º37'9''E (SAM no reg.); 3 ♂, Pooginook Conservation Park, 34º05'S, 140º06'E (SAM NN15291-3); 5 ♂, Tallaringa Conservation Park, Tallaringa Well, 10 km NE, 29º01'S, 133º25'E (SAM NN15265-9); 1 ♂, Tallaringa Conservation Park, Tallaringa Well, 2.7 km W, 29º01'S, 133º16'E (SAM NN15272); 1 ♂, 1 ♀, Taylorville Station, 34º06'S, 139º58'E (SAM NN16408, NN16409); 5 ♂, 98 Taylorville Station, 17 Mile Dam, 3.5 km W, 34º02'12''S, 140º11'04''E (SAM NN17033-7); 9 ♂, Taylorville Station, 18 Mile Dam, 3.5 km W, 34º02'12''S, 140º11'04''E (SAM NN17020-8); 1 ♀, Taylorville Station, Casuarina Dam, 33º53'10''S, 140º18'32''E (SAM NN17053); 1 ♀, Taylorville Station, Casuarina Dam, 33º53'10''S, 140º18'32''E (SAM NN17030); 4 ♂, Taylorville Station, Crows Nest Dam, 4.5 km ENE, 33º58'S, 140º11'10''E (SAM NN17042-5); 1 ♂, Taylorville Station, Middle Dam, 1.5 km SW, 33º54'37''S, 140º12'20''E (SAM no reg.); 2 ♂, Taylorville Station, Middle Dam, 1.5 km SW, 33º53'10''S, 140º18'32''E (SAM NN17018-9); 2 ♂, Taylorville Station, Mile Dam, 3.5 km W 10, 34º02'11''S, 140º11'03''E (SAM no reg.); 1 ♀, Taylorville Station, near Casuarina Dam, 33º53'10''S, 140º18'32''E (SAM no reg.); 1 ♀, Waikerie, nr Holiday House, 34º12'44''S, 140º00'30''E (SAM NN15294); 1 ♂, Wendale, 2 km SE, 34º43'33''S, 140º18'14''E (SAM NN16319); 2 ♀, Wirrulla, conservation park NE, 32º23'20''S, 134º48'45''E (SAM no reg.); 1 ♂, Yumbarra Conservation Park, Inila Rockwaters, 1.5 km S, 31º47'42''S, 133º25'44''E (SAM NN15287); 1 ♂, Yumbarra Conservation Park, Inila Rockwaters, 7 km W, 31º47'26''S, 133º20'58''E (SAM NN15288); 1 ♂, Yumbarra Conservation Park, Inila Rockwaters, 7 km W, 31º48'14''S, 133º24'05''E (SAM NN15286); 4 ♀, 1 juv., Yumbarra Conservation Park, Yumbarra camp, SW corner along dog fence, 31º39'S, 133º22'E (SAM NN15278-9, NN15276-7); 3 ♂, Yumbarra Conservation Park, Yumbarra Rockhole track, 31º39'S, 133º22'E (SAM NN15273-5); WA: 1 ♂, Goongarrie Station, 29º56'44''S, 121º07'14''E (WAM T55374); 1 ♂, Mulga Rock, 29º53'27''S, 123º36'51''E (SAM NN22290); 8 ♂, Queen Victoria Springs Nature Reserve, 30º14'S, 123º41'E (WAM T49265, T49292, T52354, T52408, T52444, T52506, T52527). 99 Summary of Section I In this first section I revised the taxonomy of the „bicolor group‟ of Australian wolf spiders and provided strong phylogenetic evidence that they represent a distinct Australian genus. According to the Rules of the International Code of Zoological Nomenclature (International Commission of Zoological Nomenclature 1999), Hoggicosa Roewer, 1960 represents the valid name for this genus. A systematic analysis based on morphological characters supported the monophyly of Hoggicosa based on a synapomorphy of the female genitalia. In addition, the combination of two characters depicting the cymbium spines of males provided a second synapomorphy. Prior to my study, spiders in this genus were incorrectly assigned to the putative Mediterranean genus Lycosa. My work has resulted in transferring nearly a third of the Australian species currently placed within Lycosa to Hoggicosa. As well as redescribing and relimiting species boundaries for the six species named prior to my work, I have also described four new Hoggicosa species. Future workers could use molecular data to test independently the species boundaries and phylogenetic hypothesis proposed here. Hoggicosa provide ideal model species for further ecological and evolutionary studies, being large, widespread within the arid zone of Australia, often very common and conspicuous, particularly the females of species with unusual sexual colour dimorphism. The fascinating colour dimorphism displayed between the sexes of four species of Hoggicosa, with females more colourful than males, begs further investigation. Indeed, spiders are ideal animals for the study of sperm competition and cryptic female choice (Eberhard 2004). What selection pressure, either sexual or natural, has driven this dimorphism remains unclear and the current lack of basic information on natural history and mating system (i.e. multiple mating and sperm priority pattern) for most species is a limiting factor for interpreting this unusual evolutionary phenomenon. Now that the species within Hoggicosa are named and identifiable, this should allow the rapid accumulation of natural history information and raise interest in the study of their evolutionary history. Along with their burrowing behaviour, which made them suitable for a detailed population study, one of the initial reasons I chose to work on the „bicolor group‟ was their large size. This meant that they would be easier to handle in ecological studies, but 100 also that their genitalia were easily visible under a light stereomicroscope. However, the palps and epigynes of wolf spiders are morphologically conservative, which made the identification of species more difficult than expected. The terminal apophysis, pars pendula and tegular apophysis of the male pedipalp proved the most useful discriminators for males. In contrast, body colour was a key distinguishing character for females. References Eberhard, W. G. (2004) Why study spider sex: special traits of spiders facilitate studies of sperm competition and cryptic female choice. Journal of Arachnology, 32, 545-556. International Commission of Zoological Nomenclature. (1999) International Code of Zoological Nomenclature. Fourth Edition, The International Trust for Zoological Nomenclature 1999 (c/o The Natural History Museum, London). 101 SECTION II: ECOLOGY SECTION II: ECOLOGY General Introduction Spinifex grasslands are a dominant habitat in Australia, covering over 25% of the continent (Allan and Southgate 2002). Spinifex is a common name used to describe over 64 species of perennial hummock grasses within the subtribe Triodiinae (Lazarides 1997). These species are characterized by a distinctive, drought-adapted leaf anatomy, in which the leaves form thin blades or spines, that are often prickly and resinous (Lazarides 1997). Their dry, resinous leaves and growth form produce large, air-filled clumps or hummocks, that create an ideal fuel (Pianka 1989, Burrows et al. 1991). Spinifex is therefore highly flammable, and large areas (> 10 000 km2) burn frequently due to lightning strikes and human ignition (Allan and Southgate 2002, Turner et al. 2008). Fire plays an integral part in ecosystem processes, providing one of the main sources of nutrient cycling, given the high aridity and slow decomposition rates (Griffin and Friedel 1985, Stafford Smith and Morton 1990). Fire was an important tool for aboriginal inhabitants and extensively used in the past (Jones 1969, Gould 1971; Kimber 1983, Bird et al. 2004, 2005). Since the arrival of Europeans, aboriginal occupation of arid Australia has changed and with it, the fire regime has been altered (Bowman 1998, Burrows et al. 2006; Bird et al. 2008). For the western desert region these changes occurred rapidly during the 1950s and 60s due to forced relocations to clear land for missile and nuclear weapons testing (Bird et al. 2008). This has led to great interest in how the flora and fauna of spinifex grasslands respond to disturbance by fire. Many studies have examined the response of vegetation (Burbidge 1943, Griffin and Friedel 1984 a, b, Westoby et al. 1988, Bogusiak et al. 1990, Griffin 1992, Rice and Westoby 1999) and vertebrate fauna (Masters 1993, 1996, Southgate and Master 1996, Letnic 2003, Letnic et al. 2004, Letnic and Dickman 2005). For small mammals, the current level of knowledge has reached a stage that allows the development of detailed state and transition models (Letnic and Dickman in press). However, only a handful of studies have examined the response of invertebrates to fire in arid Australia (Smith and Morton 1990, Gunawardene and Majer 2005, Barrow et al. 2007), and only one has examined the response of spiders (Langlands et al. 2006). I set out to examine the response of spiders to disturbance by fire in a spinifex grassland in Western Australia. I was interested in the long-term pattern in the assemblage of spiders as a whole. 102 Specifically, I had three main research questions: 1) Is there a successional pattern following disturbance by fire? 2) If so, which variables best explain this post-fire successional pattern? 3) To what extent the post-fire response of spiders can be predicted? Given the limited time available for a Ph.D. project, I sought to address these questions of long-term pattern (up to 20 years post-fire) using a chronosequence study. I recognise that the chronosequence approach carries with it a number of limitations and assumptions, and these caveats need to be acknowledged when interpreting results. Each of the following chapters focuses on one of these questions. In the second chapter of this thesis, I assess the changes in abundance, species richness, evenness and species composition of spiders with post-fire age. Previous work demonstrated changes within all these parameters following disturbance by fire for assemblages of spiders in other habitats, including eucalypt and boreal forests (Huhta 1971, Riechert and Reeder 1972, Merrett 1976, Strehlow 1993, York 1999, Buddle et al. 2000, 2006, Andersen and Mueller 2000, Moretti et al. 2002, Niwa and Peck 2002, Abbott et al. 2003, Larrivée et al. 2005, Brennan et al. 2006, Langlands et al. 2006, Gillette et al. 2008). Therefore, while I expected to find significant differences among post-fire ages, I was particularly interested in the successional trajectory. Does the assemblage return to a long-unburnt state (deterministic) or are post-fire changes largely stochastic? To help describe the successional trajectory post-fire I follow the definitions of Westman (1978). Westman defined resilience as “the ability of a natural ecosystem to restore its structure following acute or chronic disturbance”. As one of the characteristics of resilience he described the ability of an assemblage to resist change as inertia (Westman 1978). Having found successional changes following fire, in the third chapter of this thesis I assessed the factors that might potentially explain these differences. The strong influence of fire and rainfall on vertebrates in arid Australia is well established (Masters 1993, 1996, Southgate and Masters 1996, Letnic 2003, Letnic et al. 2004, Letnic and Dickman 2005). Detecting fire effects on beetles in Australian savannas is contingent on rainfall (Blanche et al. 2001), and previous work on spiders in the arid zone has suggested rainfall as a key variable (Langlands et al. 2006). Therefore, recorded detailed rainfall measurements by installing electronic tipping bucket rain gauges at or near all sites. I also measured other explanatory factors that can influence spiders, such 103 as habitat structure, species composition of plants (floristics), soil and predators. In addition, it was important to acknowledge that the spatial arrangement of sites in relation to each other can influence the fauna. I was particularly interested in quantifying the influence that each of these may have by examining the unique and shared amounts of variation in the spiders explained by each type of variable. Finally, given the insights gained from the previous two chapters, I tested if the post-fire response of spiders could become more predictive, rather than simply identifying repeating patterns. For this, I adopted an approach that has proven successful for predicting the response of plants to fire, a species-traits approach. Examining the response of traits, rather than species, ultimately leads to a greater mechanistic understanding and results are potentially more easily applied to other locations and species. Ideally, one might hope to collect a spider from any locality and from only its physical and behavioural traits predict the species‟ change in abundance following disturbance. I was interested in testing if the traits of species could predict their response to fire. In the fourth chapter, I utilise three methods to test for links between the traits of spiders and the environment (post-fire age) in which they were collected. The use of these techniques has only recently become widely accessible, and the strengths and weaknesses of each method are still being established. Additionally, the use of a traits-based approach for fauna and disturbance by fire is highly novel. References Abbott, I., Burbidge, T., Strehlow, K., Mellican, A. & Wills, A. (2003) Logging and burning impacts on cockroaches, crickets and grasshoppers, and spiders in Jarrah forest, Western Australia. Forest Ecology and Management, 174, 383399. Allan, G. E. & Southgate, R. I. (2002) Fire regimes in the spinifex landscapes of Australia. Flammable Australia: The Fire Regimes and Biodiversity of a Continent (eds R. A. Bradstock, J. E. Williams & A. M. Gill), pp. 145-176. Cambridge University Press, Cambridge. Andersen, A. N. & Muller, W. J. (2000) Arthropod responses to experimental fire regimes in an Australian tropical savannah: ordinal-level analysis. Austral Ecology, 25, 199-209. 104 Barrow, L., Parr, C. L. & Kohen, J. L. (2007) Habitat type influences fire resilience of ant assemblages in the semi-arid tropics of Northern Australia. Journal of Arid Environments, 69, 80-95. Bird, D. W., Bird, R. B. & Parker, C. H. (2004) Women who hunt with fire: Aboriginal resource use and fire regimes in Australia's western desert. Australian Aboriginal Studies, 2004, 90-96. Bird, D. W., Bird, R. B. & Parker, C. H. (2005) Aboriginal burning regimes and hunting strategies in Australia's western desert. Human Ecology, 33, 443-464. Bird, R. B., Bird, D. W., Codding, B. F., Parker, C. H. & Jones, J. H. (2008) The "fire stick farming" hypothesis: Australian Aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. Proceedings of the National Academy of Sciences, 105, 14796-14801. Blanche, K. R., Andersen, A. N. & Ludwig, J. A. (2001) Rainfall-contingent detection of fire impacts: Responses of beetles to experimental fire regimes. Ecological Applications, 11, 86-96. Bogusiak, A., Rice, B. & Westoby, M. (1990) Seedling emergence of hummock grasses in relation to the effects of fire. The Australian Rangeland Journal 12, 25-28. Bowman, D. J. M. S. (1998) Tansley review, 101: the impact of Aboriginal landscape burning on the Australian flora. New Phytologist, 140, 385-410. Brennan, K. E. C., Ashby, L., Majer, J. D., Moir, M. L. & Koch, J. M. (2006) Simplifying assessment of forest management practices for invertebrates: how effective are higher taxon and habitat surrogates for spiders following prescribed burning? Forest Ecology & Management, 231, 138-154. Buddle, C. M., Langor, D. W., Pohl, G. R. & Spence, J. R. (2006) Arthropod responses to harvesting and wildfire: implications for emulation of natural disturbance in forest management. Biological Conservation, 128, 346-357. Buddle, C. M., Spence, J. R. & Langor, D. W. (2000) Succession of boreal spider assemblages following wildfire and harvesting. Ecography, 23, 424-436. Burbidge, N. T. (1943) Ecological succession observed during regeneration of Triodia pungens R. Br. after burning. Journal of the Royal Society of Western Australia, 28, 149-156. Burrows, N. D., Burbidge, A. A., Fuller, P. J. & Behn, G. (2006) Evidence of altered fire regimes in the Western Desert region of Australia. Conservation Science Western Australia, 5, 272-284. 105 Burrows, N. D., Ward, B. & Robinson, A. (1991) Fire behaviour in spinifex fuels on the Gibson Desert Nature Reserve, Western Australia. Journal of Arid Environments, 20, 189-204. Gillette, N. E., Vetter, R. S., Mori, S. R., Rudolph, C. R. & Welty, D. R. (2008) Response of ground-dwelling spider assemblages to prescribed fire following stand structure manipulation in the southern Cascade Range. Canadian Journal of Forest Research, 38, 969-980. Gould, R. A. (1971) Uses and effects of fire among the western desert Aborigines of Australia. Mankind, 8, 14-24. Griffin, G. F. (1992) Will it burn - should it burn?: management of the spinifex grasslands of inland Australia. Desertified Grasslands: Their Biology and Management (ed G. P. Chapman), pp. 63-76. Academic Press, London. Griffin, G. F. & Friedel, M. H. (1984 a) Effects of fire on central Australian rangelands. I. Fire and fuel characteristics and changes in herbage and nutrients. Australian Journal of Ecology, 9, 381-393. Griffin, G. F. & Friedel, M. H. (1984 b) Effects of fire on central Australian rangelands. II. Changes in tree and shrub populations. Australian Journal of Ecology, 9, 395-403. Griffin, G. F. & Friedel, M. H. (1985) Discontinuous change in central Australia: some implications of major ecological events for land management. Journal of Arid Environments, 9, 63-80. Gunawardene, N. R. & Majer, J. D. (2005) The effect of fire on ant assemblages in the Gibson Desert Nature Reserve, Western Australia. Journal of Arid Environments, 63, 725-739. Huhta, V. (1971) Succession in the spider communities of the forest floor after clearcutting and prescribed burning. Annales Zoologici Fennici, 8, 483-542. Jones, R. (1969) Fire stick farming. Australian Natural History, 16, 224-228. Kimber, R. (1983) Black lightning: Aborigines and fire in central Australia and the western desert. Archaeology in Oceania, 18, 38-45. Langlands, P. R., Brennan, K. E. C. & Pearson, D. J. (2006) Spiders, spinifex, rainfall and fire: long-term changes in a community of desert spiders. Journal of Arid Environments, 67, 36-59. Larrivée, M., Fahrig, L. & Drapeau, P. (2005) Effects of recent wildfire and clearcuts on ground-dwelling boreal forest spider assemblages. Canadian Journal of Forest Research, 35, 2575-2589. 106 Lazarides, M. (1997) A revision of Triodia including Plectrachne (Poaceae, Eragrostideae, Triodiinae). Australian Systematic Botany, 10, 381-489. Letnic, M. (2003) The effects of experimental patch burning and rainfall on small mammals in the Simpson Desert, Queensland. Wildlife Research, 30, 547-563. Letnic, M. & Dickman, C. R. (2005) The responses of small mammals to patches regenerating after fire and rainfall in the Simpson Desert, central Australia. Austral Ecology, 30, 24-39. Letnic, M. & Dickman, C. R. (in press) Resource pulses and mammalian dynamics: conceptual models for hummock grasslands and other Australian desert habitats. Biological Reviews. Letnic, M., Dickman, C. R., Tischler, M. K., Tamayo, B. & Beh, C.-L. (2004) The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. Journal of Arid Environments, 59, 85-114. Masters, P. (1993) The effects of fire-driven succession and rainfall on small mammals in spinifex grassland at Uluru National Park, Northern Territory. Wildlife Research, 20, 803-813. Masters, P. (1996) The effects of fire driven succession on reptiles in spinifex grasslands at Uluru National Park, Northern Territory. Wildlife Research, 23, 39-48. Merrett, P. (1976) Changes in the ground-living spider fauna after heathland fires in Dorset. Bulletin of the British Arachnological Society, 3, 214-221. Moretti, M., Conedera, M., Duelli, P. & Edwards, P. J. (2002) The effects of wildfire on ground-active spiders in deciduous forests on the Swiss southern slope of the Alps. Journal of Applied Ecology, 39, 321-336. Niwa, C. G. & Peck, R. W. (2002) Influence of prescribed fire on carabid beetle (Carabidae) and spider (Araneae) assemblages in forest litter in southwestern Oregon. Environmental Entomology, 31, 785-796. Pianka, E. R. (1989) Desert lizard diversity: additional comments and some data. The American Naturalist, 134, 344-364. Rice, B. & Westoby, M. (1999) Regeneration after fire in Triodia R. Br. Austral Ecology, 24, 563-572. Riechert, S. E. & Reeder, W. G. (1972) Effects of fire on spider distribution in southwestern Wisconsin prairies. Proceedings of the Second Mid West Prairie Conference, Madison, Wisconsin (ed J. Zimmerman), pp. 73-90. Iowa State University, Ames, Iowa. 107 Smith, G. T. & Morton, S. R. (1990) Responses by scorpions to fire-initiated succesion in arid Australian spinifex grasslands. Journal of Arachnology, 18, 241-244. Southgate, R. & Masters, P. (1996) Fluctuations of rodent populations in response to rainfall and fire in a central Australian hummock grassland dominated by Plectrachne schinzii. Wildlife Research, 23, 289-303. Stafford Smith, D. M. & Morton, S. R. (1990) A framework for the ecology of arid Australia. Journal of Arid Environments, 18, 255-278. Strehlow, K. H. (1993) Impact of fires on spider communities inhabiting semi-arid shrublands in Western Australia's wheatbelt. Department of Biology. Murdoch University, Perth. Turner, D., Ostendorf, B. & Lewis, M. (2008) An introduction to patterns of fire in arid and semi-arid Australia, 1998-2004. The Rangeland Journal, 30, 95-107. Westman, W. E. (1978) Measuring the inertia and resilience of ecosystems. BioScience, 28, 705-710. Westoby, M., Rice, B., Griffin, G. & Friedel, M. (1988) The soil seed bank of Triodia basedowii in relation to time since fire. Australian Journal of Ecology, 13, 161169. York, A. (1999) Long-term effects of frequent low-intensity burning on the abundance of litter-dwelling invertebrates in coastal blackbutt forests of southeastern Australia. Journal of Insect Conservation, 3, 191-199. 108 CHAPTER 2 Is the successional trajectory of an arid spider assemblage following fire deterministic? Peter R Langlands Karl E C Brennan Bruce Ward Formatted for Austral Ecology 109 ABSTRACT Fire is a common disturbance in many ecosystems, including arid Australia. Understanding whether fauna respond in a deterministic manner towards a single endpoint, or to multiple states, is of crucial importance for conservation management. Why different taxa or assemblages display single or multiple end points is also important to develop a synthetic theory of succession. To examine the post-fire changes in assemblages of spiders, we established a chronosequence study in spinifex habitat of central Western Australia. Ground-active spiders were pitfall-trapped over nine months in sites representing experimental fires (0 and 0.5 years post-fire) and wildfires (3, 5, 8 and 20 years post-fire). There were significant non-linear changes in species richness, evenness and composition of spiders with increasing post-fire age. For all three measures, the assemblage appears highly deterministic, converging towards the long unburnt state. Similarity in richness, evenness and species composition to the 20-yearold sites all increased with increasing time since fire (3 to 8 years). However, experimentally burnt sites did not neatly fit this sequence. We consider two alternative hypotheses to explain this second trajectory: inertia within the system or the rapid migration and recolonization from nearby surrounding unburnt areas. Analyses indicated that half of the 179 species had significant preferences for, or where restricted to, particular post-fire ages. This suggests that adequate pyrodiversity, both in terms of post-fire ages and/ or scale and intensity of fires, may be important for the conservation of spiders in this habitat. However, due to the high number of singletons and low indicator values, the significance of this result for conservation management remains equivocal. Despite this, the high degree of determinism provides hope that managers can develop a good predictive understanding of post-fire successional changes in spider assemblages in arid Australia. 110 INTRODUCTION Fire is a conspicuous disturbance in many environments and of great importance to the maintenance of biodiversity (Bradstock et al. 2002). This is particularly true in the vast arid zone of Australia, where the vegetation is highly flammable and large swathes (> 10 000 km2) can be burnt due to lightning strikes and human ignition (Allan & Southgate 2002; Turner et al. 2008). Over the last century, the fire regime of arid Australia has been substantially altered (Edwards et al. 2008). Previously, frequent and extensive use of fire by Aborigines (Gould 1971; Kimber 1983) created a fine mosaic of post-fire ages (Burrows et al. 2006; Bird et al. 2008). The current patch mosaic burning paradigm assumes that „pyrodiversity begets biodiversity‟, but this has received limited critical assessment (Parr & Andersen 2006). Understanding the conservation implications of a changed fire regime, from many small fires to few immense fires, is a challenging task. An essential first step is examining the response of fauna and flora in relation to the time since single fires. Successional patterns with time since fire can display a continuum of responses from a single, deterministic end-point, multiple stable end-points or purely stochastic trajectories. Here, the framework of complex systems theory, and the analogy of a ball and cup or basins within a three-dimensional surface are useful (Samuels & Drake 1997; Anand & Desrochers 2004). The ball represents the community, its location represents its current state (e.g., species richness or composition) and the base of the cup or basin is the attractor or stable end-point. Therefore, disturbances will move the ball around the cup (species richness or composition changes), but if insufficient to move the ball over the lip of the cup, it will eventually return to the base (original richness or composition). The velocity of return is determined by the slope of the sides. Westman‟s (1978) terminology of resilience and inertia can describe the behaviour of the ball. The ball and cup analogy implies greater variation in community structure immediately following disturbance, but over time the ball returns to a single end point and community structure becomes more homogeneous. In the case of disturbance by fire, increased variability immediately after fire could be caused by patchy fires, which increase heterogeneity of post-fire habitats. Additionally, random colonization or lottery effects following disturbance could increase variability. Uncovering why single or multiple end-points occur is of critical interest in succession theory (Young et al. 2001; Chase 2003). 111 The response of vertebrates to fire in arid Australia has received considerable attention. Reptiles follow a strongly deterministic pattern (Masters 1996; Letnic et al. 2004), while mammal succession seems more stochastic (Masters 1993; Southgate & Masters 1996; Letnic 2003; Letnic et al. 2004). Invertebrate assemblages, however, have received limited investigation (Friend 1995; Langlands et al. 2006). Considering the high diversity and abundance of invertebrates, this should be an issue of concern to fire ecologists. Over the last decade, awareness has grown substantially amongst nature conservation agencies that there is a need to cater for the invertebrate component of biodiversity. There is a critical lack, however, of scientific studies to guide decisions about how best to manage fire other than vague notions of pyrodiversity. Identifying if species have preferences for, or are restricted to, sites of particular post-fire ages is important for assessing the potential for current fire regimes to cause local extinctions (Driscoll & Henderson 2008). Spiders are a diverse and ubiquitous predatory group, ideal for examining post-fire responses. A review of Australian studies on invertebrates and fire found that spiders were one group that showed consistent post-fire responses (Friend 1995). They are sensitive to many features that change following disturbance by fire, particularly habitat structure and complexity, along with litter depth and nutrient content (Duffey 1962; Bultman & Uetz 1982; Riechert & Gillespie 1986; Uetz 1991). They can also influence ecosystem functioning by preying on decomposers at lower trophic levels (Lawrence & Wise 2000; 2004) and are favoured prey for many insectivorous marsupials (Fisher & Dickman 1993; Bos & Carthew 2007). Indeed studies from forest ecosystems show marked changes in spider assemblages following burning (e.g. Huhta 1971; Buddle et al. 2006; Brennan et al. 2006), but we know very little about the response of spiders in arid ecosystems (but see Langlands et al. 2006). We sought to describe the post-fire changes of an assemblage of spiders in the Australian arid zone. Are there differences among post-fire ages in abundance, species richness, evenness and composition? If so, is the succession of spider assemblages highly deterministic? We define deterministic succession as sites being more similar in species composition to similar ages (e.g. following a chronological order) with similarity to long unburnt sites increasing and multivariate dispersion decreasing over time. In addition, within the „pyrodiversity promotes biodiversity paradigm‟, we were interested in how many and which species were significant indicators for, or were restricted to, particular post-fire ages. 112 METHODS Study area The study was conducted in central Western Australia at the proposed Lorna Glen (Matuwa) Conservation Park, located approximately 150 km NE of Wiluna (Fig. 1). The former sheep and cattle station receives a median annual rainfall of 235 mm (range 86-654 mm) and experiences mean minimum and maximum monthly temperatures from 21-38 ˚C in summer and 5-21 ˚C in winter (Australian Bureau of Meteorology). Lorna Glen contains a range of habitat types, with the most widespread of these being the fire-prone hummock grasslands (Bullimore). This is a vegetation assemblage dominated by hard spinifex Triodia basedowii E. Pritz, 1918, with an over storey of marble gums (Eucalyptus gongylocarpa Blakely, 1936). There are also scattered mulga (Acacia aneura Benth, 1855) and mallee (E. kingsmillii Maiden and Blakely, 1929 and E. lucasii Blakely, 1934). Therefore, the vegetation is remarkably similar to that found throughout much of the Great Victoria Desert. Although spinifex is highly flammable, fires are unlikely to spread within stands less than 10-15 years old, unless preceded by periods of high rainfall, which increases biomass and promotes growth of short-lived soft grasses (Allan & Southgate 2002). Our study was restricted to this habitat type, as improving knowledge of faunal responses to fire within the Bullimore is of critical concern to land managers. Study design We conducted our study at the landscape scale, with 27 sites selected to represent the majority of fire ages present at Lorna Glen (Fig. 1). Prior to 2000 Lorna Glen had a recent history of fire suppression by pastoralists; however, infrequent and large-scale (>100 km2) fires still occurred. We used Landsat satellite images from 1985 to 2005 to establish the recent fire history of the study area. From these natural fire histories and some experimental fires, we selected six treatments for post-fire ages: 0, 0.5, 3, 5, 8 and 20+ years since the last fire. The patch size in which each site was located was at least 9 ha and there were three to five sites per post-fire age (Fig. 1, Table 1). At each site, a 50 x 50 m (0.25 ha) quadrat was established a minimum of 150 m from the site boundary (change in fire age) or road. Many studies of the ecology of fires conducted at the landscape scale have difficulties finding multiple fires of the same age within a study area that can act as true replicates (Brennan et al. 2009). Our study design was similarly constrained by the pyrodiversity present at Lorna Glen, with very few sites burnt multiple times since 1985 113 and no very recent burns (< 1-year-old). We were therefore unable to examine the interplay of time since fire with fire frequency (multiple fires). Additionally, for some post-fire ages (3, 5 and 8 years) multiple sites were located within a single fire scar (Table 1). However, in these instances, sites were interspersed with other ages and located as far apart as possible (the minimum distance between two sites within the same fire scar was 1.9 km [sites 3-2 and 3-5] and was more often in the order of 5-10 km). To resolve the problem of a lack of very recent burns, we burnt seven patches of habitat unburnt for at least 20 years. We burnt three patches in October 2005 and four patches in April 2006 to examine two additional post-fire ages, 0 and 0.5 years respectively. Each experimentally burnt patch had a single sampling site and was 9 ha in size (300 x 300 m). For details of fire ignition procedure, weather conditions during burning and temperatures of experimental fires see Appendix A (online supplementary material). Sampling and identification of spiders At each site we installed five pitfall traps in each quadrat, one at each corner and one in the centre. A few large traps are more efficient at sampling spiders than many smaller traps (Brennan et al. 1999). Additionally, a few traps set for a longer period is more efficient than many traps set for a shorter time (Riecken 1999). Traps consisted of a sleeve (125 mm diameter PVC pipe) into which jars (2 L, 82 mm opening, 125 mm diameter body, 200 mm deep) were inserted with a plastic collar filling the 21.5 mm gap. One litre of preserving fluid was used (80% propylene glycol, 16% water and 4% formalin) with the addition of three drops of detergent as surfactant (Pekar 2002). Propylene glycol was used in preference to ethylene glycol as it is less hazardous to human health (Hall 1991) and performs equally well (Weeks & McIntyre 1997). Roofs of corrugated iron (200 mm square by 0.3 mm thick) covered each pitfall at a height of 3 cm, to minimise access by vertebrates and protect the contents from direct sunlight. We opened the traps 30 April 2006 and exchanged them at three monthly intervals until 18 January 2007 (256- 258 days per trap). The PVC sleeves ensured minimum disturbance when exchanging jars. We rinsed the trap contents with water in a 0.5 mm sieve before storing them in 75% ethanol. We sorted male spiders from each trap and recorded their abundance. We then sorted these to family (Raven et al. 2002) and identified to species level. Taxonomic nomenclature follows Platnick (2010), except for wolf spiders in the genus Hoggicosa (see Langlands & Framenau 2010). It is very difficult to match males, females and 114 juveniles of the same species correctly, because taxonomic knowledge of the Australian spider fauna is particularly poor compared to the northern hemisphere (Brennan et al. 2004). To avoid identification errors the results presented here are from males only, with species boundaries verified by taxonomic experts (see acknowledgements). We combined data for each pitfall trap and collection period to give one sample per site. All specimens are deposited in the Western Australian Museum (registration numbers T61480-T62081, T89622-T91450). Statistical analyses A permutational analysis of variance (Legendre & Anderson 1999; Anderson 2001) tested for differences in abundance, species richness, evenness and species composition of male spiders with the fixed factor AGE (levels 0, 0.5, 3, 5, 8 and 20 years). We used unrestricted permutation of the raw data with type III sums of squares and 9999 permutations in PERMANOVA+ (Anderson et al. 2008, version 1.01). For univariate analyses, a similarity matrix between all pairs of sites was calculated based on Euclidean distance. To overcome the limited number of unique permutations for some pair-wise comparisons (only 35 permutations for 0 vs 0.5), we used Monte Carlo pvalues. Anderson & Robinson (2003) demonstrate that the asymptotic permutation distribution of the entire pseudo-F statistic can be calculated in such situations. As abundance varied between sites, prior to analysis we rarefied species richness to the minimum number of spiders collected from a site (85 males) using PRIMER (Clarke & Gorley 2006, version 6.1.11). To compare evenness in the abundance of species between post-fire ages we used the Berger-Parker index. The Berger-Parker index is the inverse of the proportion of the most abundant species in a sample, and has low sensitivity to sample size. For species composition, the abundance of each species of spider was fourth root transformed, to decrease the influence of abundant species, and the Bray-Curtis measure of similarity calculated. We used non-metric multidimensional scaling (nMDS) to visualise in two dimensions the similarity in species composition between sites. We fitted ellipsoid hulls using Titterington‟s algorithm in the R package „cluster‟ (Maechler et al. 2005), so all sites of a given age were on or within the boundary of an ellipse. To uncover which species were most responsible for the observed similarity of the experimentally burnt and long unburnt sites, we used similarity percentages analysis on the transformed matrix (SIMPER in PRIMER; Clarke & Warwick 2001). We selected the 20 species that contributed the most to average similarity within a group (0, 0.5 and 115 20 years combined) and average dissimilarity between groups (compared to 3, 5 and 8 years combined). We used the distance to group centroids in the PERMDISP routine of PERMANOVA+ to test for homogeneity of multivariate dispersion among ages (Anderson 2006). Indicator species analysis (Dufrene & Legendre 1997) identified species associated with particular post-fire ages. This analysis uses the relative abundance of a species as well as its relative frequency of occurrence to calculate a value between 0 (no discrimination between ages) and 100 (perfect discrimination between ages, found only at all sites of a particular fire age). Analysis was performed using PC-ORD (McCune & Mefford 1999) on the fourth root transformed matrix (9999 permutations). Throughout the paper, we treat statistical tests with P < 0.05 as significant. We have not corrected for multiple tests (e.g. pair-wise PERMANOVA or indicator species analysis), preferring to present the raw P and data values, allowing the reader to decide significance (see Moran 2003). 116 RESULTS Of the 14 104 spiders collected nearly half were adults (48.6%), most of which were males (61.8%, 4234 specimens). The most abundant families were the Gnaphosidae (823 individuals), Corinnidae (805), Oonopidae (693), Prodidomidae (382), Miturgidae (297), Salticidae (242) and Zodariidae (240). The males represented 179 species from 24 families, with only 32 species formally described. The most speciose family was the Gnaphosidae with 34 species, followed by the Salticidae (19 spp.), Zodariidae (17 spp.), Oonopidae (16 spp.), Theridiidae (12 spp.) and Prodidomidae (10 spp.) (Appendix B). Abundance Abundance of male spiders ranged from 85 to 250 individuals per site. Permutational analysis of variance showed there was no clear pattern or significant difference in the abundance of spiders with post-fire age (d.f. 5, 21, pseudo-F = 1.509, P = 0.23; Fig. 2a). We did not consider abundance data further. Species richness and evenness Rarefied species richness ranged from 18.9 to 38.1 species per site. It was lowest in 3year-old sites and increased significantly with post-fire age to 20 years (PERMANOVA, d.f. 5, 21, pseudo-F = 10.01, P = 0.0001). The experimentally burnt sites (0 and 0.5 years since fire) were similar in richness to the 8-year-old sites (Fig. 2b). Pair-wise tests revealed significantly fewer species at 3-year-old sites than all other sites. The 5-year-old sites also had significantly fewer species than the 0 and 20-yearold sites (Fig. 2b, Appendix C). Sixty-two species (35% of total) only occurred in a single fire age: 0 years (8 species), 0.5 years (4 spp.), 3 years (9 spp.), 5 years (9 spp.), 8 years (11 spp.) and 20 years (21 spp.). However, it must be noted that the majority of these species were singletons (49 of 62) and doubletons (10 of 62) (Appendix B).Evenness increased between 3 to 20 years post-fire, with the Berger-Parker index increasing with time since fire (Fig. 2c). A permutational analysis of variance found significant differences in evenness (Berger-Parker) among post-fire ages (d.f. 5, 21, pseudo-F = 7.20, P = 0.0006). The pattern of evenness (Berger-Parker) with time was similar to that for species richness, except average evenness for experimentally burnt sites was similar to 20-year-old sites and evenness increased more slowly between 3 to 8 years. 117 Species composition There was a significant shift in the composition of species with post-fire age, and a clear successional trajectory was evident from 3 years, to 5, 8 and 20 years since fire (Fig. 3; PERMANOVA, d.f. 5, 21, pseudo-F = 2.63, P = 0.0001). The experimentally burnt sites (0 and 0.5) clustered apart from the other ages and were more similar to 20-yearold sites than were the older 3-year-old sites (Fig. 4). A three dimensional ordination (stress 0.15) was consistent with this 2-d result, in that it showed sites arranged in an orderly sequence of post-fire ages. Pair-wise comparisons showed that the 0 and 20year-old sites differed significantly from all other post-fire ages except 0.5 years. In addition, post-fire ages 3 and 8 years were also significantly different (Fig. 3, Appendix C). These differences were attributable to changes among groups in multivariate location, rather than multivariate dispersion, which was not significant (PERMDISP, d.f. 5, 21, F = 2.597, P = 0.194). The pattern of dispersion with post-fire age was somewhat hump shaped, with a maximum in 5-year-old sites: 0 years (average dispersion 27.0), 0.5 years (27.9), 3 years (29.7), 5 years (34.4), 8 years (32.2) and 20 years (30.9). The analysis of similarity percentages found sixteen species were more abundant and four species less abundant in the 0-, 0.5- and 20-year old sites compared to the 3-, 5- and 8-year old sites (Appendix D). These 20 species contributed over half (52.38%) of the average Bray-Curtis similarity (47.16%) between the experimentally and long unburnt sites. They also contributed 28.61% of the average dissimilarity (59.12%) between these sites and the other ages (3, 5 and 8 years). The 20 species represented 12 families and a wide range of life histories (Appendix D). Indicator species analysis identified 30 species from 15 families that had significant indicator values (Table 2). However, most of these species had relatively low indicator values (range 21-67%), which suggests they are not consistently good predictors of fire ages. Two-thirds of the indicator species were selected for recently burnt (0, 0.5 years) or long unburnt (20+ years) sites with fewer indicator species identified for the intermediate fire ages (3, 5, 8 years, Table 2). Graphing the abundances of several of the species selected by indicator analysis shows their distinct preferences for recently burnt (Fig. 5a) or long unburnt sites (Fig. 5c). Two lycosids [„Yalkara grp.‟ sp. 02 and Hoggicosa bicolor (Hogg, 1905)] both had higher abundance in the 0 age sites, with only a few individuals caught in 0.5-, 3- and 8-year-old sites (Fig. 5a). The reverse was true for Ceryerda cursitans Simon, 1909 and Lamponata daviesae Platnick, 2000, with most individuals collected in the 20-year-old sites (Fig. 5c). Indicators selected for the 118 intermediate ages show peaks in abundance for their particular fire age (Fig. 5b). Gnaphosidae sp. 09 for example, has very high abundance in 3-year-old sites, but declines in abundance over time. DISCUSSION Understanding the degree of determinism, why single or multiple basins of attraction occur and under what conditions they occur is an important question for theory and management (Chase 2003; Hobbs et al. 2007). From a management perspective, knowing when there is a high degree of determinism is crucial, as it provides land managers with some level of certainty as to how things will change over time. We found the post-fire response of spider assemblages in an arid habitat following wildfires to be deterministic when measured in terms of species richness, evenness and composition. Changes in these parameters with increasing post-fire age, while nonlinear, followed a chronological sequence and became increasingly similar to long unburnt sites with time. The succession of reptiles in arid Australia also follows a highly deterministic pattern (Masters 1996; Letnic et al. 2004), in contrast to mammals (e.g., Masters 1993; Southgate & Masters 1996; Letnic 2003). For arid mammals, post-fire succession is more aptly explained by multiple stable states, in which stochastic factors, particularly rainfall, shape transitions between states. It is possible that the mode of thermal regulation (ectothermic vs. endothermic) influences the level of determinism of postfire succession (Letnic et al. 2004). Thus, the pattern for spiders, being ectotherms, lends support to this hypothesis. However, we note that in other environments reptiles sometimes show little determinism (Driscoll & Henderson 2008; Lindenmayer et al. 2008) and mammals high determinism (Fox 1982). Therefore, habitat type and other factors may interact to determine the degree of determinism. Chase (2003) made a series of bold predictions as to when single or multiple states or basins of attraction would occur. He predicted single states in systems with low productivity, high rates of connectance, high disturbance and small regional species pools, while multiple states would occur under the converse. Our results fit most predictions for a single state (low productivity, high rates of connectance and high disturbance). However, they contradict the prediction of a small region species pool. We consider our study area to contain a high regional species pool, as many spiders in the arid zone have large geographical ranges (extent of occurrence) and over 200 species occur at Lorna Glen (this study & Owen 2004). 119 Post-fire changes in species richness and evenness The U-shaped pattern for species richness we found differs from chronosequence studies of the response of spiders to fire in other more mesic habitats. These have shown rapid recovery and a plateau in richness within the first three years (Moretti et al. 2002; Brennan et al. 2006), little difference between ages (Niwa & Peck 2002) and/or a hump shaped curve with time since fire (Buddle et al. 2000; 2006). In a longitudinal study, Merrett (1976) found that species richness increased slowly over the first 10 years following burning in English heathland, but richness was not high immediately following fire, as seen in our study. Likewise, species richness declined immediately after fire and increased gradually over the following two years in another longitudinal study in semi-arid Western Australia (Strehlow 1993). Working in the Great Victoria Desert of Australia, in similar vegetation to our study, Langlands et al. (2006) followed spider assemblages for 14 years. They found no clear pattern in species richness following fire. However, all three of these longitudinal studies suffer from limited replication. A further constraint limiting comparisons between studies is the oftenunderappreciated importance of standardizing species richness by the number of individuals at a site (see Gotelli & Colwell 2001; Buddle et al. 2005). Our high species richness in recently burnt sites could be driven by both survival of species in-situ and colonisation of new species and we return to these points in detail below. We found that trends in evenness were similar to changes in species richness with post-fire age. This result is not due to changes in species richness, as the Berger-Parker index depends purely on evenness (Colwell 2009). Our result is identical with post-fire changes in dominance of spiders found in the boreal forest of Canada. Buddle et al. (2000) found that evenness was initially high one year after fires, but within two years was quite low and increased steadily with increasing time since fire. Other studies have found no difference in dominance post-fire when using the Berger-Parker index (Niwa & Peck 2002). High evenness in recently burnt sites may be an indication that colonists are still establishing and have not had sufficient time to establish dominance (Buddle et al. 2000). This requires testing experimentally. 120 Shifts in species composition Changes in the species composition of spiders in arid Australia appear to follow a highly deterministic succession after wildfires, with similarity to long unburnt sites increasing markedly over time. This is consistent with previous studies, which have found species composition returns to, or converges towards, long unburnt states within 2-30 years (e.g. Huhta 1971; Buddle et al. 2000; Moretti et al. 2002; Brennan et al. 2006). We found no significant difference in multivariate dispersion, but the power of our analysis may have been limited, with a minimum of 10 sites recommended for such analyses (Anderson et al. 2008). A trend in decreasing multivariate dispersion over time may have provided further evidence of a deterministic succession. Multiple successional trajectories to long unburnt sites? All sites appear to converge towards the long unburnt state, increasing in similarity with long unburnt sites over time. However, the recently burnt sites do not fit an orderly chronological sequence with increasing post-fire age. Therefore, our results suggest that the system has a degree of inertia or that there may be two trajectories for post-fire succession, one following large intense wildfires and one following small, less intense experimental fires (Fig. 4). Although this could simply be a result of a noisy data set, this seems unlikely, as we sampled continuously over a nine-month period and had three to five sites per post-fire age. We therefore consider two biological mechanisms to explain multiple trajectories. Firstly, the lower intensities of the experimental burns allowed some species to survive the fire in-situ. This would indicate a strong degree of inertia to low intensity fires within the system. There would be evidence for this hypothesis if a longitudinal study found that the 0.5 aged sites became more similar in species composition with the 3-year-old sites, and less similar with the 20-year-old sites (Fig. 4, trajectory 2a). Secondly, the experimental burns extinguished most of the spider fauna, but the smaller scale of the fires allows species to recolonise rapidly from the surrounding long unburnt matrix. This would suggest that although there is a single attractor or cup within this system, aspects of the disturbance (scale) can result in different return trajectories or the pace of return (elasticity). If the 0.5 ages continued to increase in similarity with the 20-year-old sites, this hypothesis would be supported (Fig. 4, trajectory 2b). In Canadian forests, Buddle et al. (2000; 2006) found that succession of spiders following timber harvesting converged with sites undisturbed for 70 years much 121 faster than sites that had been burnt. They highlighted the importance of species surviving in-situ or the smaller scale of harvesting sites as potential explanations for the faster recovery following harvesting. For arid mammal assemblages, Letnic et al. (2004) suggested that the scale of fires studied could be a key factor in determining the post-fire response. Due to logistical constraints, the scale of experimental fires is often small. If the second trajectory is true and multiple return trajectories exist, it may be important to ensure not only multiple fire ages, but also multiple fire scales or intensities to maintain spider diversity (discussed further below). In an attempt to shed light on these two hypotheses, we identified the top 20 species that accounted for the similarity between the experimentally burnt and long unburnt sites. There was no clear indication amongst these species of a particular life history or phylogenetic pattern to explain their survival or recolonization of the experimentally burnt sites. The burrowing nemesiid mygalomorph Kwonkan sp.01 is likely to have survived in-situ deep it its burrow. Likewise, the pholcid Trichocyclus nigropunctatus Simon, 1908, which often live in goanna (Reptilia: Varanus) burrows on our sites, probably survived the fire. For the remaining species, it is likely that both survival in refugia and immigration and recolonization are occurring. Species responses and management implications Species that are reliant on characteristics of a specific fire age, particularly recently burnt or long unburnt habitats, may be at a heightened risk of extinction (Driscoll & Henderson 2008). Inspection of the data identified 62 species (35%) found in only a single post-fire age; however, as noted in our results, most of these were singletons, which by definition are restricted to a single post-fire age. A further 27 species (15%) were identified as significant indicator species for a post-fire age. Although again, the low indicator values we found suggest few of these were consistently restricted to or highly abundant in particular ages. We cannot rule out the possibility that these species are simply tourists or occupy a broader range of post-fire ages or habitats. For species that do survive in low densities in other post-fire ages or habitats, the risk of extinction would be limited (Driscoll & Henderson 2008 and references therein). Therefore, it is equivocal how many species are restricted to or reliant on a particular post-fire age. Clearly, surveys of other habitats within Lorna Glen are required to establish habitat preferences. 122 Lacking such data, a precautionary approach suggests we treat these species as potential post-fire age specialists. This perspective suggests that it is important to have areas representing multiple fire ages at any given time, lending support to the „pyrodiversity begets biodiversity‟ hypothesis (see Parr & Andersen 2006). However, the scale of fires required to initiate the full range of post-fire responses remains unknown. From the results of our experimental fires, it seems likely that areas greater than 9 ha are required. Actively creating pyrodiversity would also reduce the current risk of a large wildfire removing most of the long unburnt habitat simultaneously. Fire management plans for Lorna Glen have been prepared (Anon. 2003; Muller 2006), and are continuing to be revised as we develop a better understanding of the biota and its responses to fire. Trying to uncover the requirements of spiders and other fauna and incorporate them into fire plans will be a considerable and continuing challenge (see Clarke 2008). In conclusion, we have found that the post-fire succession of the arid spider assemblages we studied appears deterministic and largely in agreement with theoretical predictions for a single stable state. However, longitudinal studies are required to confirm these findings and determine the mechanism of return trajectories. Finally, for half the species, further sampling is required to determine the risk of extinction by inappropriate fire management. ACKNOWLEDGEMENTS Thanks to Bob Black,Volker Framenau, Don Driscoll and anonymous reviewers for constructive comments on the manuscript and useful conceptual insights. For field assistance we thank Karen Debski, Eliane Assis, Filomeno Cruz, Amanda McGinty, Fernanda Ferreira, Christopher Taylor, Ted Griffiths, Bob and Natasha Langlands and Graeme Liddlelow. Ryan Butler, Brad Barton, Julie Patten, Steve Toole, Luke Puertoliano, Dave Atkins and Anthony Desmond assisted with experimental fires. Fauna were collected with ethics approval (DEC AEC 2005/18). We are especially grateful to the following taxonomists for providing assistance with spider identifications: Barbara Baehr, Volker Framenau, Barbara York Main, Ricardo Ott, Robert Raven and Julianne Waldock. Thanks also to Norma and Rex Ward from Milrose Station for providing access to their property. Funding was provided by the Western Australian Department of Environment and Conservation and the School of Animal Biology at the University of Western Australia. PRL was supported by an Australian Postgraduate Award. 123 REFERENCES Allan G. E. & Southgate R. I. (2002) Fire regimes in the spinifex landscapes of Australia. In: Flammable Australia: The Fire Regimes and Biodiversity of a Continent (eds R. A. Bradstock, J. E. Williams and A. M. Gill) pp. 145-76. Cambridge University Press, Cambridge. Anand M. & Desrochers R. E. (2004) Quantification of restoration success using complex systems concepts and models. Restoration Ecology 12, 117-23. Anderson M. J. (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecology 26, 32-46. Anderson M. J. (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62, 245-53. Anderson M. J., Gorley R. N. & Clarke K. R. (2008) PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Version 1.0.1., Plymouth, UK. Anderson M. J. & Robinson J. (2003) Generalized discriminant analysis based on distances. Australian & New Zealand Journal of Statistics 45, 301-18. Anon. (2003) Fire management plan: Lorna Glen and neighbouring unallocated crown land. p. 38. Department of Conservation and Land Management, Goldfields Region. Bird R. B., Bird D. W., Codding B. F., Parker C. H. & Jones J. H. (2008) The "fire stick farming" hypothesis: Australian Aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. Proceedings of the National Academy of Sciences 105, 14796-801. Bos D. & Carthew S. M. (2007) Prey selection by the dasyurid Nigaui yvonneae. Wildlife Research 34, 632-9. Bradstock J. E., Williams M. & Gill A. M. (2002) Flammable Australia: Fire Regimes and the Biodiversity of a Continent. Cambridge University Press, Cambridge. Brennan K. E. C., Majer J. D. & Reygaert N. (1999). Determination of an optimal pitfall trap size for sampling spiders in a Western Australian Jarrah forest. Journal of Insect Conservation 3, 297-307. Brennan K. E. C., Ashby L., Majer J. D., Moir M. L. & Koch J. M. (2006) Simplifying assessment of forest management practices for invertebrates: how effective are higher taxon and habitat surrogates for spiders following prescribed burning? Forest Ecology & Management 231, 138-54. 124 Brennan K. E. C., Christie F. J. & York A. (2009) Global climate change and litter decomposition: more frequent fire slows decomposition and increases the functional importance of invertebrates. Global Change Biology 15, 2958-71. Brennan K. E. C., Moir M. L. & Majer J. D. (2004) Exhaustive sampling in a Southern Hemisphere global biodiversity hotspot: inventorying species richness and assessing endemicity of the little known jarrah forest spiders. Pacific Conservation Biology 10, 241-60. Buddle C. M., Beguin J., Bolduc E., Mercado A., Sackett T. E., Selby R. D., VaradySzabo H. & Zeran R. M. (2005) The importance and use of taxon sampling curves for comparative biodiversity research with forest arthropod assemblages. Canadian Entomologist 137, 120-7. Buddle C. M., Langor D. W., Pohl G. R. & Spence J. R. (2006) Arthropod responses to harvesting and wildfire: implications for emulation of natural disturbance in forest management. Biological Conservation 128, 346-57. Buddle C. M., Spence J. R. & Langor D. W. (2000) Succession of boreal spider assemblages following wildfire and harvesting. Ecography 23, 424-36. Bultman T. L. & Uetz G. W. (1982) Abundance and community structure of forest floor spiders following litter manipulation. Oecologia 55, 34-41. Burrows N. D., Burbidge A. A., Fuller P. J. & Behn G. (2006) Evidence of altered fire regimes in the Western Desert region of Australia. Conservation Science Western Australia 5, 272-84. Chase J. M. (2003) Community assembly: when should history matter? Oecologia 136, 489-98. Clarke K. R. & Gorley R. N. (2006) PRIMER v6: User Manual/ Tutorial. PRIMER-E Ltd, Plymouth, England. Clarke K. R. & Warwick R. M. (2001) Change in marine communitites: an approach to statistical analysis and interpretation. PRIMER-E, Plymouth. Clarke M. F. (2008) Catering for the needs of fauna in fire management: science or just wishful thinking? Wildlife Research 35, 385-94. Colwell R. K. (2009) Biodiversity: concepts, patterns, and measurement. In: The Princeton Guide to Ecology (ed S. A. Levin) pp. 257-63. Princeton University Press, Princeton. Driscoll D. A. & Henderson M. K. (2008) How many common reptile species are fire specialists? A replicated natural experiment highlights the predictive weakness of a fire succession model. Biological Conservation 141, 460-71. 125 Duffey E. (1962) A population study of spiders in Limestone Grassland. Journal of Animal Ecology 31, 571-99. Dufrene M. & Legendre P. (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecological Monographs 67, 345-66. Edwards G. P., Allan G. E., Brock C., Duguid A., Gabrys K. & Vaarzon-Morel P. (2008) Fire and its management in central Australia. The Rangeland Journal 30, 109-21. Fisher D. O. & Dickman C. R. (1993) Diets of insectivorous marsupials in arid Australia: selection for prey type, size or hardness? Journal of Arid Environments 25, 397-410. Fox B. J. (1982) Fire and mammalian secondary succession in an Australian coastal heath. Ecology 63, 1332-41. Friend G. R. (1995) Fire and invertebrates: a review of research methodology and the predictability of post-fire response patterns. In: Landscale Fires '93: Proceedings of an Australian Bushfire Conference (eds W. L. McCaw, N. D. Burrows, G. R. Friend and A. M. Gill) pp. 165-74. CALMScience Supplement 4, Perth, Western Australia. Gotelli N. J. & Colwell R. K. (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4, 379-91. Gould R. A. (1971) Uses and effects of fire among the western desert Aborigines of Australia. Mankind 8, 14-24. Hall D. W. (1991) The environmental hazard of ethylene glycol in insect pit-fall traps. The Coleopterists Bulletin 45, 193-4. Hobbs R. J., Jentsch A. & Temperton V. M. (2007) Restoration as a process of assembly and succession mediated by disturbance. In: Linking Restoration and Ecological Succession (eds L. R. Walker, J. Walker and R. J. Hobbs) pp. 150-67. Springer, New York. Huhta V. (1971) Succession in the spider communities of the forest floor after clearcutting and prescribed burning. Annales Zoologici Fennici 8, 483-542. Kimber R. (1983) Black lightning: Aborigines and fire in central Australia and the western desert. Archaeology in Oceania 18, 38-45. Langlands P. R., Brennan K. E. C. & Pearson D. J. (2006) Spiders, spinifex, rainfall and fire: long-term changes in a community of desert spiders. Journal of Arid Environments 67, 36-59. 126 Langlands P. R. & Framenau V. W. (2010) Systematic revision of Hoggicosa Roewer, 1960, the Australian 'bicolor' group of wolf spiders (Araneae: Lycosidae). Zoological Journal of the Linnean Society 158, 83-123. Lawrence K. L. & Wise D. H. (2000) Spider predation on forest-floor Collembola and evidence for indirect effects on decomposition. Pedobiologia 44, 33-9. Lawrence K. L. & Wise D. H. (2004) Unexpected indirect effect of spiders on the rate of litter disappearance in a deciduous forest. Pedobiologia 48, 149-57. Legendre P. & Anderson M. J. (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs 69, 1-24. Letnic M. (2003) The effects of experimental patch burning and rainfall on small mammals in the Simpson Desert, Queensland. Wildlife Research 30, 547-63. Letnic M., Dickman C. R., Tischler M. K., Tamayo B. & Beh C.-L. (2004) The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. Journal of Arid Environments 59, 85-114. Lindenmayer D. B., Wood J. T., MacGregor C., Michael D. R., Cunningham R. B., Crane M., Montague-Drake R., Brown D., Muntz R. & Driscoll D. A. (2008) How predictable are reptile responses to wildfire? Oikos 117, 1086-97. Maechler, M., Rousseeuw, P., Struyf, A., Hubert, M. (2005) Cluster Analysis Basics and Extensions; unpublished. Masters P. (1993) The effects of fire-driven succession and rainfall on small mammals in spinifex grassland at Uluru National Park, Northern Territory. Wildlife Research 20, 803-13. Masters P. (1996) The effects of fire driven succession on reptiles in spinifex grasslands at Uluru National Park, Northern Territory. Wildlife Research 23, 39-48. McCune B. & Mefford M. J. (1999) PC-ORD. Multivariate Analysis of Ecological Data. Version 4.41. MjM Software, Gleneden Beach, Oregon, U.S.A. Merrett P. (1976) Changes in the ground-living spider fauna after heathland fires in Dorset. Bulletin of the British Arachnological Society 3, 214-21. Moran M. D. (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100, 403-5. Moretti M., Conedera M., Duelli P. & Edwards P. J. (2002) The effects of wildfire on ground-active spiders in deciduous forests on the Swiss southern slope of the Alps. Journal of Applied Ecology 39, 321-36. 127 Muller C. (2006) Fire Management Plan: Lorna Glen and Earaheedy. pp. 46. C Muller Consulting, Perth. Niwa C. G. & Peck R. W. (2002) Influence of prescribed fire on carabid beetle (Carabidae) and spider (Araneae) assemblages in forest litter in southwestern Oregon. Environmental Entomology 31, 785-96. Owen G. (2004) Conserving biodiversity in the rangelands: are land systems effective surrogates for spider assemblages? BSc (Hons) Thesis, Curtin University of Technology, Perth. Parr C. L. & Andersen A. N. (2006) Patch mosaic burning for biodiversity conservation: a critique of the pyrodiversity paradigm. Conservation Biology 20, 1610-9. Pekar S. (2002) Differential effects of formaldehyde concentration and detergent on the catching efficiency of surface active arthropods by pitfall traps. Pedobiologia 46, 539-47. Platnick N. I. (2010) The world spider catalog, version 10.5. American Museum of Natural History. http://research.amnh.org/entomology/spiders/catalog/index.html (accessed 16-Feb-10). Raven R. J., Baehr B. C. & Harvey M. S. (2002) Spiders of Australia: Interactive identification to subfamily. CSIRO Publishing, Canberra. Riechert S. E. & Gillespie R. G. (1986) Habitat choice and utilization in web-building spiders. In: Spiders: Webs, Behavior and Evolution (ed W. A. Shear) pp. 23-48. Stanford University Press, Stanford, California, USA. Riecken U. (1999). Effects of short-term sampling on ecological characterization and evaluation of epigeic spider communities and their habitats for site assessment studies. Journal of Arachnology 27, 189-195. Samuels C. L. & Drake J. A. (1997) Divergent perspectives on community convergence. Trends in Ecology & Evolution 12, 427-32. Southgate R. & Masters P. (1996) Fluctuations of rodent populations in response to rainfall and fire in a central Australian hummock grassland dominated by Plectrachne schinzii. Wildlife Research 23, 289-303. Strehlow K. H. (1993) Impact of fires on spider communities inhabiting semi-arid shrublands in Western Australia's wheatbelt. BSc (Hons) Thesis, Murdoch University, Perth. Turner D., Ostendorf B. & Lewis M. (2008) An introduction to patterns of fire in arid and semi-arid Australia, 1998-2004. The Rangeland Journal 30, 95-107. 128 Uetz G. W. (1991) Habitat structure and spider foraging. In: Habitat Structure: The Physical Arrangement of Objects in Space (eds S. S. Bell, E. D. McCoy and H. R. Mushinsky) pp. 325-48. Chapman and Hall, Melbourne. Weeks jr. R. D. & McIntyre N. E. (1997) A comparison of live versus kill pitfall trapping techniques using various killing agents. Entomologia Experimentalis et Applicata 82, 267-73. Westman W. E. (1978) Measuring the inertia and resilience of ecosystems. BioScience 28, 705-10. Young T. P., Chase J. M. & Huddleston R. T. (2001) Community succession and assembly. Ecological Restoration 19, 5-18. 129 Table 1. The post-fire age of study sites with location (latitude and longitude) and the date when last burnt. Post-fire age 0 0 0 0 0.5 0.5 0.5 3 3 3 3 3 5 5 5 5 5 8 8 8 8 8 20 20 20 20 20 Location Number 26°S 121°E 1 21' 43'' 25' 27'' 2 15' 51'' 06' 12'' 3 16' 13'' 06' 02'' 4 21' 26'' 14' 14'' 1 21' 26'' 24' 50'' 2 13' 58'' 11' 48'' 3 21' 42'' 25' 16'' 1 14' 12'' 17' 00'' 2 09' 35'' 16' 46'' 3 11' 58'' 18' 10'' 4 12' 13'' 19' 42'' 5 08' 45'' 16' 05'' 1 16' 13'' 02' 34'' 2 16' '52' 21' 42'' 3 17' 03'' 11' 43'' 4 21' 24'' 13' 29'' 5 11' 00'' 02' 36'' 1 20' 15'' 26' 58'' 2 15' 22'' 07' 07'' 3 13' 33'' 11' 36'' 4 16' 41'' 21' 12'' 5 11' 57'' 17' 47'' 1 16' 00'' 06' 19'' 2 14' 01'' 11' 35'' 3 21' 22'' 25' 06'' 4 21' 39'' 14' 11'' 5 11' 05'' 02' 11'' Date Burnt 27 April 2006 26 April 2006 28 April 2006 27 April 2006 11 October 2005 9 October 2005 14 October 2005 25 November 2002 25 November 2002 25 November 2002 25 November 2002 25 November 2002 8 January 2001 8 January 2001 8 January 2001 8 January 2001 15 November 2000 23 December 1997 1 February 1998 1 February 1998 1 February 1998 1 February 1998 before 1988 before 1988 before 1988 before 1988 before 1988 130 Table 2. Species that were significant indicators (p < 0.05) for each post-fire age. Post-fire Family Species Indicator p age value Lycosidae „Yalkara grp.‟ sp. 02 66 0.002 Segestriidae Genus 1 sp. 02 60 0.007 Lycosidae Hoggicosa bicolor 43 0.026 Gnaphosidae Encoptarthria sp. 01 43 0.028 Nemesiidae Kwonkan sp. 01 32 0.021 Theridiidae Steatoda sp. 01 31 0.024 Miturgidae Genus 1 sp. 02 22 0.048 Thomisidae Genus 1 sp. 02 67 0.009 Lamponidae Lampona quinqueplagiata 50 0.040 Corinnidae Poecilipta sp. 03 49 0.016 Pholcidae Trichocyclus nigropunctatus 42 0.017 Oxyopidae Oxyopes rubicundus 24 0.029 Theridiidae Steatoda sp. 02 21 0.031 Gnaphosidae Genus 1 sp. 09 48 0.001 Prodidomidae Nomindra leeuweni 29 0.006 Corinnidae Supunna picta 21 0.013 5 Gnaphosidae Genus 1 sp. 03 43 0.033 8 Gnaphosidae Genus 1 sp. 28 53 0.001 Segestriidae Genus 1 sp. 01 45 0.044 Pholcidae Trichocyclus arabana 45 0.037 Theridiidae Steatoda sp. 05 41 0.030 Nemesiidae Genus 1 sp. 02 36 0.023 Zoridae Argoctenus sp. 01 27 0.001 Gnaphosidae Ceryerda cursitans 63 0.006 Gnaphosidae Genus 1 sp. 13 60 0.008 Prodidomidae Wydundra sp. 04 60 0.016 Zoridae Argoctenus sp. 08 60 0.016 Lamponidae Lamponata daviesae 55 0.010 Trochanteriidae Fissarena castanea 44 0.008 Oonopidae 28 0.035 0 0.5 3 20 Opopaea sp. 13 131 Fig. 1. The location of sites (*) within the proposed Lorna Glen conservation park (inset: park location in Western Australia). Site names denote fire age in years followed by replicate number. Non-spinifex vegetation is shaded grey and lines mark unsealed roads. 132 a Abundance ±s.e. 250 a 200 a a 150 a a a 100 0 b 5 10 15 20 Species richness ±s.e. 40 a 35 a ad ab bd 30 c 25 20 0 Berger-Parker index ±s.e. c 5 10 15 20 10 ab 8 a a abd 6 c cd 4 2 0 0 5 10 15 20 Post-fire age (years) Fig. 2. Mean a) spider abundance, b) species richness rarefied to 85 individuals and c) Berger-Parker evenness index with post-fire age in years. Unlike letters indicate significant differences (p < 0.05) from PERMANOVA pair-wise tests and bars indicate standard errors. 133 1.0 8-1 20-1 8-5 20-4 20-2 20-5 8 20 0.5 5-2 8-2 5 3-4 5-1 5-5 0.0 nmds axis 2 5-3 20-3 0.5-2 8-4 8-3 5-4 0.5 -0.5 0.5-1 0-1 3-2 0.5-3 3 0-2 3-3 0-3 0 -1.0 3-1 3-5 -1.5 -1.0 0-4 -0.5 0.0 0.5 1.0 nmds axis 1 Fig. 3. Non-metric multidimensional scaling (nMDS) of species composition of spiders based on Bray-Curtis similarity from fourth root transformed data (Stress = 0.23). Small numbers indicate sites (post-fire age in years – site number, see Table 1). As a visual aid, for each post-fire age, sites are enclosed within ellipses and the centre of the ellipse is indicated by large numbers. 134 Average similarity to 20 years 55 50 2b 45 2a 1 40 35 Hypothetical trajectories 30 0 5 10 15 20 Post-fire age (years) Fig. 4. Trajectories of post-fire re-assembly as shown by the average similarity (BrayCurtis) of each post-fire age compared to 20-year-old sites. For 20-year-old sites it is the average similarity within the age. Solid arrows mark the two inferred recovery trajectories: 1) following wildfires (3- 20 years) and 2) experimental burns (0 & 0.5 years). Dashed arrows mark two possible outcomes for the experimentally burnt sites: 2a) inertia in the system or 2b) alternative recovery trajectory (see discussion). For clarity, standard errors are shown above or below only. 135 a Mean abundance +s.e. 81 Lycosidae 'Yalkara grp.' sp.02 Lycosidae Hoggicosa bicolor Thomisidae Genus 1 sp.02 Corinnidae Poecilipta sp.03 16 1 0 0 0.5 3 5 8 20 b Gnaphosidae Genus 1 sp.09 Gnaphosidae Genus 1 sp.03 Gnaphosidae Genus 1 sp.28 Mean abundance +s.e. 81 16 1 0 0 0.5 3 5 8 20 c Mean abundance +s.e. 81 Gnaphosidae Ceryerda cursitans Gnaphosidae Genus 1 sp.13 Prodidomidae Wydundra sp.04 Lamponidae Lamponata daviesae 16 1 0 0 0.5 3 5 8 20 Time since fire (years) Fig. 5. Mean abundance for selected species that were identified by indicator analysis as significant indicators of post-fire ages a) 0 and 0.5 years, b) 3, 5, and 8 years and c) 20 years (see Table 2). Analysis used fourth-root transformed data, but for ease of viewing, graphs show untransformed abundance values with standard errors. There were five sites each for 3 to 20 years, while 0 and 0.5 years had four and three sites respectively. 136 Appendix A Detailed explanation of fire ignition procedure, weather conditions and temperatures of experimental fires. A 9 hectare area (300 x 300 m) was marked by 2 m wide fire breaks and sites were prescribe burnt (lit in 5-10 m strips along the side opposite to the prevailing wind). Ignition by drip torches ensured that almost all vegetation on each site was burnt. Table A1 shows the time of ignition and completion for experimental fires (24 hrs), with the observed weather conditions (ambient air temperature, humidity and wind speed). Table A1. Site Ignition time Finish (24 hrs) Avg. relative Avg. wind Ambient temperature (˚C) humidity (%) speed (m.s-1) 0-1 10:30 (17) 12:10 (22) 31 2.2 0-2 14:00 (27) 17:00 (23) 34 2.8 0-3 14:00 (25) 16:40 (20) 25 1.7 0-4 14:31 (26) 21:35 (17) 26 1.7 0.5-1 18:00 (24) 21:35 (17) 15 0.6 0.5-2 17:10 (31) 20:10 (17) 13 0.3 0.5-3 16:46 (29) 22:30 (21) 20 0.8 To provide an indication of the intensity of the experimental fires, thermocouples were used to record temperatures in five of the seven sites. Due to the small scale, the experimental fires were unlikely to be as nearly as intense as large wildfires. However, for all sites measured, the maximum temperature at ground level (0 cm) within spinifex ranged between 456 and 709˚C. The maximum temperature recorded was 825˚C at 10 cm above ground within spinifex (Fig. A1). 137 Site 0-1: in spinifex, above ground 30 cm 10 cm 5 cm 2 cm 0 cm 800 700 600 500 400 300 200 100 0 Site 0-1: in bare soil, below ground 900 0 60 120 180 240 Temperature *C Temperature *C 900 300 500 400 300 200 100 0 Site 0-3: in spinifex, above ground 30 cm 10 cm 5 cm 2 cm 0 cm 0 60 120 180 240 300 200 100 0 30 cm 0 cm 2 cm 5 cm 10 cm 0 120 240 360 480 600 Site 0.5-2: in spinifex, above ground 800 700 600 500 400 300 200 100 0 0 60 120 180 240 300 0 cm 2 cm 5 cm 10 cm 0 60 120 180 240 300 0 cm 2 cm 5 cm 10 cm 500 400 300 200 100 0 0 60 120 180 240 300 900 800 700 600 500 400 300 200 100 0 2 cm 5 cm 10 cm 15 cm 0 60 120 180 240 300 300 Seconds 2 cm 10 cm 20 cm 800 700 600 500 400 300 200 100 0 0 60 120 180 240 300 Site 0.5-3: in spinifex, below ground Temperature *C Temperature *C 500 400 300 200 100 0 240 Site 0.5-2: in bare soil, below ground Site 0.5-3: in spider burrow, bare ground 900 800 700 600 180 800 700 600 900 90 cm 60 cm 30 cm 0 cm Temperature *C Temperature *C 900 120 Site 0.5-2: in bare soil, below ground Temperature *C Temperature *C Site 0.5-1: in spinifex, above & below ground 900 800 700 600 500 400 300 60 900 Temperature *C Temperature *C 500 400 300 200 100 0 0 Site 0-3: in spinifex, below ground 900 800 700 600 0 cm 2 cm 5 cm 10 cm 800 700 600 900 800 700 600 500 400 300 200 100 0 0 cm 2 cm 5 cm 10 cm 0 60 120 180 240 300 Seconds Figure A1: The temperatures (˚C) recorded by thermocouples in some of the experimentally burnt sites. For each graph the site, habitat (bare ground, spinifex or spider burrow) and location (above or below ground) are indicated. 138 Species Cheiracanthium sp.05 Clubiona sp.01 Clubiona sp.02 Clubiona sp.03 Clubiona sp.04 Battalus sp.04 Genus 1 sp.01 Poecilipta sp.02 Poecilipta sp.03 Supunna funerea Simon, 1896 Supunna picta (L. Koch, 1873) Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.04 Phryganoporus sp.01 Genus 1 sp.01 Genus 1 sp.02 Family Clubionidae Clubionidae Clubionidae Clubionidae Clubionidae Corinnidae Corinnidae Corinnidae Corinnidae Corinnidae Corinnidae Desidae Desidae Desidae Desidae Dictynidae Dictynidae collected only from a single post-fire age. 8 20 0 0 8 1 6 0 25 0 21 0 0 0 0 1 0 1 0 0 0 0 0 1 1 40 0 18 0 0 0 0 0 0 1 0 139 1 0 0 0 3 2 283 1 1 1 1 0 0 0 0 2 0 0 2 42 0 5 0 212 2 0 0 0 0 2 0 0 2 0 0 3 59 1 7 4 119 1 0 1 0 0 0 0 2 0 0 0 2 5 0 4 1 75 2 0 0 0 2 1 0 0 0 1 20 15 25 14 17 13 21 15 49 10 20 40 27 25 40 12 20 3* 20 5 0 0 8 3 20 0.5 3 3* 20* 5 0* 8* 3 20* Age IndVal 3 0 0.5 Indicator analysis Post-fire age 5 1.000 0.604 0.311 0.565 0.900 0.013 0.685 0.016 0.551 1.000 1.000 0.142 0.286 0.252 0.143 0.933 1.000 p abundance and frequency of occurrence, low values denote the opposite. Significant p values are bolded and the symbol * indicates species that were the maximum value and p value (9999 permutations). High Indicator values denote species that were found in a post-fire age in high relative Abundance of all 179 species collected within each post-fire age, ordered by family, with the indicator species value (IndVal, 0-100) for the age with Appendix B Species Ceryerda cursitans Simon, 1909 Eilica sp.07 Eilica sp.11 Encoptarthria sp.01 Encoptarthria sp.08 Encoptarthria sp.15 Encoptarthria sp.33 Encoptarthria sp.34 Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.04 Genus 1 sp.05 Genus 1 sp.06 Genus 1 sp.09 Genus 1 sp.10 Genus 1 sp.12 Genus 1 sp.13 Genus 1 sp.16 Genus 1 sp.17 Genus 1 sp.18 Genus 1 sp.21 Genus 1 sp.22 Family Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Appendix B cont. 5 8 20 0 0 1 0 0 0 0 1 1 0 24 9 0 0 0 0 0 17 4 50 1 0 0 0 0 2 0 1 0 11 6 14 1 7 0 0 1 0 0 12 0 45 1 0 140 4 1 2 0 0 0 1 18 91 13 0 6 1 0 0 0 0 5 0 35 8 0 16 0 0 0 0 0 0 6 14 4 5 16 15 2 0 0 0 3 2 0 10 0 0 0 0 0 0 0 0 8 5 6 11 10 3 2 0 0 0 1 0 2 12 2 4 0 0 1 1 12 0 4 6 17 15 7 1 0 1 1 1 21 1 1 1 7 18 20 25 22 20 60 20 26 48 28 22 16 43 25 21 20 20 29 43 35 18 63 5 3* 3 0.5 20* 20 3* 0.5 3 20 20 5 5 5 0.5 20* 20* 0.5 0 0.5 5 20 Age IndVal 3 0 0.5 Indicator analysis Post-fire age 0.374 1.000 0.345 0.235 0.008 1.000 1.000 0.001 0.186 0.216 0.489 0.033 0.941 0.330 0.315 1.000 1.000 0.028 0.203 0.078 0.006 0.634 p Species Genus 1 sp.24 Genus 1 sp.25 Genus 1 sp.27 Genus 1 sp.28 Genus 1 sp.29 Genus 1 sp.30 Genus 1 sp.31 Genus 1 sp.32 Hemicloea sp.20 Hemicloea sp.23 Hemicloea sp.26 Laronius sp.19 Asadipus banjiwarn Platnick, 2000 Lamponina asperrima (Hickman, 1950) Lamponata daviesae Platnick, 2000 Lamponina elongata Platnick, 2000 Lampona quinqueplagiata Simon, 1908 Lamponina scutata (Strand, 1913) Erigone prominens Bösenberg & Strand, 1906 Genus 1 sp.01 Hoggicosa alfi Langlands & Framenau, 2010 Hoggicosa bicolor (Hogg, 1905) Family Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Lamponidae Lamponidae Lamponidae Lamponidae Lamponidae Lamponidae Linyphiidae Liocranidae Lycosidae Lycosidae Appendix B cont. 5 8 20 10 1 0 0 2 0 0 0 0 0 0 1 0 0 0 0 0 4 1 1 0 7 1 0 0 1 0 3 0 0 0 0 0 0 0 1 0 1 0 4 0 0 0 3 141 1 0 0 0 0 0 2 0 0 0 0 0 0 0 0 0 0 0 6 8 0 15 0 1 0 3 1 0 0 1 0 0 0 0 0 0 0 0 1 4 4 8 1 8 1 2 1 1 8 0 0 1 1 0 0 0 1 0 0 2 0 10 28 8 0 5 0 1 0 0 7 2 0 7 0 2 1 0 0 0 3 0 0 4 1 9 1 21 43 7 20 18 22 50 20 55 20 40 20 25 20 33 40 20 20 17 53 30 10 23 0 0 8* 5 20 0.5 3* 20 8* 20* 20* 0* 8* 0.5* 20* 0.5 5* 8 8 3 20 3 Age IndVal 3 0 0.5 Indicator analysis Post-fire age 0.026 0.965 1.000 0.509 0.040 0.361 0.010 1.000 1.000 0.136 1.000 0.252 1.000 0.116 0.139 0.333 1.000 0.001 0.719 0.139 1.000 0.326 p 1 0 11 0 Knoelle clara (L. Koch, 1877) „Pexa grp.' sp.04 „Yalkara grp.' sp.02 „Yalkara grp.' sp.03 Genus 1 sp.01 Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.04 Miturga sp.05 Genus 1 sp.06 Miturga sp.07 Genus 1 sp.08 Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.05 Kwonkan sp.01 Kwonkan sp.06 Yilgarnia sp.04 Cavisternum sp.07 Grymeus sp.04 Grymeus sp.09 Myrmopopaea sp.01 Lycosidae Lycosidae Lycosidae Lycosidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Nemesiidae Nemesiidae Nemesiidae Nemesiidae Nemesiidae Nemesiidae Oonopidae Oonopidae Oonopidae Oonopidae 5 8 20 0 0 0 44 2 4 13 1 1 2 17 0 2 8 6 11 31 7 0 1 0 13 0 5 3 0 0 0 3 1 0 6 7 8 6 1 1 2 0 0 142 0 0 0 9 0 0 1 0 0 2 5 0 0 0 1 7 15 6 0 0 1 1 5 0 1 0 0 1 4 0 0 7 6 0 1 2 2 11 24 4 0 0 3 0 1 2 1 22 0 1 4 0 0 6 9 1 0 2 5 13 16 2 0 2 0 0 0 2 2 54 0 12 4 0 0 0 16 0 0 9 5 3 17 1 0 0 1 0 12 14 8 30 25 23 32 25 25 36 23 21 15 30 33 22 22 15 33 66 8 14 5 0.5 20 20 0* 0.5 0 0* 0* 8 0 0.5 0 0 0.5 0 0 3 0.5* 0 5 0 Age IndVal 3 0 0.5 Indicator analysis Post-fire age Species Family Appendix B cont. 0.885 0.599 1.000 0.185 0.252 0.021 0.323 0.258 0.023 0.262 0.500 0.318 0.461 0.161 0.134 0.048 0.707 0.868 0.002 0.116 1.000 0.569 p Species Myrmopopaea sp.05 Myrmopopaea sp.08 Myrmopopaea sp.14 Myrmopopaea sp.15 Opopaea sp.02 Opopaea sp.03 Opopaea sp.06 Opopaea sp.12 Opopaea sp.13 Opopaea sp.16 Xestaspis sp.10 Xestaspis sp.11 Oxyopes sp.01 Oxyopes sp.02 Oxyopes dingo Strand, 1913 Oxyopes rubicundus L. Koch, 1878 Genus 1 sp.01 Genus 1 sp.02 Trichocyclus arabana Huber, 2001 Trichocyclus nigropunctatus Simon, 1908 Cryptoerithus halli Platnick & Baehr, 2006 Cryptoerithus occultus Rainbow, 1915 Family Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oxyopidae Oxyopidae Oxyopidae Oxyopidae Philodromidae Philodromidae Pholcidae Pholcidae Prodidomidae Prodidomidae Appendix B cont. 5 8 20 3 1 0 0 0 0 4 0 0 1 0 0 0 7 1 3 46 1 0 0 0 9 0 0 14 0 0 1 15 5 0 1 0 0 6 2 0 3 41 0 0 1 0 23 143 0 1 2 0 0 1 9 5 0 0 0 0 0 1 0 6 56 0 1 1 0 12 0 2 2 0 0 1 14 1 0 0 0 0 0 1 0 0 28 1 0 0 0 2 1 1 1 6 1 0 14 1 0 0 0 3 3 7 0 0 86 0 0 0 1 32 0 3 15 2 0 0 11 0 1 0 1 2 9 15 0 1 64 0 0 1 0 59 37 22 42 45 20 15 24 39 20 19 20 36 27 28 25 30 19 14 20 15 20 26 0 20 0.5 8 8* 0.5 0.5 3 20* 0.5 20* 8 20 20 0* 3 8 0 3* 0.5 8* 20 Age IndVal 3 0 0.5 Indicator analysis Post-fire age 0.069 0.017 0.398 0.037 1.000 0.029 0.549 0.050 1.000 0.340 1.000 0.073 0.035 0.285 0.263 0.157 0.378 0.570 1.000 0.550 1.000 0.124 p 1 0 3 0 Molycria vokes Platnick & Baehr, 2006 Nomindra sp.01 (cf. cooma) Nomindra sp.02 Nomindra leeuweni Platnick & Baehr, 2006 Wesmaldra learmonth Platnick & Baehr, 2006 Wydundra sp.03 Wydundra sp.04 Wydundra kennedy Platnick & Baehr, 2006 ‘Clynotis albobarbatus grp.' sp.10 Damoetas sp.13 Damoetas sp.17 Genus 1 sp.02 'big embolus grp.' ‘Neon' sp.15 Genus 1 sp.23 Genus 1 sp.27 'big embolus grp.' Grayenulla sp.01 Holoplatys sp.07 (cf. planissima) Holoplatys sp.14 Lycidas sp.08 Lycidas grp. sp.11 Lycidas grp. sp.12 Lycidas chlorophthalmus (Simon, 1909) Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae 5 8 20 0 1 0 0 0 0 0 4 0 0 0 0 0 0 10 1 8 0 0 0 0 17 0 0 3 11 0 0 0 0 1 0 0 0 0 0 12 0 1 0 144 1 0 0 5 0 0 4 23 0 0 2 0 0 0 3 0 0 1 124 2 30 0 1 1 0 12 1 1 8 23 0 0 3 0 0 0 1 0 1 0 25 0 49 0 0 0 3 7 0 0 4 19 0 0 0 0 0 1 1 0 1 0 60 0 16 0 0 0 1 15 0 0 4 32 1 1 0 1 0 0 5 7 2 0 3 0 5 1 10 14 28 21 20 20 21 21 20 20 26 20 33 20 14 60 20 20 29 25 18 20 3 0 8 20 5* 5* 5 20 20* 20* 0 20* 0.5* 8* 20 20* 20 3* 3 3 3 20* Age IndVal 3 0 0.5 Indicator analysis Post-fire age Species Family Appendix B cont. 1.000 0.570 0.224 0.550 1.000 1.000 0.420 0.775 1.000 1.000 0.175 1.000 0.112 1.000 0.016 0.643 0.400 0.006 1.000 0.347 0.766 1.000 p 1 0 Ocrisiona yakatunyae Żabka, 1990 Paraplatoides sp.03 Paraplatoides sp.06 Pellenes bitaeniata (Keyserling, 1882) Zebraplatys keyserlingi Żabka, 1992 Genus 1 sp.01 Genus 1 sp.02 Genus 1 sp.01 Genus 1 sp.02 Genus 1 sp.07 Genus 1 sp.08 Genus 1 sp.12 Genus 1 sp.13 Hadrotarsus sp.10 Hadrotarsus sp.11 Latrodectus sp.09 Steatoda sp.01 Steatoda sp.02 Steatoda sp.03 Steatoda sp.04 Steatoda sp.05 Genus 1 sp.01 Salticidae Salticidae Salticidae Salticidae Salticidae Segestriidae Segestriidae Sparassidae Sparassidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Thomisidae 5 8 20 0 0 2 4 5 27 1 0 0 0 0 0 0 1 0 5 0 0 0 1 0 1 1 0 11 9 1 0 0 2 1 0 0 0 0 0 0 1 1 0 0 6 145 0 1 0 2 9 3 0 0 0 2 0 0 0 0 0 0 0 1 0 0 0 6 1 1 0 0 7 8 1 0 1 0 0 0 1 0 0 0 0 0 1 1 1 1 0 9 0 1 6 8 0 0 1 0 0 2 0 1 1 1 3 1 0 0 2 1 0 1 7 2 12 3 1 2 1 0 9 0 0 3 0 0 1 1 0 0 1 1 20 41 19 33 21 31 11 40 7 20 25 40 20 20 20 60 45 12 14 20 8 20 5* 8 0 0 0.5 0 0.5 20* 20 3 20 8* 5* 20 8* 0 8 0.5 0.5 5* 8 3 Age IndVal 3 0 0.5 Indicator analysis Post-fire age Species Family Appendix B cont. 0.030 1.000 0.332 0.031 0.100 0.024 0.855 0.145 1.000 0.394 0.279 0.142 1.000 0.387 0.007 1.000 0.044 0.753 0.629 1.000 1.000 0.545 p Species Genus 1 sp.02 Genus 1 sp.04 Genus 1 sp.05 Genus 1 sp.06 Stephanopis sp.03 Fissarena castanea (Simon, 1908) Australutica quaerens Jocqué, 1995 Australutica sp.01 (cf. quaerens) Cavasteron sp.32 (cf. crassicalcar) Genus 1 sp.31 Genus 1 sp.33 Genus 1 sp.34 Habronestes bicornis Baehr, 2003 Habronestes sp.13 (cf. longiconductor) Habronestes sp.16 (cf. grahami) Habronestes sp.18 (cf. hamatus) Habronestes sp.21 Habronestes sp.30 Masasteron piankai Baehr, 2004 Neostorena sp.02 Neostorena sp.03 Neostorena sp.05 Family Thomisidae Thomisidae Thomisidae Thomisidae Thomisidae Trochanteriidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Appendix B cont. 5 8 20 0 1 6 8 14 0 0 0 0 0 2 0 2 1 5 0 4 1 0 0 0 0 0 0 1 8 0 0 0 0 0 0 2 0 1 10 3 0 2 0 0 0 0 2 146 0 0 5 0 25 0 1 0 0 0 0 1 4 1 2 5 0 0 0 0 1 0 0 0 2 1 2 1 0 0 2 1 0 0 2 0 3 13 4 0 1 0 0 0 1 0 4 4 8 0 0 0 0 0 1 0 6 5 16 9 5 0 0 1 0 0 0 0 0 17 0 0 0 2 1 0 0 0 2 14 3 4 20 0 0 0 1 0 20 25 16 28 27 20 20 40 11 20 23 20 15 28 27 30 44 25 20 20 10 67 8* 0* 3 20 3 5* 3* 20* 5 5* 0 3* 8 0.5 0 5 20 0* 5* 8* 3 0.5* Age IndVal 3 0 0.5 Indicator analysis Post-fire age 1.000 0.262 0.862 0.207 0.215 1.000 1.000 0.145 1.000 1.000 0.189 1.000 0.785 0.175 0.292 0.008 0.117 0.262 1.000 1.000 0.009 1.000 p Species Spinasteron cavasteroides Baehr & Churchill, 2003 Argoctenus sp.01 Argoctenus sp.02 Argoctenus sp.03 Argoctenus sp.04 Argoctenus sp.05 Argoctenus sp.08 Genus 1 sp.10 Family Zodariidae Zoridae Zoridae Zoridae Zoridae Zoridae Zoridae Zoridae Appendix B cont. 5 8 20 0 0 0 0 0 0 4 1 0 0 0 1 0 0 2 0 147 0 0 0 0 0 0 9 0 0 0 0 0 0 2 8 0 0 0 0 0 0 8 17 1 2 4 1 1 1 6 5 6 40 60 20 21 20 26 27 36 20* 20* 20* 0.5 20* 8 8 20 Age IndVal 3 0 0.5 Indicator analysis Post-fire age 0.016 0.136 1.000 0.314 1.000 0.001 0.206 0.096 p Comparison Post-fire age 0 vs 0.5 0 vs 3 0 vs 5 0 vs 8 0 vs 20 0.5 vs 3 0.5 vs 5 0.5 vs 8 0.5 vs 20 3 vs 5 3 vs 8 3 vs 20 5 vs 8 5 vs 20 8 vs 20 Unique perms 35 126 126 126 126 56 56 56 56 126 126 126 126 126 126 t 0.662 5.507 2.378 0.745 1.260 5.423 1.842 0.222 2.018 2.547 3.961 7.220 1.456 3.779 1.939 Richness p values perm 0.661 0.008 0.065 0.534 0.231 0.017 0.138 0.911 0.108 0.049 0.020 0.009 0.165 0.008 0.093 MC 0.529 0.001 0.049 0.487 0.247 0.001 0.115 0.829 0.090 0.034 0.004 0.000 0.181 0.005 0.089 are provided with significant (< 0.05) p values in bold. 148 Berger-Parker p values t perm 0.126 0.914 3.142 0.009 2.566 0.031 1.341 0.229 0.321 0.757 5.918 0.018 3.475 0.021 2.544 0.053 0.235 0.861 0.180 0.820 3.647 0.023 5.319 0.008 2.259 0.076 3.954 0.016 2.543 0.057 MC 0.910 0.018 0.038 0.220 0.759 0.001 0.012 0.044 0.817 0.865 0.007 0.001 0.051 0.004 0.034 Species composition p values t perm MC 1.381 0.027 0.130 2.060 0.008 0.007 1.806 0.008 0.016 1.666 0.006 0.030 1.842 0.017 0.014 1.547 0.018 0.050 1.531 0.035 0.064 1.566 0.015 0.054 1.345 0.035 0.127 1.335 0.039 0.121 1.636 0.007 0.026 2.158 0.009 0.005 1.125 0.111 0.283 1.613 0.015 0.034 1.527 0.008 0.042 (PERMANOVA pair-wise tests). For each comparison the value of the test statistic (t) and both permutational (perm) and Monte Carlo (MC) values Differences in species richness, Berger-Parker index and species composition between post-fire ages from a permutational analysis of variance Appendix C Species Poecilipta sp.03 Supunna picta (L. Koch, 1873) Phryganoporus sp.01 Eilica sp.11 Encoptarthria sp.08 Genus 1 sp.05 Genus 1 sp.06 Miturga sp.05 Kwonkan sp.01 Cavisternum sp.07 Myrmopopaea sp.05 Opopaea sp.13 Trichocyclus nigropunctatus Simon, 1908 Nomindra sp.01 (cf. cooma) Nomindra leeuweni Platnick & Baehr, 2006 Family Corinnidae Corinnidae Desidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Miturgidae Nemesiidae Oonopidae Oonopidae Oonopidae Pholcidae Prodidomidae Prodidomidae dis/similarity and cumulative contribution. 0.73 0.60 0.77 1.08 1.44 1.70 0.88 1.06 0.79 0.88 1.12 1.10 0.59 1.80 0.87 1.69 1.07 0.33 0.49 0.83 0.36 0.49 0.27 0.72 0.47 0.44 0.53 0.84 2.45 0.07 149 0.61 0.45 0.66 1.64 2.03 2.86 1.1 1.6 0.66 0.81 1.34 1.07 0.45 3.04 0.85 average 0/0.5/20 3/5/8 Similarity Average abundance 0.66 0.53 0.66 2.12 2.11 8.01 1.07 2.14 0.66 0.84 1.06 0.77 0.53 8.15 0.66 /SD 1.30 0.96 1.41 3.49 4.31 6.07 2.34 3.40 1.39 1.72 2.84 2.26 0.95 6.44 1.81 (%) Cont. 40.69 39.39 38.43 37.02 33.53 29.22 23.15 20.81 17.41 16.02 14.30 11.46 9.20 8.25 1.81 (%) Cumul. 1.11 0.89 0.71 0.69 0.88 1.40 0.66 0.84 0.72 0.77 0.89 1.03 0.87 0.69 0.88 average Dissimilarity 1.49 1.28 1.16 1.25 1.34 2.14 1.19 1.61 1.22 1.18 1.41 1.24 1.23 1.68 1.16 /SD 1.88 1.51 1.20 1.17 1.49 2.37 1.11 1.42 1.21 1.30 1.50 1.74 1.47 1.16 1.49 (%) Cont. 22.02 20.14 18.63 17.43 16.26 14.77 12.40 11.29 9.87 8.66 7.36 5.86 4.12 2.65 1.49 (%) Cumul. within age groups along with average dis/similarity, dis/similarity divided by standard deviation (SD), percentage contribution to the total average similarity between the experimentally burnt (0 and 0.5 years) and the long unburnt sites (20 years). For each species, there is the average abundance (versus 3, 5 and 8 years combined) from an analysis of similarity percentages (SIMPER). These species are therefore important contributors to the The twenty species that contributed the most to average Bray-Curtis similarity within the group (0, 0.5 and 20 years combined) and between groups Appendix D Species Genus 1 sp.27 'big embolus grp.' Lycidas sp.08 Steatoda sp.01 Fissarena castanea (Simon, 1908) Masasteron piankai Baehr, 2004 Family Salticidae Salticidae Theridiidae Trochanteriidae Zodariidae Appendix D cont. 0.91 0.87 1.10 0.73 1.18 0.16 0.26 0.58 0.65 1.06 150 0.92 0.93 1.44 0.54 1.69 average 0/0.5/20 3/5/8 Similarity Average abundance 0.83 0.85 1.33 0.52 2.06 /SD 1.94 1.97 3.06 1.15 3.57 (%) Cont. 52.38 50.44 48.47 45.41 44.26 (%) Cumul. 0.85 0.79 0.81 0.76 0.69 average Dissimilarity 1.30 1.26 1.30 1.21 1.23 /SD 1.44 1.33 1.36 1.29 1.17 (%) Cont. 28.61 27.17 25.84 24.48 23.19 (%) Cumul. CHAPTER 3 Untangling the drivers of structure in animal assemblages in arid ecosystems: the influence of fire, rainfall, vegetation, predators and spatial structure on spiders (Araneae) Peter R Langlands Karl E C Brennan Bruce Ward Formatted for Ecography 151 Abstract A vast array of variables can act singly or in combination to influence an area‟s biota. A critical challenge in ecology is to understand the degree to which these variables, or combination of variables, influence assemblages of species. For those variables under anthropogenic control, such knowledge also assists prioritising actions to conserve biodiversity. In arid Australia, fire and rainfall are two key and potentially interacting forces that contribute in shaping assemblages of plants and animals. However, the degree to which spatial processes, environmental variables and biotic processes combine with fire and rainfall to shape assemblages of animals remains poorly quantified. Using a chronosequence approach in spinifex habitat we studied the influence of fire (0-20 years previously) on spiders, while also examining the short-term effect of rainfall, the influence of space, environmental variables (Soil, Floristics, Habitat structure) and biotic processes (Predation by scorpions, small insectivorous mammals and reptiles). Predictor variables explained 79% of the variation in species richness and 32% in composition of spiders using distance-based redundancy analysis. Variation partitioning identified post-fire age as explaining the largest proportion of this variation, 61% for species richness and 78% for composition. However, spatially and non-spatially structured vegetation variables (Floristics and Habitat structure) explained much of this variation. Rainfall explained a large proportion of the explained variation for species richness (59%), but not species composition (30%). There was little variation attributable to predation (0 & 14% for species richness and composition, respectively) or pure spatial structure (5 & 5%). These findings provide support for environmental variables structuring assemblages of spiders in the arid zone and suggest that actively managing fire effects on habitat structure and floristics is critical to conserving spider biodiversity. 152 Introduction Quantifying the degree to which different variables interact in structuring assemblages of animal and plant species is a central theme in community ecology (Quinn and Dunham 1983, Parris 2004, Cottenie 2005, Driscoll and Lindenmayer 2009, Legendre et al. 2009). Environmental conditions can structure the species composition of assemblages (e.g. species-habitat or niche-based models Whittaker 1956, Bray and Curtis 1957). This concept appears similar to the species sorting perspective within metacommunity theory (see Leibold et al. 2004). Additionally, biotic processes (dispersal, competition, mortality and predation) can structure species composition (Hairston et al. 1960, Paine and Vadas 1969, Connell 1983). Historical legacies from past natural (fires, droughts and storms) or anthropogenic events (clearing and agriculture), may impose further structure on species composition (e.g. Sousa 1984, Pickett and White 1986, Borcard and Legendre 1994). Ecologists now recognise that variables can consist of spatially and non-spatially structured patterns (Legendre and Legendre 1998). Spatially structured environmental variables can produce spatial patterns in assemblages, as species track their habitat (Fortin and Dale 2005, Legendre and Legendre 1998). Alternatively, the biotic processes described above may be contagious, with sites closer together being more similar in species composition (Legendre 1993). The latter reveals itself as a pure effect of spatial location on assemblages of species (Borcard et al. 1992). There is, however, a dearth of empirical studies quantifying the influences of environmental variables and spatial processes for terrestrial invertebrates in arid ecosystems in the Southern Hemisphere (Cottenie 2005). Quantifying the pure and interacting influences of an often-vast array of environmental, biotic and historical variables (while explicitly considering the influence of space) on assemblages of species is a crucial, but formidable challenge. Although methods such as variation partitioning have existed for more than 15 years (Borcard et al. 1992, Borcard and Legendre 1994), it is only recently that such techniques have been refined (e.g. Peres-Neto et al. 2006) and become widely accessible (e.g. Oksanen et al. 2008). Indeed, until the recent development of a recursive algorithm by Økland (2003), such analyses have been limited to no more than four groups of variables. These developments permit answers to fundamental but hitherto unresolved questions for many land managers seeking to conserve biodiversity, where there are many potential variables influencing assemblages of species. Such questions include: 1) how much of a system is under anthropogenic control (Pozzi 153 and Borcard 2001, de Warnaffe and Dufrene 2004)? and 2) how much of the composition of species are they likely to be able to influence solely by influencing environmental conditions? The latter question recognises that control of intrinsic processes within an assemblage may not always be possible or desirable. Fire and rainfall are two variables that ecologists have long recognized as potentially important in governing the structure of faunal assemblages in arid ecosystems (Stafford Smith and Morton 1990, Masters 1993, Letnic et al. 2004, Langlands et al. 2006) (Fig. 1, arrow 1). For example, within the vast arid zone of Australia the vegetation is highly flammable, and large swathes (> 10 000 km2) can be burnt due to lightning strikes and human ignition (Allan and Southgate 2002, Turner et al. 2008). Additionally, extensive use of fire by Aboriginal people can create a landscape characterised by a mosaic of different post-fire ages (Burrows et al. 2006, Bird et al. 2008). In arid Australia, many studies document marked changes in assemblages of animals following fire for vertebrates as well as some invertebrate groups (Gunawardene and Majer 2005, Letnic et al. 2004, Smith and Morton 1990). Rainfall is highly variable, leading to populations of many species undergoing a cyclic pattern of boom followed by bust (Masters 1993, Dickman et al. 1999a, b). This is because massive downpours, often associated with low-pressure systems that are the remnants of cyclones (hurricanes), occasionally interrupt long periods of drought. For example, our study location received 110 mm, nearly half the median annual rainfall, in just two days in January 2007 (Langlands unpubl. data). It is important to appreciate, however, that fire and rainfall can be linked. Fuel-load and fire-interval are strongly influenced by rainfall, with more fires following periods of high rainfall (Turner et al. 2008, Fig. 1 arrow 2), making it important to consider the influence of rainfall when examining faunal responses to fire (Blanche et al. 2001, Letnic et al. 2004, Langlands et al. 2006, Fig. 1 arrow 3). Yet it remains poorly quantified to what degree fire, rainfall and the interplay of these or other variables can explain variation in assemblages of species. Here we consider this question on an assemblage of spiders. Other variables, such as habitat structure, exert strong influences on assemblages of spiders, with simplification of structure decreasing abundance (see meta-analysis by Langellotto and Denno 2004). Experimental manipulations of habitat structure that increase complexity cause increases in abundance and species richness and can alter species composition of spiders (Uetz 1979, Gunnarsson 1990, Hatley and MacMahon 1980, Fig. 1 154 arrow 4). In our system, the dominate ground cover, spinifex grass (Triodia basedowii), is removed by fire, and with sufficient rainfall, grows gradually with increasing time since fire. This leads to a gradual increase in the vertical structure of vegetation over time as spinifex and long-lived plants develop (Griffin 1992, Fig.1 arrow 5). Floristics and soil properties may also influence assemblages of spiders (Owen 2004, Beals 2006, Schaffers et al. 2008, Fig. 1 arrows 6 and 7). Marked difference in species composition of spiders among species of plants may arise through differences in architecture of plants, flowering and the species of prey used by spiders (Robinson 1981, Uetz 1991). The species richness of vegetation in spinifex habitats increases rapidly after a fire with the germination of many short-lived herbs, forbs and grasses (Allan and Baker 1990, Fig. 1 arrow 8). Reduced competition from spinifex and increased nutrients following the fire aid the flush of plant growth (Griffin 1990, Fig. 1 arrow 9). Within three years, the flush of plants following the fire will senesce, but the shrubs, trees and spinifex are still establishing. While many studies have implicated post-fire changes in vegetation as driving post-fire changes in faunal assemblages within the Australian arid zone, there has been far less consideration of other processes, such as predation and parasitism. For spiders, these are potentially important variables. Many studies have demonstrated strong top-down control from predacious birds and lizards causing decreases in abundance or species richness of spiders (Evelegh et al. 2001, Spiller and Schoener 1988, 1998, Gunnarsson 2007, Manicom et al. 2008, Fig. 1 arrow 10). Indeed, in a global context, the reptile fauna of our study site is exceptionally rich (Pianka 1969). However, rainfall, seasonality and perhaps habitat structure can mediate the strength of top-down control (Gunnarsson 1990, Recher and Majer 2006, Spiller and Schoener 2008, Fig. 1 arrow 11). Parasitoids and intraguild predation can also exert strong influences by decreasing the abundance of spider populations (Polis et al. 1989, 1998, Wise 1993, Fig. 1 arrow 12). The aim of our study was to use variation partitioning to quantify the degree to which fire, rainfall, along with environmental and biotic variables and spatial processes, combine to shape a spider assemblage within fire-prone spinifex grasslands of arid Australia. Although our approach is clearly correlative, it is an important initial step for determining which variables might be most influential and generating hypotheses of potential causal mechanisms (Legendre and Legendre 1998). 155 Materials and methods Study area and design Our study was conducted in central Western Australia at the proposed Lorna Glen (Matuwa) Conservation Park, located approximately 150 km NE of Wiluna (Fig. 2). Lorna Glen contains a range of habitat types with the most widespread of these being the fireprone hummock grasslands (Bullimore). This is a vegetation assemblage dominated by spinifex Triodia basedowii E. Pritz, 1918, an over storey of marble gums (Eucalyptus gongylocarpa Blakely, 1936) with scattered mulga (Acacia aneura Benth, 1855) and mallee (E. kingsmillii Maiden and Blakely, 1929 and E. lucasii Blakely, 1934). We conducted our study at the landscape scale, with 27 sites selected to represent a range of fire and potentially rainfall histories (described below) (Fig. 2). At each site, a 50 x 50 m quadrat was established a minimum of 150 metres from the site boundary (change in fire age) or road. Fire history We used Landsat satellite images from 1988 to 2005 to establish the fire history of the study area. From these natural fire histories and some experimental fires, six treatments for post-fire ages were selected: 0, 6 months, 3, 5, 8 and 20 plus years since the last fire. There were three to five sites per post-fire age (Fig. 2). Patch size for each site was at least 9 ha. To establish some recently burnt sites, seven sites that had not been burnt for at least twenty years were burnt experimentally (three sites were burnt October 2005 [0.5 years treatment] and four sites were burnt April 2006 [0 years treatment]). For details of fire ignition procedure and weather conditions during burning see Langlands et al. (accepted). Rainfall Twenty-six rain gauges (Davis tipping buckets [165 mm diameter, 0.2 mm accuracy ± 5% at 100 mm/hr] connected to Odyssey data loggers [http://www.odysseydatarecording.com]), were installed within 200 m of most sites in August 2005 (for site 8-1 the nearest gauge was 1500 m away). We installed rain gauges on 200 L (44 gallon) drums (~ 850 mm high), which we positioned from surrounding vegetation at a distance of at least twice the height of the vegetation (Australian Bureau of Meteorology). We ensured gauges were level. 156 We calculated the total monthly rainfall at each site from August 2005 to January 2007, along with total rainfall for blocks of three and six months. The total rainfall before trapping (August 2005 – April 2006) and total rainfall during trapping was also calculated. Habitat structure and floristics We used the line intercept method to estimate vegetation cover and structure. At each plot taut measuring tapes along the diagonals created two 70 metre transects (140 metres total). Using a pole marked in 5 cm sections we recorded the ground cover (height ≤ 50 cm) and over storey cover (height > 50 cm). At each point on the tape where there was a change in ground cover we recorded the distance and cover category. We did not record cover changes of less than 5 cm length. There were eight categories of ground cover: bare ground, live spinifex, dead spinifex, leaf litter, grass, dead grass, woody shrub and dead woody shrub. Over storey cover was recorded as the length of transect covered by vegetation in six categories: alive or dead vegetation of either woody shrubs, marble gums or mulga. The proportion of each category of ground cover and over storey for the two transects was calculated and averaged, giving the average proportion of ground covered by each category of ground cover and over storey for a site. We recorded the composition of plant species within each quadrat (50 x 50 m) at each site. The abundance of each species was scored in five categories: 1, 2-10, 11-50, 51-100 and >100 plants. Soil Soil texture, pH, conductivity, organic carbon (%), and the concentration of nitrogen, ammonium, phosphorus, potassium and sulphur were assessed using a sample of the top 5cm of soil. This was collected 1 m from each pitfall trap in August 2006 and bulked for each site. 157 Predators As a measure of potential predation pressure, we used scorpions and an unintended bycatch of small mammals (Dasyuridae) and reptiles that prey on spiders caught in pitfall traps. For each site, we calculated the abundance and species richness of reptilian and mammalian predators, along with abundance of scorpions. Spatial We used trend surface analysis with the easting and northing values of sites from Universal Transverse Mercator (UTM) coordinates used to create polynomial combinations up to the third order with Spacemaker2 (Borcard and Legendre 2002). An irregular distribution of sites over the study area prevented the use of principal coordinate analysis of neighbour matrices (PCNM, Borcard and Legendre 2002). Sampling and identification of spiders Each quadrat contained five pitfall traps, which we opened 30 April 2006 and exchanged at three-monthly intervals until 18 January 2007 (256-258 days per trap session). We combined the data for each pitfall trap and collection period to give one sample per site. We identified spiders to species level. It is very difficult to match males, females and juveniles of the same species correctly, because taxonomic knowledge of the Australian spider fauna is particularly poor compared with the northern hemisphere (Brennan et al. 2004). To avoid identification errors the results presented here are from males only with species boundaries verified by taxonomic experts. For further details of the pitfall trapping and identification procedures see Langlands et al. (accepted). Data analysis Spiders To test for differences in abundance, species richness and species composition of male spiders, we used a permutational analysis of covariance (Legendre and Anderson 1999, Anderson 2001) with the fixed factor AGE (levels 0, 0.5, 3, 5, 8 and 20 years). As the spatial coordinate northing3 explained the most variation with step-wise selection, it was subsequently used as a covariate to account for the influence of site locations. These 158 analyses used PERMANOVA+ (Anderson et al., 2008, version 1.01). To account for differences in abundance between sites, prior to analysis, we rarefied the richness of species using PRIMER (Clarke and Gorley, 2006, version 6.1.11) to the minimum number of spiders collected from a site (85 males). For analyses of species composition, the abundance of each species of spider was fourth root transformed, to decrease the influence of abundant species and Bray-Curtis similarity calculated. To visualise the correlation of selected explanatory variables with species composition we used distance-based redundancy analysis (db-RDA, McArdle and Anderson 2001). Throughout the paper we treat statistical tests with p < 0.05 as significant, unless otherwise specified. We have not corrected for multiple tests (e.g. pair-wise PERMANOVA), preferring to present the raw p and data values, allowing the reader to decide significance (see Moran 2003). Variation partitioning We used the distance-based linear models (DISTLM) function in PERMANOVA+ to perform variation partitioning. This allowed us to choose an appropriate similarity measure for the data, Euclidean distance for richness and Bray-Curtis similarity for composition of species. As the DISTLM function fits a linear model, we transformed predictor variables to improve normality. We took the natural logarithm of soil variables, except for pH. Habitat proportions were arcsine transformed, with Predator and Rainfall variables square root transformed. To allow for non-linear relationships between the predictors and response variables we calculated the squares and cubes of variables (Rainfall, Habitat, Soil and Predators) after centering data on their means (Makarenkov and Legendre, 2002; Jones et al., 2008). We coded post-fire age as a dummy variable to allow a non-linear response and the fire age surrounding each site was included as a variable. The similarity between sites in floristic composition was calculated using Bray-Curtis similarity. Principal coordinates analysis (PCO) was then used to reduce the dimensionality of the floristic data to make it suitable for incorporation into variation partitioning analysis. For each group of explanatory variables (Fire, Rainfall, Habitat, Floristics, Soil, Predators and Spatial, see Fig. 1), we used step-wise selection (9999 permutations) to identify variables which explained a significant (p < 0.01) amount of variation in the species richness or species composition of spiders. We were conservative in our selection of 159 variables for variation partitioning, as like regression, it is not possible to have more explanatory variables than sites. Although the selection process chose variables with the highest explanatory power, many variables within a group are likely to be inter-correlated. Therefore, we used spearman correlation to find variables that had a correlation (> 0.60) with the selected variables. We used adjusted R2 values to allow comparison between groups with differing numbers of variables selected (Peres-Neto et al., 2006). Adjusted R2 values are also displayed as a percentage of the total variation explained, as it is more important and informative to compare the relative amounts of variation explained between groups rather than the absolute amount (Økland, 1999). This approach is also more useful for comparisons between studies. As the number of explanatory groups (n) included in variation partitioning increases, the number of components also increases (2n-1). We therefore used the simplification method of Økland (2003) to show only the significant components. This uses a value (l) as a threshold to decide what higher order components are large/significant enough to be kept and which higher order components should be distributed onto lower orders (see Økland, 2003, and calculations provided in Appendix B (supplementary online material)). We used the more conservative method of calculating l, which uses the variation explained by single predictor variables that corresponds to a chosen significance level (p = 0.05). Results Of the 14 104 spiders collected, nearly half were adults (48.6%), of which most were males (61.8%, 4 234 specimens). The males represented 179 species from 24 families, with only 32 species formally described. The predator by-catch considered to prey on spiders consisted of 426 vertebrates (313 reptiles and 113 mammals) and 172 scorpions. These represented 26 species of reptile species, 5 mammals and an unknown number of species of scorpion. 160 Abundance Abundance of male spiders ranged from 85 to 250 individuals per site. There was no clear pattern or significant difference in the abundance of spiders with post-fire age, based on a permutational analysis of covariance; therefore we did not consider the data further (d.f. 5, 20; pseudo-F = 0.695, p = 0.62). Species richness Rarefied species richness of spiders ranged from 18.9 to 38.1 species per site, with the lowest mean richness found in 3-year-old sites. The spatial covariate northing3 was significant, and results reported henceforth are adjusted accordingly. Species richness, varied significantly (Table 1), with similar richness at the experimentally burnt and 8-yearold sites, but with richness increasing with post-fire age from 3 to 20 years (Fig. 3a). Pairwise tests revealed significantly fewer species at 3-year-old sites than at the 0, 0.5, 8 and 20-year-old sites (Table 1). The 20-year-old sites had significantly more species than the 0.5- and 5-year-old sites. In comparison, the post-fire response of the species richness of plants did not display the same pattern. Plant richness increased rapidly in the first six months following fire, but subsequently decreased, increased and decreased in the following years (Fig. 3a). Habitat structure was markedly different between post-fire ages. For example, bare ground increased from around 40%, to over 90% immediately after fire, but decreased over time to return to 40% after 20 years (Fig. 3b). Part of this increase in bare ground was due to the removal of live spinifex, which gradually regrew with increasing time since fire (Fig. 3b). Eight explanatory variables from five groups (Fire, Rainfall, Habitat, Floristics and Spatial location of the study sites) explained 79% of the total variation in the species richness of spiders (Table 2). While the variables selected for Rainfall (Jan 20072, Nov 20053), Habitat (bare ground2) and Spatial (northing3) were the best in terms of explanatory power, they were also correlated with several other variables within each group (Appendix C). Rainfall in January 20072 was strongly correlated with rain from February to April in 2006, while November 20053 was correlated with rainfall during July to November 2006 (Appendix C). The Habitat variable bare ground2 was correlated with woody shrub cover, as well as with dead and live spinifex. 161 Overall, Fire (61%) and Rainfall (59%) explained the greatest amounts of the total variation explained, followed by Floristics (47%), Spatial (41%) and Habitat (22%) (Fig. 4). That said, there was a large degree of overlap between groups, with 51% of the total variation explained shared in common between three or more groups (Fig. 4). All groups shared 14% of the total variation explained, with Fire, Rainfall and Spatial sharing a further 22% in common. Fire, Rainfall and Floristics also shared 15% of the explained variation (Table 2). Species composition Adjusting for the spatial covariate northing3, there were marked differences in the species composition of spiders with increasing post-fire age (Table 3). Previous analyses indicated a clear pattern from 3 years, to 5, 8 and 20 years since fire, while the experimentally burnt (0 and 0.5 years since fire) sites were located away from the other ages (Langlands et al. accepted). Pair-wise comparisons showed that the most recently burnt sites (0 years) differed significantly from all other post-fire ages. Similarly, the long unburnt sites (20 years) differed significantly from all other ages, except 0.5 years (Table 3). Other differences between site ages are shown in Table 3. Partitioning of the variation in the species composition of spiders found that twelve explanatory variables, representing six groups (Fire, Rainfall, Habitat, Floristics, Predators and Spatial location), were significant in explaining 32% of the variation (Table 4). The selected Rainfall variable (Feb-Apr 2006) was strongly correlated with rainfall from February to July 2006 and January 2007 (Appendix C). Likewise, the selected Habitat variables (live and dead spinifex) correlated strongly with themselves, and upper storey woody shrubs. Predictably, the Predator variable of reptile abundance was correlated with the number of reptile species and the spatial variable northing3 was correlated with northing, as for species richness (Appendix C). Five groups of variables (Fire, Rainfall, Habitat, Floristics and Spatial) shared 28% of the total variation explained (Table 4). Fire, Habitat and Predators also shared 11%, with Fire and Habitat explaining a further shared 19%. Fire explained the greatest proportion of the total variation explained (78%) followed by Habitat (64%), Floristics (34%), Spatial (33%), Rainfall (30%) and Predators (14%) (Table 4, Fig. 4). Similar variation partitioning results were also obtained using the Hellinger transformation with traditional Redundancy 162 Analysis (RDA), indicating a high degree of robustness to similarity measure and ordination technique. A constrained ordination (distance-based RDA) of the species composition, using the selected explanatory variables, identified which ages and variables were strongly correlated (Fig. 5). The first axis was dominated by the Age of surrounds, being positively correlated with recently burnt and long unburnt sites (all surrounded by 20+ years). The second axis was strongly correlated with live spinifex, greater in older sites, as well as abundance of reptiles and rainfall between February to April 2006, which were higher in recently burnt sites. The Floristics principal coordinate 2 was strongly positively correlated with the third axis (not shown). Discussion Untangling the myriad of variables influencing assemblages of species is a critical challenge for developing a deeper mechanistic understanding of arid ecosystems, allowing better management to conserve biodiversity. In this study, we have quantified for the first time the degree to which Rainfall, Habitat structure, Floristics, Predators, Soil and Spatial pattern explain the variation in an arid assemblage of spiders following disturbance by fire. Although post-fire age explained the greatest amount of the variation explained for species richness (61%) and composition (78%) of the spiders, much of this variation was also explained by Floristics or Habitat structure (i.e. was not unique to fire). This suggests fire is instrumental in shaping the plant assemblage, which in turn exerts a strong influence on the spider assemblage. The variables representing Fire, Rainfall, Floristics and Habitat also shared most of the variation explained by spatial location, suggesting that where the landscape burnt and rain fell, along with underlying environmental gradients, generated spatial structure in the vegetation to which the spiders are responding. The spider assemblage we studied is explained best by changes in vegetation, which in turn is strongly influenced by fire, with only a minor contribution of predators or intrinsic species processes at this scale. There was very little pure spatial structuring or spatial autocorrelation, which would have provided support for contagious species processes (Borcard and Legendre 1994, Legendre and Legendre 1998). Additionally, the crude measures of Predators we included failed to account for any variation in species richness and explained only a minor part of species 163 composition. This suggests that the contribution of contagious species processes is small relative to fire and rainfall mediated changes in vegetation. These findings provide strong support for land managers wishing to conserve spider biodiversity in arid Australia: they can have a large influence on the spider community through changes to the habitat and floristics, particularly via fire. However, they will need to consider carefully the influence of rainfall on the fire regime, as rainfall is largely beyond anthropogenic control. Despite there being strong arguments as to why the top-down control via predators might be particularly important in structuring assemblages of spiders (see introduction), our results are consistent with findings from a recent meta-analysis across a range of non-spider taxa in predominantly Northern Hemisphere temperate ecosystems. Cottenie (2005) found that environmental variables were dominant, and the spatial or neutral processes were important for only a few communities. Our results are also consistent with two previous studies of spiders that have included environmental variables and spatial location, but did not explicitly consider predators (Pozzi and Borcard 2001, Jeanneret et al. 2003). Environmental variables and spiders As noted above, amongst our explanatory variables, post-fire age explained the most variation of species richness and composition of spiders. This result was expected, given that, firstly, vegetation structure and floristics are well known to change in spinifex habitats as greater time elapses following burning (see Introduction). Although habitat structure in our study changed as described in previous work, changes in the species richness of plants were less congruent. Although species richness of plants increased rapidly after the experimental fires, and decreased by three years, there was not a consistent decline thereafter. It is not clear why our results differ. Secondly, assemblages of spiders respond strongly to alterations in habitat structure and floristics (discussed below). The finding of a large amount of the variation explained by habitat structure is consistent with many studies and the long-held importance of habitat in determining assemblages of spiders (Hatley and MacMahon 1980, Robinson 1981, Halaj et al. 1998, 2000, Heikkinen and MacMahon 2004, Brennan et al. 2006). Several mechanisms may explain changes in assemblages of spiders with increasing habitat complexity, including access to alternative prey, more effective prey capture, architectural attachments for webs, and particularly, refuge from intraguild predation (Langellotto and Denno 2004). Interestingly, habitat 164 explained the least variation of the five groups selected for species richness. This may be because whereas habitat structure generally increases in a continuous manner with post-fire age in this habitat, species richness may follow a more complex pattern (see Fig. 3a). Simplifying this pattern by excluding the recently burnt sites and re-running our analysis resulted in habitat explaining the most variation and slightly more than floristics (for brevity results not shown). Rainfall explained nearly as much as post-fire age for species richness, but little for species composition. Previous work in arid Australia suggested that rainfall in the year before sampling was correlated with the species richness of spiders, but that was not statistically significant (Langlands et al., 2006). Increased plant growth and florescence following rain may lead to increased invertebrates and therefore spiders. Rainfall is clearly a strong driving force in arid Australia, and trying to understand how it influences vegetation development following fire and the subsequent influence on fauna will be vital for management (see Letnic and Dickman 2006). Although changes in soil properties (e.g NH4+, NO3-) are known following fire (Wan et al. 2001), which presumably affects the subsequent regeneration of floristics and vegetation structure, we found few differences in nutrient values among our study sites. As such, it is not surprising that soil was unimportant in explaining variation within the spider assemblage. This result agrees with Sanderson et al. (1995), who found no relationship with soil in northeast England. However, a study conducted at our location, which contrasted assemblages of spiders between habitat types associated with differing geologies, found a correlation between the composition of spider species and soil texture and ammonium nitrogen (Owen 2004). Predation and spiders Several studies have demonstrated the strong influence predators, particularly reptiles, can have on spiders (Spiller and Schoener 1988, 1998, Manicom et al. 2008). However, we found the effect of predation alone explained none of the variation in the species richness of spiders and very little for composition. Most of the variation explained by predation for species composition was shared with post-fire age and habitat. We interpret this as reptiles and scorpions potentially responding to post-fire changes in habitat structure in a similar manner to the spiders, rather than exerting direct control over the spider assemblage. 165 Additionally, our collection methods may have been inadequate for assessing predation pressure on spiders, or the influence of top-down control may be ephemeral in our system. Thus, before top-down control in our study system is considered negligible, more detailed measures of the predator and parasitoid assemblage through periods of boom and bust should be undertaken. Future studies should also consider the implications of intraguild predation (Polis et al. 1989, Wise 1993). Study limitations While we were able to explain most of the variation in species richness (79%), we were unable to explain 68% of the variation in species composition. This level of unexplained variation is common in partitioning analyses, which range from 20 to 80% of species composition explained (Cottenie 2005). The unexplained variation may be due to unmeasured variables or random noise (Legendre and Legendre 1998). For example, our study was constrained by the absence of long-term rainfall records for our study sites. It is clear that the long-term history of rainfall plays an important role in the post-fire regeneration of vegetation. Other aspects of the fire regime (patch size, frequency and intensity) may also account for unexplained variation. Additionally, Økland (1999) has suggested that a large proportion of unexplained variation in such analyses (50-85%) may be due to lack-of-fit of data to the model. This is why we presented the proportion of the total variation explained. Another limitation of our study is that because it is correlative it does not provide proof of causality. Individual variables selected as the best predictors within explanatory groups were correlated with other variables within and among groups. Different variables were also selected for species richness and composition, although these were often strongly correlated. Therefore, although step-wise selection chose particular variables, other measured or unmeasured variables may be causing the relationships. That said, the variables selected should be considered as good candidates around which to generate hypotheses for future manipulative experiments. 166 Concluding remarks To develop a mechanistic understanding of arid ecosystems and conserve biodiversity, we must untangle the influence of multiple variables on assemblages of species. We found that an assemblage of spiders in the arid zone of Western Australia had significant responses in the number of species and its species composition to post-fire age. Post-fire variation in the spider assemblage we studied is largely explained by vegetation (Floristics and Habitat structure), which in turn is strongly influenced by Fire, with only a minor influence of Predators at this scale. These findings suggest that land managers striving to conserve spider diversity in arid Australia have the potential to influence a large part of the variation within the assemblage. Acknowledgements - We thank the many people who provided assistance in the field, particularly T Griffiths, B and N Langlands and G Liddlelow. Experimental fires were conducted with the help of staff from the Kalgoorlie office of the Western Australian Department of Environment and Conservation (DEC). Thanks for identifications to R Cranfield (plants) and M Cowan (vertebrates). Fauna were collected with ethics approval (DEC AEC 2005/18). We are especially grateful to B Baehr, VW Framenau, BY Main, R Ott, RJ Raven and JM Waldock for providing assistance with identifications of spiders. Rainfall data were generously provided by the WA Bureau of Meterology. Thanks to P Legendre and R Økland for answers to questions regarding variation partitioning. GIS data and assistance were provided by G Behn and L Shu. Thanks to R Black, BY Main and VW Framenau for helpful comments that improved the manuscript. Funding was provided by DEC and the School of Animal Biology at the University of Western Australia. 167 References Allan, G. E. and Baker, L. M. 1990. Uluru (Ayers Rock - Mt. Olga) National Park: an assessment of a fire management programme. - In: D. A. Saunders, et al. (eds), Australian Ecosystems: 200 years of Utilization, Degradation and Reconstruction. Proceedings of a symposium held in Geraldton, Western Australia, 28 August- 2 September, 1988. Chipping Norton, N.S.W. Surrey Beatty & Sons for the Ecological Society of Australia, pp. 215-220. Allan, G. E. and Southgate, R. I. 2002. Fire regimes in the spinifex landscapes of Australia. - In: R. A. Bradstock, et al. (eds), Flammable Australia: The Fire Regimes and Biodiversity of a Continent. Cambridge University Press, pp. 145-176. Anderson, M. J. 2001. A new method for non-parametric multivariate analysis of variance. - Austral Ecology 26: 32-46. Anderson, M. J., et al. 2008. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. - PRIMER-E, Version 1.0.1. Beals, M. 2006. Understanding community structure: a data-driven multivariate approach. Oecologia 150: 484-495. Bird, R. B., et al. 2008. The "fire stick farming" hypothesis: Australian Aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. - Proceedings of the National Academy of Sciences 105: 14796-14801. Blanche, K. R., et al. 2001. Rainfall-contingent detection of fire impacts: Responses of beetles to experimental fire regimes. - Ecological Applications 11: 86-96. Borcard, D., et al. 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045-1055. Borcard, D. and Legendre, P. 1994. Environmental control and spatial structure in ecological communities: an example using oribatid mites (Acari, Oribatei). Environmental and Ecological Statistics 1: 37-61. Borcard, D. and Legendre, P. 2002. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. - Ecological Modelling 153: 51-68. Bray, J. R. and Curtis, J. T. 1957. An ordination of the upland forest communities of southern Wisconsin. - Ecological Monographs 27: 325-349. 168 Brennan, K. E. C., et al. 2006. Simplifying assessment of forest management practices for invertebrates: how effective are higher taxon and habitat surrogates for spiders following prescribed burning? - Forest Ecology & Management 231: 138-154. Brennan, K. E. C., et al. 2004. Exhaustive sampling in a Southern Hemisphere global biodiversity hotspot: inventorying species richness and assessing endemicity of the little known jarrah forest spiders. - Pacific Conservation Biology 10: 241-260. Burrows, N. D., et al. 2006. Evidence of altered fire regimes in the Western Desert region of Australia. - Conservation Science Western Australia 5: 272-284. Clarke, K. R. and Gorley, R. N. 2006. PRIMER v6: User Manual/ Tutorial. - PRIMER-E Ltd. Connell, J. H. 1983. On the prevalence and relative importance of interspecific competition: evidence from field experiments. - The American Naturalist 122: 661. Cottenie, K. 2005. Integrating environmental and spatial processes in ecological community dynamics. - Ecology Letters 8: 1175-1182. de Warnaffe, G. d. B. and Dufrêne, M. 2004. To what extent can management variables explain species assemblages? A study of carabid beetles in forests. - Ecography 27: 701-714. Dickman, C. R., et al. 1999a. Population dynamics of two species of dragon lizards in arid Australia: the effects of rainfall. - Oecologia 119: 357-366. Dickman, C. R., et al. 1999b. Long-term dynamics of rodent populations in arid Australia: the influence of rainfall. - Wildlife Research 26: 389-403. Driscoll, D. A. and Lindenmayer, D. B. 2009. Empirical tests of metacommunity theory using an isolation gradient. - Ecological Monographs 79: 485-501. Everlegh, N. C. P., et al. 2001. The effects of reducing bird predation on canopy arthropods of marri (Eucalyptus calophylla) saplings on the Swan Coastal Plain, Western Australia. - Journal of the Royal Society of Western Australia 84: 13-21. Fortin, M.-J. and Dale, M. 2005. Spatial Analysis: A Guide for Ecologists. - Cambridge University Press. Griffin, G. F. 1990. Characteristics of three spinifex alliances in Central Australia. - Journal of Vegetation Science 1: 435-444. 169 Griffin, G. F. 1992. Will it burn - should it burn?: management of the spinifex grasslands of inland Australia. - In: G. P. Chapman (ed) Desertified Grasslands: Their Biology and Management. Academic Press, pp. 63-76. Gunawardene, N. R. and Majer, J. D. 2005. The effect of fire on ant assemblages in the Gibson Desert Nature Reserve, Western Australia. - Journal of Arid Environments 63: 725-739. Gunnarsson, B. 1990. Vegetation structure and the abundance and age size distribution of spruce living spiders. - Journal of Animal Ecology 59: 743 - 752. Gunnarsson, B. 2007. Bird predation on spiders: ecological mechanisms and evolutionary consequences. - Journal of Arachnology 35: 509-529. Hairston, N. G., et al. 1960. Community structure, population control, and competition. The American Naturalist 94: 421-425. Halaj, J., et al. 1998. Habitat structure and prey availability as predictors of the abundance and community organization of spiders in western Oregon forest canopies. - Journal of Arachnology 26: 203-220. Halaj, J., et al. 2000. Importance of habitat structure to the arthropod food-web in Douglasfir canopies. - Oikos 90: 139-152. Hatley, C. L. and MacMahon, J. A. 1980. Spider community organization: seasonal variation and the role of vegetation architecture. - Environmental Entomology 9: 632639. Heikkinen, M. W. and MacMahon, J. A. 2004. Assemblages of spiders on models of semiarid shrubs. - Journal of Arachnology 32: 313-323. Jeanneret, P., et al. 2003. Arthropod reaction to landscape and habitat features in agricultural landscapes. - Landscape Ecology 18: 253-263. Jones, M., et al. 2008. Explaining variation in tropical plant community composition: influence of environmental and spatial data quality. - Oecologia 155: 593-604. Langellotto, G. A. and Denno, R. F. 2004. Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. - Oecologia 139: 1-10. Langlands, P. R., et al. 2006. Spiders, spinifex, rainfall and fire: long-term changes in a community of desert spiders. - Journal of Arid Environments 67: 36-59. Langlands, P. R., et al. accepted. Is the successional trajectory of an arid spider assemblage following fire deterministic? - Austral Ecology 170 Legendre, P. 1993. Spatial autocorrelation: trouble or new paradigm? - Ecology 74: 16591673. Legendre, P. and Legendre, L. 1998. Numerical Ecology. Second English Edition. Elsevier Science B.V. Legendre, P. and Anderson, M. J. 1999. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. - Ecological Monographs 69: 1-24. Legendre, P., et al. 2009. Partitioning beta diversity in a subtropical broad-leaved forest of China. - Ecology 90: 663-674. Leibold, M. A., et al. 2004. The metacommunity concept: a framework for multi-scale community ecology. - Ecology Letters 7: 601-613. Letnic, M. and Dickman, C. R. 2006. Boom means bust: interactions between the El Niño/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia. - Biodiversity and Conservation 15: 3847-3880. Letnic, M., et al. 2004. The responses of small mammals and lizards to post-fire succession and rainfall in arid Australia. - Journal of Arid Environments 59: 85-114. Makarenkov, V. and Legendre, P. 2002. Nonlinear redundancy analysis and canonical correspondence analysis based on polynomial regression. - Ecology 83: 1146-1161. Manicom, C., et al. 2008. Self-made shelters protect spiders from predation. - Proceedings of the National Academy of Sciences 105: 14903-14907. Masters, P. 1993. The effects of fire-driven succession and rainfall on small mammals in spinifex grassland at Uluru National Park, Northern Territory. - Wildlife Research 20: 803-813. McArdle, B. H. and Anderson, M. J. 2001. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. - Ecology 82: 290-297. Moran, M. D. 2003. Arguments for rejecting the sequential Bonferroni in ecological studies. - Oikos 100: 403-405. Økland, R. H. 1999. On the variation explained by ordination and constrained ordination axes. - Journal of Vegetation Science 10: 131-136. Økland, R. H. 2003. Partitioning the variation in a plot-by-species data matrix that is related to n sets of explanatory variables. - Journal of Vegetation Science 14: 693-700. 171 Oksanen, J., et al. 2008. Vegan: Community Ecology Package. R package version 1.15-0. http://vegan.r-forge.r-project.org/. Owen, G. 2004. Conserving biodiversity in the rangelands: are land systems effective surrogates for spider assemblages? – Honours Thesis, Dept. Environmental Biology. Curtin University of Technology. Paine, R. T. and Vadas, R. L. 1969. The effects of grazing by sea urchins, Strongylocentrotus spp., on benthic algal populations. - Limnology and Oceanography 14: 710-719. Parris, K. M. 2004. Environmental and spatial variables influence the composition of frog assemblages in sub-tropical eastern Australia. - Ecography 27: 392-400. Peres-Neto, P. R., et al. 2006. Variation partitioning of species data matrices: estimation and comparison of fractions -Ecology 87: 2614-2625. Pianka, E. R. 1969. Habitat specificity, speciation, and species density in Australian desert lizards. - Ecology 50: 498-502. Pickett, S. T. and White, P. S. 1986. The Ecology of Natural Disturbance and Patch Dynamics. - Academic Press. Polis, G. A., et al. 1989. The ecology and evolution of intraguild predation: potential competitors that eat each other. - Annual Review of Ecology and Systematics 20: 297330. Polis, G. A., et al. 1998. Multifactor population limitation: variable spatial and temporal control of spiders on gulf of California islands. - Ecology 79: 490-502. Pozzi, S. and Borcard, D. 2001. Effects of dry grassland management on spider (Arachnida: Araneae) communities on the Swiss occidental plateau. - Ecoscience 8: 32-44. Quinn, J. F. and Dunham, A. E. 1983. On hypothesis testing in ecology and evolution. The American Naturalist 122: 602-617. Recher, H. F. and Majer, J. D. 2006. Effects of bird predation on canopy arthropods in wandoo Eucalyptus wandoo woodland. - Austral Ecology 31: 349-360. Robinson, J. V. 1981. The effect of architectural variation in habitat on a spider community: an experimental field study. - Ecology 62: 73-80. Sanderson, R. A., et al. 1995. Soil, vegetation and space: an analysis of their effects on the invertebrate communities of a moorland in North-East England. - The Journal of Applied Ecology 32: 506-518. 172 Schaffers, A., et al. 2008. Arthropod assemblages are best predicted by plant species composition. - Ecology 89: 782-794. Smith, G. T. and Morton, S. R. 1990. Responses by scorpions to fire-initiated succesion in arid Australian spinifex grasslands. - Journal of Arachnology 18: 241-244. Sokal, R. R. and Rohlf, F. J. 1995. Biometry: The Principles and Practice of Statistics in Biological Research. - W. H. Freeman and company. Sousa, W. P. 1984. The role of disturbance in natural communities. - Annual Review of Ecology and Systematics 15: 353-391. Spiller, D. A. and Schoener, T. W. 1988. An experimental study of the effect of lizards on web-spider communities. - Ecological Monographs 58: 57-77. Spiller, D. A. and Schoener, T. W. 1998. Lizards reduce spider species richness by excluding rare species. - Ecology 79: 503-516. Spiller, D. A. and Schoener, T. W. 2008. Climatic control of trophic interaction strength: the effect of lizards on spiders. - Oecologia 154: 763-771. Stafford Smith, D. M. and Morton, S. R. 1990. A framework for the ecology of arid Australia. - Journal of Arid Environments 18: 255-278. Turner, D., et al. 2008. An introduction to patterns of fire in arid and semi-arid Australia, 1998-2004. - The Rangeland Journal 30: 95-107. Uetz, G. A. 1979. The influence of variation in litter habitats on spider communities. Oecologia 40: 29-42. Uetz, G. W. 1991. Habitat structure and spider foraging. - In: S. S. Bell, et al. (eds), Habitat Structure: The Physical Arrangement of Objects in Space. Chapman and Hall, pp. 325348. Wan, S., et al. 2001. Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: a meta-analysis. - Ecological Applications 11: 1349-1365. Whittaker, R. H. 1956. Vegetation of the Great Smoky Mountains. - Ecological Monographs 26: 1-80. Wise, D. H. 1993. Spiders in Ecological Webs. - Cambridge University Press. 173 Table 1. a) The species richness of spiders (rarefied to 85 individuals) among post-fire ages as tested by a permutational analysis of covariance (PERMANOVA), with the spatial coordinate northing3 as the covariate. There was no significant interaction between the covariate and post-fire age (p = 0.89), therefore this term was not included in the final analysis. b) Pair-wise comparisons amongst fire ages with pseudo-t and p values (significant in bold). See Fig. 3. a Permutations Source df 3 b SS MS Pseudo-F p unique Northing 1 257.31 257.31 34.93 0.0001 9847 Post-fire age 5 211.19 42.24 5.73 0.0032 9952 Residual 20 147.35 7.37 Total 26 615.85 Post-fire age 0 0.5 3 5 8 t p 0.5 1.53, 0.212 3 3.21, 0.035 3.12, 0.047 5 1.78, 0.138 1.10, 0.328 1.56, 0.156 8 0.05, 0.967 0.22, 0.782 2.78, 0.037 1.47, 0.174 20 2.54, 0.055 3.55, 0.029 5.18, 0.004 3.94, 0.005 174 1.61, 0.121 Table 2. The adjusted R2 and relative percentage of the total variation explained in species richness of spiders by five groups of explanatory variables: Fire, Rainfall, Habitat, Floristics and Spatial location of sites. The individual variables representing each group and selected as having the highest explanatory power, are shown in brackets. No variables from Soil or Predators explained significant (p < 0.01) variation. Results from a simplification value of l < 0.05 (see methods). Partitioned components Variation explained R2 adj Relative % 0.08 10 Rainfall (January 07 , November 05 ) 0.06 8 Habitat (bare ground2) 0.06 8 Floristics (principal coordinate 5 and 8) 0.14 18 Spatial (northing ) 0.04 5 Fire, Rainfall, Floristics 0.12 15 Fire, Rainfall, Spatial 0.18 22 Fire, Rainfall, Habitat, Floristics, Spatial 0.11 14 Total 0.79 100 Fire (post-fire age dummy variables 3 and 5) 2 3 3 175 Table 3. a) The species composition of spiders among post-fire ages as tested by a permutational analysis of covariance (PERMANOVA), with the spatial coordinate northing3 as the covariate. The interaction between the covariate and post-fire age was not significant, but the p value was not sufficiently large (p > 0.25) to justify removing the term from the analysis (Sokal and Rohlf, 1995). b) Pair-wise comparisons amongst fire ages with pseudo-t and p values (significant in bold). See Fig. 5. a Permutations Source df 3 b SS MS Pseudo-F p Unique Northing 1 3901.7 3901.7 3.43 0.0001 9929 Post-fire age 5 14109.0 2821.8 2.48 0.0001 9847 Northing3 x Time since fire 5 6891.1 1378.2 1.21 0.0934 9812 Residual 15 17040.0 1136.0 Total 26 41942.0 Post-fire age 0 0.5 3 5 8 t p 0.5 1.57, 0.028 3 1.89, 0.001 1.26, 0.110 5 1.78, 0.007 1.51, 0.048 1.20, 0.166 8 1.58, 0.023 1.35, 0.114 1.42, 0.016 1.14, 0.203 20 1.96, 0.003 1.35, 0.073 1.68, 0.009 1.62, 0.019 176 1.50, 0.024 Table 4. The adjusted R2 and relative percentage of the total variation explained in the species composition of spiders by six groups of explanatory variables: Fire, Rainfall, Habitat, Floristics, Spatial location of sites and Predators. The individual variables representing each group and selected as having the highest explanatory power, are shown in brackets. No variables from Soil explained significant (p < 0.01) variation. Results using a simplification value of l < 0.05 (see methods). Partitioned components Variation explained R2 adj Relative % Fire (post-fire age dummy variables 0, 0.5, 3, 20 and age of surrounds) 0.063 20 Rainfall (February-April 2006) 0.008 2 Habitat (live spinifex, dead spinifex) 0.019 6 Floristics (principle coordinate 2) 0.020 6 Spatial (northing ) 0.016 5 Predators (reptile abundance, scorpion abundance) 0.009 3 Fire, Habitat 0.062 19 Fire, Habitat, Predators 0.034 11 Fire, Rainfall, Habitat, Floristics, Spatial 0.090 28 Total 0.32 100 3 177 2 Rainfall Spatial 11 11 Predators prey of spiders 9 Floristics 3 Soil 7 6 4 (birds, lizards & small mammals) 8 1 5 Habitat structure Fire 7 11 10 Spiders 12 parasitoids (wasps + flies) intraguild predation 12 Fig. 1. Simplified conceptual diagram of some of the key variables that can influence assemblages of spiders either directly (solid arrows) or indirectly (dashed arrows). A spatial box bounds all variables to signify that spatial processes can influence each variable or variables can generate spatial pattern. Arrow numbers indicate a selection of supporting references, see text for further details. Note, for clarity, we do not show all possible connections (e.g. the influence of rainfall on floristics or in mediating parasitoid populations). We did not assess the factors associated with grey arrows and lower case font in this study. 178 Fig. 2. The location of sites (*) within the proposed Lorna Glen (Matuwa) Conservation Park (inset: park location in Western Australia). Site names denote fire age in years followed by replicate number. Non-spinifex vegetation is shaded grey and lines mark unsealed roads. 179 a Species richness ±s.e. 40 30 spiders 20 plants 10 0 Proportion of ground cover ±s.e. b 1.0 0 3 5 8 20 5 5 5 5 n=7 4 3 0.8 bare ground 0.6 0.4 0.2 live spinifex 0.0 0 3 5 8 20 Post-fire age (years) Fig. 3. a) Mean species richness of spiders rarefied to 85 individuals and adjusted for the covariate northing3 with species richness of plants. b) Changes in mean proportion of bare ground and spinifex with post-fire age. Experimental fires indicated by arrow, with pre-fire vegetation samples for the seven sites burnt experimentally to the left of arrow. 180 Variation explained (%) 100 80 Richness R2adj = Composition Total variation explained 0.79 R2adj = 0.32 60 40 20 Fire Rainfall Habitat Floristics Predators Spatial Soil Fire Rainfall Habitat Floristics Predators Spatial Soil 0 Groups of explanatory variables Fig. 4. The percentage of the total variation explained in the species richness and composition of spiders by each of seven groups of explanatory variables. Shared patterns indicate components of variation explained by multiple groups (e.g. Fire, Rainfall or Floristics data explain equally well the component of variation in species richness represented by horizontal parallel lines). Solid black indicates variation unique to an explanatory group. The sum of the total variation explained by all the groups equals more than 100% due to shared components. For species richness, the variables selected within each group were as follows: Fire (post-fire age dummy variables 3 and 5), Rainfall (January 072, November 053), Habitat (bare ground2), Floristics (principal coordinate 5 and 8) and Spatial location of sites (northing3). For species composition, the variables selected were as follows: Fire (post-fire age dummy variables 0, 0.5, 3, 20 and age of surrounds), Rainfall (February-April 2006), Habitat (live spinifex, dead spinifex), Floristics (principle coordinate 2), Spatial location of sites (northing3) and Predators (reptile abundance, scorpion abundance). See Tables 2 and 4 for the values of each component. Soil variables did not explain significant (p < 0.01) variation in either species richness or composition. 181 d b R D A 2 ( 1 8 .9 % o f f itte d , 1 2 % o f to ta l v a r ia tio n ) 40 Live spinifex 20 8-38-2 5-3 5-4 3-1 8-4 0 20-1 8-1 2-3 ns S co rpio 8-5 5-1 5-5 20-2 20-5 20-4 Dead spinifex 20 5-2 Northing3 3-5 3-3 3 PCO 2 3-4 Age of surrounds 0.5 0.5-2 3-2 Reptiles -20 0.5-1 Feb 0 0.5-3 -A p 0-4 r 06 0-3 0-2 0-1 -40 -40 -20 0 20 40 dbRDA1 (25.1% of fitted, 15.9% of total variation) Fig. 5. Ordination from a distance-based RDA of species composition of spiders with study sites. Non bold numbers represent the post-fire age for each site. The environmental variables selected for variation partitioning are shown as multiple partial correlation vectors, with the length of the vector indicating the strength of the relationship between the variable and each axis, while taking into account the other variables. Fire (post-fire age dummy variables 0, 0.5, 3, 20, age of surrounds), Rainfall (February-April 2006), Habitat (live spinifex, dead spinifex), Floristics (principal coordinate 2), Predators (reptile abundance, scorpion abundance) and Spatial location of sites (northing3). 182 Appendix A The post-fire age of study sites with location (latitude and longitude) and the date when last burnt. Post-fire age Location Number 26°S 121°E Date Burnt 0 1 21' 43'' 25' 27'' 27 April 2006 0 2 15' 51'' 06' 12'' 26 April 2006 0 3 16' 13'' 06' 02'' 28 April 2006 0 4 21' 26'' 14' 14'' 27 April 2006 0.5 1 21' 26'' 24' 50'' 11 October 2005 0.5 2 13' 58'' 11' 48'' 9 October 2005 0.5 3 21' 42'' 25' 16'' 14 October 2005 3 1 14' 12'' 17' 00'' 25 November 2002 3 2 09' 35'' 16' 46'' 25 November 2002 3 3 11' 58'' 18' 10'' 25 November 2002 3 4 12' 13'' 19' 42'' 25 November 2002 3 5 08' 45'' 16' 05'' 25 November 2002 5 1 16' 13'' 02' 34'' 8 January 2001 5 2 16' '52' 21' 42'' 8 January 2001 5 3 17' 03'' 11' 43'' 8 January 2001 5 4 21' 24'' 13' 29'' 8 January 2001 5 5 11' 00'' 02' 36'' 15 November 2000 8 1 20' 15'' 26' 58'' 23 December 1997 8 2 15' 22'' 07' 07'' 1 February 1998 8 3 13' 33'' 11' 36'' 1 February 1998 8 4 16' 41'' 21' 12'' 1 February 1998 8 5 11' 57'' 17' 47'' 1 February 1998 20 1 16' 00'' 06' 19'' before 1988 20 2 14' 01'' 11' 35'' before 1988 20 3 21' 22'' 25' 06'' before 1988 20 4 21' 39'' 14' 11'' before 1988 20 5 11' 05'' 02' 11'' before 1988 183 Appendix B Excel file with calculations used to simplify results from variation partitioning, following the method of Økland (2003). See enclosed CD. 184 Species Richness (rarefied 85 inds) Env. Factors selected by Distlm, step-wise, in PERMANOVA, P<0.01, 9999 perms Groups F = fire age X = Spatial R = Rainfall H = Habitat V = Vegetation (floristics) step No. RDA Variables 3, 5 y3 Jan07 2, Nov05 3 BG2 PCO5, PCO8 unique partial union VE intersection VE 0.01878 0.0015 -0.0058 -0.0015 0.06733 constraining F X R H V partial XRHV RHVF HVFX FXRV FXRH 6 7 8 9 10 11 12 13 14 15 FX FR FH FV XR XH XV RH RV HV RHV HVX VXR XRH HVF VFR FRH XFV XFH XFR 0.02801 0.05412 0.04903 0.07044 0.03238 0.00095 0.08148 -0.0113 0.10886 0.11867 0.00773 0.04114 0.03175 -0.01567 0.03668 0.00095 0.01265 -0.004 0.04733 0.05284 3 3 3 3 3 3 3 3 3 3 16 17 18 19 20 21 22 23 24 25 XFR XFH XFV XRH XRV XHV FRH FHV RHV FRV HV RV RH FV FH FR XV XR XF XH 0.26186 0.06364 0.08795 0.02552 0.15123 0.14395 0.07109 0.18131 0.14412 0.24422 0.16183 0.00443 -0.00437 -0.00231 -0.00846 0.01018 -0.00928 0.02778 -0.01208 0.09111 4 4 4 4 4 26 27 28 29 30 XFRH XRHV XHVF FRHV XFRV V F R X H 0.2897 0.18995 0.22454 0.39365 0.50043 0.0078 -0.00536 0.01016 0.06392 0.04865 5 31 XFRHV 0.78818 0.11247 1 1 1 1 1 1 2 3 4 5 2 2 2 2 2 2 2 2 2 2 L = <0.05 0.11 Distributed 3rd 5 31 XFRHV 0.11247 4 4 4 4 4 26 27 28 29 30 XFRH XRHV XHVF FRHV XFRV V F R X H 0.0078 -0.00536 0.01016 0.06392 0.04865 3 3 3 3 3 3 3 3 3 3 16 17 18 19 20 21 22 23 24 25 XFR XFH XFV XRH XRV XHV FRH FHV RHV FRV HV RV RH FV FH FR XV XR XF XH 0.16183 0.00443 -0.00437 -0.00231 -0.00846 0.01018 -0.00928 0.02778 -0.01208 0.09111 0.175943 0.00892 0.010333 -0.0017 0.002363 0.01138 0.00865 0.0463 0.00256 0.119253 2 2 2 2 2 2 2 2 2 2 6 7 8 9 10 11 12 13 14 15 FX FR FH FV XR XH XV RH RV HV RHV HVX VXR XRH HVF VFR FRH XFV XFH XFR 0.00773 0.04114 0.03175 -0.01567 0.03668 0.00095 0.01265 -0.004 0.04733 0.05284 0.014148 0.044023 0.05304 0.003207 0.039874 0.004177 0.020675 -0.00083 0.048971 0.07292 1 1 1 1 1 1 2 3 4 5 F X R H V XRHV RHVF HVFX FXRV FXRH 0.01878 0.0015 -0.0058 -0.0015 0.06733 2nd After = 1st 0.00195 -0.0013 0.00254 0.01598 0.01216 0.00195 -0.0013 0.00254 0.01598 0.01216 0.00195 0.00195 0.00254 0.00195 -0.0013 -0.0013 0.00195 0.00254 -0.0013 0.01598 0.01216 0.00254 0.01216 -0.0013 0.01216 0.00254 0.01598 0.01598 0.01598 0.01216 0.003 0.0034 -6E-04 0.0008 0.0038 0.0029 0.0154 0.0009 0.14 0.1759 0.22 0.1193 0.15 0.0760 0.0409 0.0617 0.0617 0.1402 0.096 0.052 0.078 0.078 0.178 0.003 0.0034 -6E-04 0.0008 0.0038 0.0029 0.0154 0.0009 0.003 0.0034 0.0029 0.0029 0.0154 0.0034 0.0154 0.003 -6E-04 -6E-04 0.0038 0.0034 0.0008 -6E-04 0.0029 0.0008 0.0009 0.0038 0.0154 0.0071 0.0071 0.0235 0.025 0.0016 0.1125 0.0235 0.0199 0.0199 0.0021 0.0103 0.0071 0.022 0.0265 0.0016 0.0199 0.0021 0.0103 -4E-04 0.0245 0.0365 0.003 0.0008 0.0038 0.0009 0.0009 0.025 0.0021 -4E-04 -4E-04 0.0245 0.0016 0.0103 0.0245 0.0365 0.0365 0.0071 0.0235 0.025 0.0016 0.0199 0.0021 0.0103 -4E-04 0.0245 0.0365 Appendix C The Spearman correlation between selected variables (bold) and other variables. Species Richness Rho Species composition Jan 20072 Rainfall Rainfall Feb-Apr 2006 2 0.73 Feb-Jul 2006 1.00 Feb-Jul 20062 0.68 Apr 2006 0.83 0.67 3 0.79 3 Feb 2006 Feb-Jul 2006 Feb-Apr 2006 2 Feb-Apr 2006 Feb-Apr 2006 0.66 0.65 Feb-Jul 2006 Mar 2006 0.78 0.72 Feb 20063 0.64 Nov-Jan 20073 0.71 Feb-Jul 20063 0.64 Feb 20063 0.71 3 Feb-Apr 2006 Aug 2005-Apr 2006 0.64 0.63 3 Apr 2006 Feb 2006 0.70 0.69 Nov-Jan 20073 0.61 Feb 20062 0.69 0.61 2 Jan 2007 Jan 2007 0.65 0.63 Aug 2005-Apr 20063 0.63 Aug 2005-Apr 2006 3 Nov 20053 2 Nov 2005 Nov 2005 0.97 0.88 Habitat live spinifex Sept 2006 3 Sept 2006 2 0.71 Aug-Oct 2006 3 -0.66 upper storey woody shrub 0.69 Sept 2006 -0.63 2 -0.63 3 -0.62 3 -0.61 Jul 2006 Jan 2006 -0.72 live spinifex live spinifex upper storey woody shrub dead spinifex 0.71 0.67 woody shrub3 -0.67 dead spinifex 3 0.63 dead spinifex 2 0.61 live spinifex 2 0.60 -0.71 dead spinifex2 0.92 3 0.92 3 -0.71 2 0.67 dead spinifex -0.76 2 0.90 3 dead spinifex bare ground woody shrub 3 bare ground 2 Habitat Spatial Rho bare ground bare ground Predators Reptile abundance Reptile species Spatial northing3 northing 0.93 185 0.61 northing3 northing 0.93 Appendix D The ten plant species most correlated with the three floristics axes that were selected as significant variables for variation partition of species richness or species composition of spiders. Axes were generated using principle coordinate analysis (PCO) of floristic data. PCO Species Species richness PCO 5 -0.59 Acacia aneura -0.55 Ptilotus sessilifolius -0.45 Kennedia prorepens 0.42 Eucalyptus gongylocarpa -0.41 Solanum centrale 0.39 Genus 1 sp.46 0.36 Glischrocaryon aureum 0.36 Euphorbia boophthona 0.36 Eucalyptus lucasii -0.35 Codonocarpus cotinifolius PCO 8 0.54 Halgania cyanea 0.50 Acacia burkittii 0.46 Ptilotus obovatus 0.41 Spartothamnella teucriiflora 0.38 Acacia grasbyi -0.38 Goodenia triodiophila 0.38 Hakea rhombales -0.35 Solanum sturtianum -0.33 Glischroncaryon aureum -0.33 Goodenia mueckeana Species composition PCO 2 -0.76 Solanum lasiophyllum -0.62 Euphorbia boophthona -0.58 Codonocarpus cotinifolius -0.53 Dicrastylis brunnea -0.53 Triodia basedowii -0.53 Aristida contorta -0.52 Abutilon otocarpum -0.45 Eragrostis lanipes 0.45 Aluta maisonneuvei -0.45 Eragrostis eriopoda Family Fabaceae Amaranthaceae Papilionaceae Myrtaceae Solanaceae Poaceae Haloragaceae Euphorbiaceae Myrtaceae Gyrostemonaceae Boraginaceae Fabaceae Amaranthaceae Lamiaceae Fabaceae Goodeniaceae Proteaceae Solanaceae Haloragaceae Goodeniaceae Solanaceae Euphorbiaceae Gyrostemonaceae Lamiaceae Poaceae Poaceae Malvaceae Poaceae Myrtaceae Poaceae 186 CHAPTER 4 Predicting the post-fire responses of animal assemblages: testing a trait-based approach using spiders Peter R Langlands Karl E C Brennan Volker W Framenau Barbara Y Main Formatted for the Journal of Animal Ecology 187 Summary 1. Developing a predictive understanding of how species assemblages respond to fire is a key conservation goal. In moving from solely describing patterns following fire to predicting changes, plant ecologists have successfully elucidated generalisations based on functional traits. Using species-traits might also allow better predictions for fauna but there are few empirical tests of this approach. 2. We examined whether species traits changed with post-fire age for spiders in 27 sites, representing a chronosequence of 0-20 years post-fire. We predicted a priori whether spiders with ten traits associated with survival, dispersal, reproduction, resource-utilization and microhabitat occupation would increase or decrease with post-fire age. We then tested these predictions using a direct (fourth-corner on individual traits and composite traits) and an indirect (emergent groups) approach, comparing the benefits of each and also examining the degree to which traits were inter-correlated. 3. For the seven individual traits that were significant in the fourth-corner analysis, three followed predictions (body size, abundance of burrow ambushers and burrowers was greater in recently burnt sites); two were opposite (species with heavy sclerotisation of the cephalothorax and longer time to maturity were in greater abundance in long unburnt and recently burnt sites respectively); and two displayed response patterns more complex than predicted (abdominal scutes displayed a U-shaped response and dispersal ability a hump shaped curve). However, within a given trait, there were few significant differences among post-fire ages. 4. Several traits were inter-correlated and scores based on composite traits used in a fourthcorner analysis found significant patterns, but slightly different to those using individual traits. Changes in abundance with post-fire age were significant for three of the five emergent groups. The fourth-corner analysis yielded more detailed results, but overall we consider the two approaches complementary. 5. While we found significant differences in traits with post-fire age, our results suggest that a trait-based approach may not increase predictive power, at least for the assemblages of spiders we studied, as shown for plants. That said, there are many refinements to faunal traits that could increase predictive power. 188 Introduction Ecologists have long sought to understand and predict the consequences of disturbances (flooding, grazing, habitat fragmentation, fire etc.) on ecosystems and assemblages of plants and animals (Connell 1978; Lavorel et al. 1997). A formidable challenge is elucidating how individual species of plants and animals might respond to a particular disturbance, especially for highly diverse groups, where taxonomic knowledge is poor and/or there is an absence of knowledge on the autecology of individual species. Therefore, studying solely the responses of individual species is unlikely to lead to a rapid increase in predictive power for unstudied species because it only generates location and taxa-specific information (Weiher & Keddy 1995). However, an understanding of which traits determine a species‟ response to disturbance may lead to generalizations, which can predict how other species with the same traits will respond (Lavorel & Garnier 2002; McGill et al. 2006). We define a trait following McGill et al. (2006) as “a well-defined, measurable property of organisms, usually measured at the individual level and used comparatively across species”. Ideally, we might hope to be able to collect an animal from any locality and from only its morphological and behavioural traits predict the species‟ change in abundance following disturbance. A species-traits approach has yielded impressive advances in increasing predictive power for plants (Shipley, Vile & Garnier 2006; Keith et al. 2007). A prominent example is the marked distinction in post-fire responses between seeders versus resprouters (Noble & Slayter 1980). However, correlations between traits cause most species to occur only within a limited portion of the total multidimensional trait-space (Hubbell 2001). Therefore, to develop a better mechanistic understanding of post-fire responses it is important to identify groups of traits that are inter-correlated and might be functional (strongly influence organismal performance). The adoption of a trait-based approach for fauna and fire lags behind that for plants (notable exceptions are Jonas & Joern 2007; Moretti et al. 2009; Moretti & Legg 2009). Using previous approaches for fire, we adapt the vital attributes (Noble & Slatyer 1980) and critical life cycles approaches (Whelan et al. 2002) to classify traits under five categories: survival, dispersal, reproduction, resource-utilization and microhabitat occupation. The interest of our search is convergent traits (Grime 2006; Pillar et al. 2009). That is, due to environmental filters, the species within a community and the traits they 189 possess are more similar or under-dispersed (Weiher & Keddy 1995). We use current theories and functional understandings (existing mechanisms) of traits to predict how each trait might respond with increasing post-fire age. We then compare these qualitative predictions to the observed results. Several methods explore potential links between species-traits and environmental conditions. These can either test the link indirectly (e.g. emergent groups), or directly (e.g. fourth-corner or RLQ analysis). Emergent groups are suites of species selected for sharing similar traits (Lavorel et al. 1997). The fourth-corner is an approach that combines information on species traits and environmental characteristics via a matrix of species abundances. While RLQ analysis is complementary to the fourth-corner, it focuses primarily on ordination and interpretation, rather than testing the significance of relationships (Dolédec et al. 1996; Legendre, Galzin & Harmelin-Vivien 1997). Most applications use an indirect approach, partly due to the initial limitation of the fourth-corner to presence-absence data only. However, the fourth-corner approach now incorporates abundance data and may yield additional insights compared to emergent groups (Aubin et al. 2009). That said, emergent groups easily incorporate links between traits, but for the fourth-corner this remains an outstanding problem (Dray & Legendre 2008). Inclusion of linked traits into fourth-corner analyses may increase the chance of type I error (Aubin et al. 2009) and ignores biological reality. Ultimately, a direct approach that can incorporate linked traits is required. In the interim, however, it is unclear whether researchers should follow a direct or indirect approach, as only a single study has explicitly compared these approaches for the same data (see Aubin et al. 2009). Despite the usefulness of the approaches described above, some problems remain. Neither the fourth-corner or emergent groups can yet incorporate potential spatial autocorrelation of sites or phylogenetic signal between species (See Dray & Legendre 2008, p.3411). For faunas that are poorly known taxonomically and where autecological information is scant, there will be a reliance on inferring behavioural traits at higher taxonomic levels using information gleaned from related taxa. Thus, greater caution will be required when considering behavioural traits compared to morphological traits measured directly from each taxon. Even with these unresolved issues, however, the fourth-corner and emergent groups approaches represent the best techniques currently available and offer new insights into trait-environment relationships. As such, and because the search for a 190 responsive set of traits for fauna is still in its infancy, to assist future workers we have included both morphological and behavioural traits. Spiders are an interesting group for considering linkages between post-fire environments and traits because they show marked changes in species composition with fire (Buddle, Spence & Langor 2000; Brennan et al. 2006; Langlands, Brennan & Pearson 2006) and possess a diverse array of potentially influential traits. Spiders represent a range of body sizes; our species differed by an order of magnitude (0.6-9.4 mm). While spiders are unable to fly, many families possess the ability to drift passively (“ballooning”) by using extensions of silk (Bell et al. 2005). Whilst spiders generally have soft abdomens, some species also possess morphological adaptations that reduce desiccation, such as the presence of hard plates (scutes) on the abdomen or heavy sclerotisation of the cephalothorax (Main 1984). While almost all spiders are obligate carnivores, there is a vast array of different hunting strategies, which have attracted interest as feeding guilds (Wise 1993; Uetz, Halaj & Cady 1999). Additionally, while many have a broad diet others show increasing specialization. For example, some species are specialist predators of ants (myrmecophagous) or other spiders (araneophagous) (Polis & Yamashita 1991). In this paper, we examine if the species-traits of spiders can make better predictions of post-fire responses using both the fourth-corner and emergent groups approaches. We address four questions: 1) Are individual traits of spiders linked with post-fire age? In short, we hypothesized that the abundance of spiders with the following traits would decrease with increasing post-fire age: larger body size, heavy sclerotisation (cephalothorax plus abdominal scutes), greater dispersal ability (ballooning) and using burrows. We hypothesized the abundance of spiders with the traits of slow maturation, short seasonal activity, diet specialization and a flattened body would increase with post-fire age. In our methods, we describe how each of these traits might act to structure assemblages of spiders following disturbance. 2) If significant relationships with traits are found, are these traits inter-correlated? and if so, 3) Do combinations of these inter-correlated traits have significant associations with post-fire age? Finally, 4) Are emergent groups of spiders linked with post-fire age and what additional insights does the fourth-corner approach offer over this method? 191 Materials and Methods STUDY LOCATION AND DESIGN The study site was the proposed Lorna Glen (Matuwa) Conservation Park in central Western Australia (26°13'33" S, 121°33'28" E). Satellite images were used to select sites representing six treatments of post-fire ages: zero (4 sites), six months (3 sites), three years (5 sites), five (5 sites), eight (5 sites) and twenty plus (5 sites) years since the last fire. See Appendix S1 and Langlands et al. (accepted) for further details of study location, sites and fires. SAMPLING AND IDENTIFICATION OF SPIDERS Ground active spiders were sampled continuously over a nine-month period (30 April 2006-18 January 2007) using five pitfall traps (2 L jars with a mouth of 82 mm) per site. Traps were exchanged every three months. Male spiders were identified to species level. Due to taxonomic difficulties of correctly matching females and juveniles to males for many of the undescribed taxa, the results presented here are only for males. All specimens are deposited in the Western Australian Museum. TRAITS We scored morphological and behavioural traits where there was a potential mechanism to explain post-fire responses. As noted in the introduction, owing to limited autecological information, behavioural traits were inferred at the family or sub-family level. We included only behavioural traits that we were confident had sufficient information available for such inferences. As we measured morphological traits directly from the specimens, these data are robust and independent of whether they have been named by taxonomists. We classified these traits into five key categories: survival, dispersal, reproduction, resource-utilization and microhabitat occupation. The placement of traits within these categories is not mutually exclusive because burrowing could be placed under survival or microhabitat, but we consider the categories a useful framework on which to build. Those working with spiders in other areas or indeed other groups may find that some traits such as flat bodies specialized to live under eucalypt bark are not relevant, but the categories may 192 help to find other relevant traits. A summary of each trait follows with a full list of species and traits in the online supplementary material (Table 1, Appendix S2). Survival Body size (measured as carapace length): Having soft abdomens, spiders are vulnerable to desiccation, particularly small spiders (Main 1984). As body size increases, the surface area to volume ratio decreases. Therefore, the body size of arthropods was predicted to increase with aridity (Remmert 1981), as shown for spiders in Europe (Entling et al. 2010). We hypothesised that species with a larger body size and therefore a decreased surface area to volume ratio, may be better able to cope with the greater variability in temperature and humidity associated with recently burnt habitats. Indeed, the large body size of lycosids might be an adaptation to withstand larger temperature variation in open habitats (Coyle 1981). As an estimate of body size for each species, we measured the mean carapace length of five individuals. Abdomen length was not included as spider abdomens are soft and can change greatly in size when preserved. Burrowing: The intensity of heat from fires decreases rapidly with increasing soil depth (Whelan 1995). Some species of spiders can construct burrows in the ground, creating a microhabitat that shields them from direct disturbance by fire. Species exhibiting this behaviour might have a greater chance of surviving the passage of fire relative to nonburrowing species and therefore occur in greater abundance in recently burnt areas. Degree of sclerotisation: The development of thick plates or shields on soft body parts is considered an adaptation which reduces water loss, particularly for small-bodied species which, when compared with larger bodied species, have a higher surface area to volume ratio (Main 1984). We scored two traits for sclerotisation. Heavy sclerotisation of the cephalothorax (head-thorax) was scored as present (the cephalothorax was a rigid case, often pitted and dark red) or absent. Secondly, we scored the presence of scutes (hard plates) on either the dorsal or ventral side of the abdomen as absent, partial coverage or full coverage. 193 Dispersal Ballooning: The ability to disperse would be advantageous in colonising recently disturbed habitats. Previous studies of spiders and some insect groups show that dispersal ability is important for re-colonising disturbed sites (Crawford, Sugg & Edwards 1995; Moir et al. 2005). Many species of spiders can disperse over large distances by ballooning, releasing silk threads as juveniles to catch wind currents while others can disperse only by walking (Main 1984). Species were scored as ballooners if there was a published record of ballooning for the genus or family (Bell et al. 2005). While species may possess the ability to balloon, the distance can vary greatly along a continuum from only a few meters to many kilometres (Bell et al. 2005). To simplify the analysis, we treated ballooning as a binary response. Reproduction Time to maturity: Species can be divided into two broad life history categories, r- or Kselected (Southwood 1977). Although not without limitations, this can provide a useful dichotomy of the life history of species (Polis & Yamashita 1991). One aspect of these categories is time to maturity, short for r- selected and long for K- selected species. Species with short maturity times would have an advantage in colonising and reproducing in recently disturbed areas. The time taken to mature can vary widely between species of spiders from one to several years (Main 1984). To differentiate between species we scored maturation time as less than or greater than 3 years. We did not attempt a finer separation in time to maturity ages due to the lack of natural history information. Phenology: The reproductive activity of spiders can be highly seasonal with males sexually mature and actively searching for females for limited periods (Merrett 1967; Framenau & Elgar 2005). Fire may disadvantage those species with highly specific environmental triggers for mating activity compared with species that can mate anytime throughout the year. As a crude measure of phenology, species were scored according to the length of time over which they were collected: three, six or nine months. 194 Resource-utilization Hunting strategy: Spiders are almost all obligate predators, but the strategies they use to catch prey differ substantially. Some are active hunters, others may sit-and-wait, while many use different types of webs to capture prey (Turnbull 1973; Wise 1993). We used taxonomic literature and expert opinion to allocate species to one of five hunting strategies: active hunters, sit-and-wait hunters, burrow-dwelling sedentary hunters, terrestrial webs and aerial webs. Diet specialization: While as a group spiders are viewed as polyphagous predators, some have specialised feeding habits (Marc & Canard 1997). For example, zodariids are specialist ant predators (Jocqué 1991). Species with restricted diets may be limited to habitats that support suitable supply of the required food. Hence, diet specialization may increase with time since disturbance. We scored species that are known ant specialists as a binary trait. We did not score species that mimic ants as potential predators of ants, because mimicry can also evolve to evade predators (see Cushing 1997). Microhabitat Flattened body: Several groups of Australian spiders exhibit an extremely flattened body shape, which might have evolved with the rapid speciation of Eucalyptus trees and the crevice habitat provided under the peeling bark (Żabka 1991). In instances where the bark is thick and fire intensity is low, by sheltering under bark some spiders might survive the passage of fire (Brennan, Moir & Wittkuhn in press). However, for species of eucalypt with thin bark (or for trees with thicker bark but where fire intensity is high), most of the bark may be consumed by the fire or slough off in ensuing months. Thus, there may be limited microhabitat for crevice-dwelling spiders for several years. At our study site, fire resulted in a loss of bark crevices and so a flattened body might not confer a survival advantage during burning and the lack of suitable crevices post-fire may inhibit recolonization. STATISTICAL ANALYSIS To test the link between environmental conditions and individual spider traits directly, we used the fourth-corner analysis (Legendre, Galzin & Harmelin-Vivien 1997; Dray & 195 Legendre 2008). This analysis uses three data matrices (Matrix R: environment by sites, Matrix L: species by sites, Matrix Q: species by traits) to test directly the link between environmental characteristics and species-traits via the observed abundance of species (Appendix S3). We used the two-stage permutation test recommended by Dray & Legendre (2008), which combines the results from permutation models 2 and 4 (see Dray & Legendre 2008: Figures 2 & 3). Previous work indicated that the post-fire age of sites in years was a good predictor of the spider community (Langlands et al. accepted); we therefore chose this variable to represent the matrix R (environmental characteristics). Matrix R was coded as dummy variables following Aubin et al. (2009), which allows for non-linear patterns with increasing post-fire age. Matrix L was represented by a matrix of abundances of spiders that were fourth root transformed to reduce the dominance of abundant species. A matrix of the ten traits scored for each species represented Matrix Q, all traits were coded as qualitative variables except for body size, which was quantitative. Analyses were conducted using the „fourthcorner‟ function with 9999 permutations in the R package ade4 (Dray & Dufour 2007). To examine which traits were linked, we calculated a similarity matrix between all species based on the ten traits. We used the Gower coefficient because it can incorporate both quantitative and qualitative variables. A principal coordinate analysis (PCoA) was then employed on the resulting matrix to simplify the inter-correlated traits to a reduced number of axes. We chose this method as it allows any similarity measure. To relate the resulting axes back to the original traits we used vector overlays of the correlation between axes and traits. To test the link between environment and combined traits directly, and address the inability of the fourth-corner to incorporate linked traits, we devised the following approach. We incorporated the axis scores for each species from the PCoA into a fourthcorner analysis as matrix Q (Appendix S3). We recognise that this method, although theoretically sound, is somewhat crude as it contains multiple steps. We again used the two-stage permutational test (models 2 & 4). To test the link between environmental conditions and traits indirectly we used emergent group analyses based on the similarity matrix constructed from the ten traits (Appendix S3). This method is indirect as it requires two steps (Dray and Legendre 2008). Traits and species abundances (matrices Q & L) are first linked, and then related to 196 environmental conditions (Matrix R). Ward‟s method was used to cluster species and the resulting dendrogram was arbitrarily split into five emergent groups. The abundance of species within each emergent group was then summed for each site. Permutational analysis of variance (PERMANOVA) was then used to test for significant differences in the abundances of groups between post-fire ages. PERMANOVA is highly robust as it uses permutation for significance testing and any dissimilarity measure may be used (Legendre and Anderson 1999). We used Euclidean distance with unrestricted permutation of the raw data, type III sums of squares and 9999 permutations. As argued by Moran (2003), we have not corrected for multiple analyses. While none of the above methods can currently incorporate spatial autocorrelation, we have shown elsewhere that spatial location explained less than 4% of the variation in species richness or composition of our spider assemblages (Langlands 2010). Analyses were performed using PERMANOVA+ (Anderson, Gorley & Clarke 2008, version 1.01) and R (R Development Core Team 2009). Results Our dataset comprised 4234 male specimens representing 179 species of spider from 24 families, of which only 32 species (=17.9%) had scientific names. Previous analyses indicated significant differences in the species composition of spiders with increasing postfire age (Langlands et al. accepted, Appendix S4). Matching these differences in species composition, were shifts in the abundance of species with particular traits among post-fire ages. The fourth-corner analysis showed significant links between post-fire age and spiders for seven of the ten traits measured (Table 1). There were significant results within all of the groups of traits except microhabitat occupation. There were more large-bodied individuals at recently burnt sites (0-year-old) (Fig. 1a). Abundance of species with heavy sclerotisation of the cephalothorax increased in 8-year-old sites and was significantly higher in 20-year-old sites (Fig. 1b). Abdominal scutes displayed a U-shaped pattern with significantly fewer scutes in 5-year-old sites and high abundances in recently burnt and long unburnt sites (Figs. 1c). The abundance of spiders that can disperse via ballooning was lowest in 0 aged sites and highest in 5-year-old sites (Fig. 1d). There were significantly more long-lived individuals in 0-year-old sites, compared to all other ages (Fig. 1e). Likewise, both burrow-dwelling sedentary hunters and burrowing spiders were in significantly greater abundance in 0 aged sites compared to all other ages (Figs. 1f, g). 197 However, within a particular trait there was usually only one post-fire age that differed significantly from all others. The first three axes of the principle coordinate analysis (PCoA) explained 91.7% of the variation between species based on the ten traits. High values along axis 1 were associated with the presence of partial abdominal scutes, an active hunting strategy and to a lesser extent, a more specialized diet (ants), and not using burrows (Fig. 2, Appendix S5). High values along axis 2 were strongly associated with high dispersal ability (ballooning) and to a lesser extent hunting from terrestrial webs. Axis 3 was associated with phenology. Plotting of unique vectors for each trait onto the PCoA ordination showed that ballooning and hunting using a terrestrial web were strongly inter-correlated (Fig. 2). Similarly strongly inter-correlated were heavy sclerotisation of the cephalothorax, diet specialization on ants, the presence of full abdominal scutes and active hunting. Correlated to a lesser extent were body length, time to maturity, and burrowing Ordination also showed a clear influence of phylogeny as many species within a family clustered together in trait space. For example, five of the six species of Nemesiidae clustered very tightly in the lower left corner of the ordination (Fig. 2). Other families such as the Zodariidae clustered together more loosely in trait space. Using the PCoA axes in a fourth-corner analysis found PCoA axes 1 and 2 to be highly significant with the 0-year-old sites (Table 2). The negative correlation of 0-year-old sites with axis 1 indicated that recently burnt sites had a greater abundance of species without abdominal scutes and a lesser abundance of species with partial abdominal scutes or an active hunting strategy. It also indicated to a lesser extent that 0-year-old sites had greater abundance of species using burrows and a lesser abundance of species with a specialized diet (ants) (Appendix S5). For axis 2 there was a positive correlation; hence the 0-year-old sites had fewer individuals that disperse via ballooning. For the indirect analysis, five emergent groups of species chosen from the cluster analysis contained 12 to 65 species each. Several families had species within multiple groups (Table 3, Appendix S6). Subsequently, permutational analysis of variance (PERMANOVA) found abundances of only three of the groups displayed significant responses to post-fire age (Groups III, IV & V, Fig. 3). Group III consisted of small to large, active hunters, which were mostly myrmecophageous and non-ballooning, with a complex pattern in abundances with post-fire age (d.f. 5, 21; pseudo-F = 2.89; p = 0.04). 198 Group IV were small, active hunters with full scutes, predominately Oonopidae, and were more abundant in recently burnt and long unburnt sites (d.f. 5, 21; pseudo-F = 5.17; p = 0.004). Group V consisted mainly of mygalomorphs and lycosids, which were more abundant in 0-year-old sites (d.f. 5, 21; pseudo-F = 6.93; p = 0.002, Fig. 3). Discussion We found significant patterns in the species-traits of spiders with post-fire age, namely recently burnt sites (0-years-old) had more individuals with a large body, limited dispersal ability (i.e. presumed unable to balloon), burrowing habit and an ambushing hunting strategy from burrows. Although a majority (7 of 10) of our traits differed among post-fire ages, within traits there were few significant differences between post-fire ages. In four instances out of seven, only the recently burned sites differed from all other ages (Fig. 1). Furthermore, the patterns of difference did not consistently match our predictions. Of the seven spider traits that were significantly different among post-fire ages, three followed predictions (body size, hunting strategy and burrowing behaviour), two were opposite (heavy sclerotisation of cephalothorax & time to maturity) and two displayed more complicated response patterns than predicted (abdominal scutes & ballooning). Given the limited research effort into the trait-based approach for fauna and fire, it is not surprising that our results are not as clear as found recently for plants (e.g. Keith et al. 2007; Aubin et al. 2009). ARE INDIVIDUAL TRAITS LINKED WITH POST-FIRE AGE? Survival All four traits within the survival category had significant links with post-fire age; however, as noted above, only two of these followed predictions. Body size was expected to influence survival via differences in desiccation rate, with larger bodied spiders having an advantage in recently burnt and open habitats. In Europe, the body size of female Linyphiidae increased with increasing disturbance from flooding; however, Lycosidae were larger in less disturbed sites (Lambeets et al. 2008). However, body size often correlates with other traits (Peters 1983; Gaston & Blackburn 2000), and in our study it was correlated with burrowing. It is therefore unclear whether body size or burrowing is functional. 199 The response of spiders with sclerotisation of the cephalothorax was opposite to the prediction of greater sclerotisation immediately post-fire. Thus, the mechanism for heavy sclerotisation of the cephalothorax and abdomen also requires attention. It is unclear why there would be more spiders with this trait in long unburnt sites. The response of abdominal scutes was similar with high abundance in long unburnt sites, but there was also relatively high abundance in recently burnt sites. Most of the species with full scutes were from the Oonopidae, which are leaf-litter dwellers. At our study sites the proportion of ground covered by leaf-litter followed a U-shaped pattern and remained high in the recently burnt sites as scorched trees shed leaves and bark (Langlands unpublished data). Oonopid spiders selecting post-fire ages with more leaf-litter may explain why spiders with full scutes were more common in recently burnt and long unburnt sites. If so, the reduction in desiccation that spiders receive from possessing full scutes may not act directly to influence changes in the composition of species of spiders with post-fire age. Dispersal Contrary to our predictions, and to previous studies of primary and secondary successions, we found spiders that had greater dispersal ability via ballooning most abundant in 5-yearold sites, rather than those burnt recently. Although clearly different to disturbance by fire, ballooners dominated early colonists during primary succession following glaciations and volcanic eruptions (Crawford, Sugg & Edwards 1995; Hodkinson, Webb & Coulson 2002), and for two spider families in areas frequently disturbed by flooding (Lambeets et al. 2008). Perhaps this inconsistency relates to two things. Firstly, although we were able to discriminate between ballooning and non-ballooning species, we were unable to consider differences in ballooning or walking ability. Secondly, patch size might play an important role in determining the influence of traits associated with dispersal ability. Logistical constraints meant that our recently burnt sites were only 300 by 300 m, so dispersal by walking may have been sufficient for colonization. It may be beneficial to incorporate the walking abilities of non-ballooning species. Reproduction Time to reach maturity was also opposite to our predictions with a very high abundance of long-lived species in recently burnt sites. This might be because males from long-lived burrowing species (Nemesiidae) survived the fire in situ and then left to find mates and fell into our pitfall traps. This possibly indicates a degree of inertia immediately following 200 burning (see Langlands et al. accepted). Another explanation that cannot be excluded is that large wandering spiders are more likely to be caught by pitfall traps in burnt areas with little ground cover (Topping and Sunderland 1992). This may partially explain the significant results found for body size and burrowing in the 0-year-old sites. However, inspection of the raw data indicated clearly that long-lived species did not solely drive the significant 0-year-old result. Two large lycosid species, one of which burrows [Hoggicosa bicolor (Hogg, 1905)] were abundant in the 0-year-old sites. The phenology of species was not significantly different among post-fire ages, although our measure of seasonal activity was rather crude, and data with finer temporal resolution may yet again provide better insights. Resource-utilization Although abundance of burrowing ambushers was significantly higher in recently burnt sites, a lack of significant differences between other post-fire ages suggests hunting strategy does not differ markedly with time since fire. However, many studies show that web-hunting spiders require specific habitat architecture and respond to changes in vegetation accordingly (Riechert & Gillespie 1986; Uetz 1991). Therefore, our results for web building spiders may be due in part to our sampling method. Due to the remote location and low catch rates from hand collecting and vacuuming in this habitat we were restricted to using pitfall traps (Owen 2004). Pitfall traps sample many of the foliage and web building species (Brennan, Moir & Majer 2004). However, these taxa will probably be underrepresented in our data set and thus constrain our ability to detect post-fire changes in abundance of species utilizing an aerial web for hunting. For our study, it seems that specialised food sources are not limiting with no detectable result for diet specialization on ants. This result is contrary to predictions that niche breadth decreases with increasing time since disturbance (Odum 1969; Brown & Southwood 1983). ARE TRAITS INTER-CORRELATED? Several traits were inter-correlated from different categories of traits (e.g. survival, dispersal, reproduction etc.). Uncovering the functional significance of each trait is critical to developing a better mechanistic understanding of post-fire response patterns. Methodological issues also demand the consideration of links between traits. Aubin et al. (2009) suggest including highly correlated or linked traits in the fourth-corner analysis 201 could increase the level of Type-I errors. Their proposed solution is to test for correlated traits and exclude redundant traits. Our maximum correlation was only 0.69 (burrowing and time to maturity) which we considered moderately high. Our preferred solution however, was to use PCoA to incorporate axes representing combinations of traits into a fourthcorner analysis. ARE COMBINATIONS OF TRAITS LINKED WITH POST-FIRE AGE? Fourth-corner analysis showed the composite scores of traits from the PCoA had some significant links with the 0-year-old sites. This more biologically realistic approach detected some of the pattern from the analysis on individual traits; there was lower abundance of heavy sclerotisation of the cephalothorax and higher abundance of burrowers and burrow hunters in 0-year-old sites. However, it did not detect significant links between other post-fire ages and suggested some different patterns for traits. A strong loading of abdominal scutes for axis 1 suggested this trait is significantly less abundant in the 0-yearold sites, compared with 5-year-old sites as identified by the analysis on individual traits. It also suggests diet specialisation on ants is less abundant in 0-year-old sites, a finding not found analysing traits separately. A major problem with this approach however is that a large number of traits correlated with axis 1 made it difficult to interpret the significant result. Interpretation of the second axis is easy, with only ballooning strongly correlated; however, this approach did not detect greater abundance of ballooners in 5-year-old sites. ARE EMERGENT GROUPS LINKED WITH POST-FIRE AGE? The indirect method of using emergent groups found links between post-fire age and the post-fire response of spiders. Of the five groups defined, three had significant changes in abundance. However, the post-hoc tests found these changes with post-fire age were not linear or simple. The responses of Group IV appear strongly related to abdominal scutes, while group V are related to body size, burrowing and time to maturity. Therefore, the results from the emergent group approach, particularly for these traits, were largely congruous with those of the fourth-corner. However, the significance of traits such as dispersal ability and heavy sclerotisation of the cephalothorax were less clear compared to the fourth-corner approach with individual traits. 202 A key advantage of emergent groups is that it generates response groups ultimately leading to the creation of functional types (e.g. plant functional types). We strived to select a limited number of groups that were biologically meaningful. However, there is a trade-off inherent with the size and number of emergent groups selected. While selecting more groups of smaller size may increase predictive accuracy and generate more statistically significant responses, this ultimately reduces practicality and the ability to generalize (Noble & Gitay 1997; Keith et al. 2007). Indeed, we note that the three response groups that had significant responses had the fewest species. CURRENT CONSTRAINTS AND FUTURE DIRECTIONS Our natural history knowledge and taxonomic understanding of Australian spiders is still poor for many families (Brennan, Moir & Majer 2004). This means that while we can have strong confidence in morphological traits measured directly from specimens, the quality of data available for behavioural traits may continue to hinder a trait-based approach. Indeed, we have spent much of the discussion of this paper wondering if a finer resolution of detail for behavioural traits would have distinguished more of the post-fire ages. This is somewhat disappointing. It is for faunas where autecological knowledge at species level is poor that the trait-based approach might offer the greatest benefit to develop rapidly a predictive understanding of faunal responses to disturbance. Two other potentially contributing factors also deserve mention. Firstly, Moretti et al. (2009) have questioned whether the response of traits to fire can be generalized between regions where fire history differs at evolutionary time scales. They suggest that for areas with a long history of fire, community level changes in traits may exhibit greater inertia/resilience. Thus, the long history of fire in arid Australia may have contributed to the limited functional responses we observed. Secondly, while searching for convergent traits, the suite of traits we measured may contain divergent traits (limiting similarity). These function to allow species to coexist under competition, rather than under similar environmental conditions (Weiher & Keddy 1995). As noted in our introduction, our interest was the ability of morphological and behavioural traits to predict changes in the abundance of species following disturbance. Therefore, we focused on traits at the individual level, and chose not to include community level traits such as rarity (e.g. geographic range, habitat specificity). These traits can be 203 powerful for well-known faunas (e.g., Bonte, Lens & Maelfait 2006; Lambeets et al. 2008), but will often be unavailable for poorly known faunas. Finally, we echo previous calls for statistical advances. The most important of these are the ability to incorporate phylogeny and spatial autocorrelation of sites into the fourth-corner approach (Dray & Legendre 2008, Aubin et al. 2009). Conclusions We set out to test, using spiders, if the species-traits of fauna might increase the predictive power of post-fire responses. Ideally, we might hope to be able to collect a spider from a location and predict its change in abundance following fire from only morphological traits measured from specimens and inferred behavioural traits. We found significant relationships between species-traits of spiders associated with survival, dispersal, reproduction and resource-utilization and post-fire age. Both a direct and an indirect method of assessing links between traits and the environment showed significant links and we consider the combination of the two approaches complementary. Overall, while our analysis suggests caution over the predictive power of a trait-based approach of faunal responses post-fire, it is still too early to assess its full potential. We look forward to further studies using novel data sets, refined traits and experimental studies that test underlying mechanisms. 204 Acknowledgements We thank R. Raven, B. Baehr and J. Waldock for useful insights into the biology of Australian spiders. Thanks to R. Black, D. Reed, two anonymous reviewers and the associate editor for useful comments that improved the manuscript. Fauna were collected under licenses and with ethics approval (WA Dept. of Environment and Conservation, AEC 2005/18). The WA Department of Environment and Conservation and the University of Western Australia provided funding. Literature cited Anderson, M. J., Gorley, R. N. & Clarke, K. R. (2008) PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods, PRIMER-E, Plymouth, UK. Aubin, I., Ouellette, M.-H., Legendre, P., Messier, C. & Bouchard, A. (2009) Comparison of two plant functional approaches to evaluate natural restoration along an old-field - deciduous forest chronosequence. Journal of Vegetation Science, 20, 185-198. Bell, J., Bohan, D., Shaw, E. M. & Weyman, G. S. (2005) Ballooning dispersal using silk: world fauna, phylogenies, genetics and models. Bulletin of Entomological Research, 95, 69-114. Bonte, D., Lens, L. & Maelfait, J. P. (2006) Sand dynamics in coastal dune landscapes constrain diversity and life-history characteristics of spiders. Journal of Applied Ecology, 43, 735-747. Brennan, K. E. C., Ashby, L., Majer, J. D., Moir, M. L. & Koch, J. M. (2006) Simplifying assessment of forest management practices for invertebrates: how effective are higher taxon and habitat surrogates for spiders following prescribed burning? Forest Ecology & Management, 231, 138-154. Brennan, K. E. C., Moir, M. L. & Majer, J. D. (2004) Exhaustive sampling in a Southern Hemisphere global biodiversity hotspot: inventorying species richness and assessing endemicity of the little known jarrah forest spiders. Pacific Conservation Biology, 10, 241-260. Brennan, K. E. C., Moir, M. L. & Wittkuhn, R. S. (in press) Fire refugia: the mechanism governing animal survivorship within a highly flammable plant. Austral Ecology. 205 Brown, V. K. & Southwood, T. R. E. (1983) Trophic diversity, niche breadth and generation times of exopterygote insects in a secondary succession. Oecologia, 56, 220-225. Buddle, C. M., Spence, J. R. & Langor, D. W. (2000) Succession of boreal spider assemblages following wildfire and harvesting. Ecography, 23, 424-436. Connell, J. H. (1978) Diversity in tropical rainforests and coral reefs. Science, 199, 13021310. Coyle, F. A. (1981) Effects of clearcutting on the spider community of a South Appalachian forest. Journal of Arachnology, 9, 285 - 298. Crawford, R. L., Sugg, P. M. & Edwards, J. S. (1995) Spider arrival and primary establishment on terrain depopulated by volcanic eruption at Mount St. Helens, Washington. American Midland Naturalist, 133, 60-75. Cushing, P. E. (1997) Myrmecomorphy and Myrmecophily in spiders: a review. Florida Entomologist, 80, 165-193. Dolédec, S., Chessel, D., ter Braak, C. & Champely, S. (1996) Matching species traits to environmental variables: a new three-table ordination method. Environmental and Ecological Statistics, 3, 143-166.Dray, S. & Dufour, A.-B. (2007) The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software, 22, 1-20. Dray, S. & Legendre, P. (2008) Testing the species traits-environment relationships: the fourth-corner problem revisited. Ecology, 89, 3400-3412. Entling, W., Schmidt-Entling, M. H., Bacher, S., Brandl, R. & Nentwig, W. (2010) Body size-climate relationships of European spiders. Journal of Biogeography, 37, 477485. Framenau, V. W. & Elgar, M. A. (2005) Cohort dependent life-history traits in a wolf spider (Aranea: Lycosidae) with a bimodal life cycle. Journal of Zoology, London, 265, 179-188. Gaston, K. J. & Blackburn, T. M. (2000) Pattern and Process in Macroecology, Blackwell Science, Oxford. Grime, J. P. (2006) Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. Journal of Vegetation Science, 17, 255-260. 206 Hodkinson, I. D., Webb, N. R. & Coulson, S. J. (2002) Primary community assembly on land - the missing stages: why are the heterotrophic organisms always there first? Journal of Ecology, 90, 569-577. Hubbell, S. P. (2001) The Unified Neutral Theory of Biodiversity and Biogeography, Princeton University Press, Princeton. Jocqué, R. (1991) A generic revision of the spider family Zodariidae (Araneae). Bulletin of the American Museum of Natural History, 201, 1-160. Jonas, J. & Joern, A. (2007) Grasshopper (Orthoptera: Acrididae) communities respond to fire, bison grazing and weather in North American tallgrass prairie: a long-term study. Oecologia, 153, 699-711. Keith, D. A., Holman, L., Rodoreda, S., Lemmon, J. & Bedward, M. (2007) Plant functional types can predict decade-scale changes in fire-prone vegetation. Journal of Ecology, 95, 1324-1337. Lambeets, K., Vandegehuchte, M. L., Jean-Pierre & Bonte, M. D. (2008) Understanding the impact of flooding on trait-displacements and shifts in assemblage structure of predatory arthropods on river banks. Journal of Animal Ecology, 77, 1162-1174. Langlands, P. R. (2010) Conservation of Spiders (Araneae) in the Western Australian Rangelands, with Particular Reference to Disturbance by Fire. School of Animal Biology. University of Western Australia, Perth. Langlands, P. R., Brennan, K. E. C. & Pearson, D. J. (2006) Spiders, spinifex, rainfall and fire: long-term changes in a community of desert spiders. Journal of Arid Environments, 67, 36-59. Langlands, P. R., Brennan, K. E. C. & Ward, B. (accepted) Is the successional trajectory of an arid spider assemblage following fire deterministic? Austral Ecology Lavorel, S. & Garnier, E. (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology, 16, 545-556. Lavorel, S., McIntyre, S., Landsberg, J. & Forbes, T. D. A. (1997) Plant functional classifications: from general groups to specific groups based on response to disturbance. Trends in Ecology & Evolution, 12, 474-478. 207 Legendre, P. & Anderson, M. J. (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs, 69, 1-24. Legendre, P., Galzin, R. & Harmelin-Vivien, M. L. (1997) Relating behavior to habitat: solutions to the fourth-corner problem. Ecology, 78, 547-562. Main, B. Y. (1984) Spiders, Collins, Sydney. Marc, P. & Canard, A. (1997) Maintaining spider biodiversity in agroecosystems as a tool in pest control. Agriculture, Ecosystems & Environment, 62, 229-235. McGill, B. J., Enquist, B. J., Weiher, E. & Westoby, M. (2006) Rebuilding community ecology from functional traits. Trends in Ecology & Evolution, 21, 178-185. Merrett, P. (1967) The phenology of spiders on heathland in Dorset: I. Families Atypidae, Dysderidae, Gnaphosidae, Clubionidae, Thomisidae and Salticidae. Journal of Animal Ecology, 36, 363-374. Moir, M. L., Brennan, K. E. C., Koch, J. M., Majer, J. D. & Fletcher, M. J. (2005) Restoration of a forest ecosystem: the effects of vegetation and dispersal capabilities on the reassembly of plant-dwelling arthropods. Forest Ecology and Management, 217, 294-306. Moran, M. D. (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos, 100, 403-405. Moretti, M., de Bello, F., Roberts, S. & Potts, S. G. (2009) Taxonomical vs. functional responses of bee communities to fire in two contrasting climatic regions. Journal of Animal Ecology, 78, 98-108. Moretti, M. & Legg, C. (2009) Combining plant and animal traits to assess community functional responses to disturbance. Ecography, 32, 299-309. Noble, I. R. & Gitay, H. (1996) A functional classification for predicting the dynamics of landscapes. Journal of Vegetation Science, 7, 329-336. Noble, I. R. & Slatyer, R. O. (1980) The use of vital attributes to predict successional changes in plant communities subject to recurrent disturbances. Vegetatio, 43, 5-21. Odum, E. P. (1969) The strategy of ecosystem development. Science, 164, 262-270. Owen, G. (2004) Conserving Biodiversity in the Rangelands: Are Land Systems Effective Surrogates for Spider Assemblages? Dept. Environmental Biology. Curtin University of Technology, Perth. 208 Peters, R. H. (1983) The Ecological Implications of Body Size, Cambridge University Press, Cambridge. Pillar, V. D., Duarte, L. d. S., Sosinski, E. E. & Joner, F. (2009) Discriminating traitconvergence and trait-divergence assembly patterns in ecological community gradients. Journal of Vegetation Science, 20, 334-348. Polis, G. A. & Yamashita, T. (1991) The ecology and importance of predaceous arthropods in desert communities. The Ecology of Desert Communities (ed G. A. Polis), pp. 180-222. The University of Arizona Press, Tucson. R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org. Remmert, H. (1981) Body size of terrestrial arthropods and biomass of their populations in relation to the abiotic parameters of their milieu. Oecologia, 50, 12-13. Riechert, S. E. & Gillespie, R. G. (1986) Habitat choice and utilization in web-building spiders. Spiders: Webs, Behavior and Evolution (ed W. A. Shear), pp. 23-48. Stanford University Press, Stanford, California, USA. Shipley, B., Vile, D. & Garnier, E. (2006) From plant traits to plant communities: a statistical mechanistic approach to biodiversity. Science, 314, 812-814. Southwood, T. R. E. (1977) Habitat, the templet for ecological strategies? Journal of Animal Ecology, 46, 337-365. Topping, C. J. & Sunderland, K. D. (1992) Limitations to the use of pitfall traps in ecological studies exemplified by a study of spiders in a field of winter wheat. Journal of Applied Ecology, 29, 485-491. Turnbull, A. L. (1973) Ecology of the true spiders (Araneomorphae). Annual Review of Entomology, 18, 305-348. Uetz, G. W. (1991) Habitat structure and spider foraging. Habitat Structure: The Physical Arrangement of Objects in Space (eds S. S. Bell, E. D. McCoy & H. R. Mushinsky), pp. 325-348. Chapman and Hall, Melbourne. Uetz, G. W., Halaj, J. & Cady, A. B. (1999) Guild structure of spiders in major crops. Journal of Arachnology, 27, 270-280. Weiher, E. & Keddy, P. A. (1995) Assembly rules, null models and trait dispersion: new questions from old patterns. Oikos, 74, 159-164. 209 Whelan, R. J. (1995) The Ecology of Fire, Cambridge University press, Cambridge. Whelan, R. J., Rodgerson, L., Dickman, C. R. & Sutherland, E. F. (2002) Critical life cycles of plants and animals: developing a process-based understanding of population changes in fire-prone landscapes. Flammable Australia: The Fire Regimes and Biodiversity of a Continent (eds R. A. Bradstock, J. E. Williams & A. M. Gill), pp. 94-125. Cambridge University Press, Cambridge. Wise, D. H. (1993) Spiders in Ecological Webs, Cambridge University Press, Cambridge, UK. Żabka, M. (1991) Salticidae (Arachnida: Araneae) of Oriental, Australian and Pacific regions: V. Genus Holoplatys Simon, 1885. Records of the Australian Museum, 43, 171-240. 210 Table 1. Test of the direct link between the species-traits of spiders and increasing post-fire age from a combined fourth-corner analysis (permutation models 2 & 4) using 9999 permutations. For each trait are, the states scored, the test statistic, and the value of the test statistic for each post-fire age with significance indicated (* denotes p <0.05, ** denotes p < 0.01). Traits Scored states Test Post-fire age (years) statistic 0 0.5 3 5 8 20 Chi 15.9** 0.7 3.0 0.0 0.4 3.2 pseudo-F 21.6** 0.0 4.5 0.1 0.1 2.4 Survival Burrowing yes/ no Body size (length) mean carapace length (mm) Heavy sclerotisation of the cephalothorax normal/ sclerotised Chi 2.0 0.1 2.4 1.4 1.9 7.0* Abdominal scutes none/ some/ whole surface Chi 4.0 1.0 3.7 8.6* 0.5 7.7 yes/ no Chi 7.3* 1.2 3.1 5.3* 2.3 0.5 < 3 years/ > 3 years 3/ 6 / 9 months Chi 6.6* 0.0 3.0 0.4 0.1 1.7 Chi 1.3 3.5 5.1 0.4 0.7 1.6 Hunting strategy active/ burrow/ sit & wait/ aerial webs/ terrestrial webs Chi 16.1** 1.6 5.1 0.0 1.2 3.4 Diet specialization (ants) yes/ no Chi 0.2 1.3 0.0 0.3 1.3 0.1 yes/ no Chi 0.4 0.1 0.5 0.0 0.0 0.5 Dispersal Ballooning Reproduction Time to maturity Phenology Resource-utilization Microhabitat Flattened body 211 Table 2. Test of the direct link between combinations of traits and post-fire age. Principal coordinate analysis (PCoA) was used to reduce the ten species-traits of spiders (with varying degrees of inter-correlation) to three axes, which were then used as matrix Q in a fourth-corner analysis. Values represent a pseudo-F statistic. For details of axis correlation with traits, see Fig. 2 and Appendix S4. ** denotes p < 0.01. PCoA axes Post-fire age (years) 0 1 2 3 0.5 11.28** 0.14 9.48** 1.66 0.02 0.15 Variation (%) 3 5 8 20 4.70 4.75 0.65 0.44 4.14 0.22 0.17 2.19 0.08 2.82 0.43 0.15 Cumulative variation (%) 37.5 32.3 21.9 37.5 69.8 91.7 Table 3: The five groups of species selected from cluster analysis with the number of species, shared traits and taxa for each group. Note, species from some families are within multiple groups. For each family, superscripts denote the fidelity of species within a group, A : all species in a family, S: Some species, F: Few species. Cluster Number groups I of species 65 Small to large, with no scutes, active, sit-&-wait hunters and terrestrial webs II 48 III 27 IV 27 V 12 Shared traits Taxa A A A Clubionidae , Desidae , Dictynidae , S A F Gnaphosidae , Linyphiidae , Lycosidae , A A A Miturgidae , Oxyopidae , Philodromidae , F F A Prodidomidae , Salticidae , Sparassidae , A A Thomisidae , Zoridae F S A Small to Medium, some with flattened bodies, ballooning, mostly active hunters with some terrestrial webs Small to large, ant eaters, active hunters and mostly non-ballooning Small, with full scutes and active hunters Corinnidae , Gnaphosidae , Pholcidae , S S Salticidae , Theridiidae Large, without sclerotisation, mostly slow maturing and burrowing behaviour Nemesiidae , Segestriidae , Lycosidae 212 F S F Corinnidae , Lamponidae , Prodidomidae , A Zodariidae F F F Corinnidae , Gnaphosidae , Lamponidae , A A F Liocranidae , Oonopidae , Salticidae , F Theridiidae A A S b Body size Mean abundance of species with sclerotised cephalothorax Mean length (mm) 3.5 * 3.0 2.5 2.0 1.5 0 5 c 10 15 20 d Abdominal scutes Mean abundance of species with a full scute 15 10 * 5 0 0 10 15 20 Time to maturity 4 1 * 3 2 1 0 0 5 10 15 20 * 2.0 1.5 1.0 * 0.5 0.0 0 5 10 15 20 Hunting Strategy- Burrowing 8 * 2 4 Ballooning f 3 Heavy sclerotisation of cephalothorax 2.5 Mean abundance of burrow hunters Mean abundance of long lived species e 5 Ratio: ballooning/ non-ballooning a * 6 4 2 0 0 0 5 10 15 0 20 5 10 15 20 Post-fire age (years) Ratio:Burrowing/ non-burrowing species g Burrowing 0.15 * 0.10 0.05 0.00 0 5 10 15 20 Post-fire age (years) Fig. 1. Mean abundance (4th root) or ratio, ± SE, with post-fire age (years). Asterisk denotes significant ages based on fourth-corner analysis not graphs (Table 1). 213 Family Gnaphosidae Lamponidae Lycosidae Nemesiidae Oonopidae Zodariidae Other 0.4 Ballo Ph en -9 cu A bS l artia te-P it ent AbScute-Abs Ab Sc g wi n rro rrow u B Bu ntHu to m at Le ng urity t Phe h n-3 mth s ute -F Hu ull ntAc Ce tive p Di ha lot et sp h o ra ec x ia li z at ion (a Bo -0.2 dy e Ti m PCoA 2 (32.3% of total variation) d b o dy 0 it&wa Flattene eb errW nt-T Hu s th m -6 en Ph HuntS m ths g oni n 0.2 nt s) -0.4 -0.4 -0.2 0 0.2 PCoA 1 (37.5% of total variation) 0.4 Fig. 2. Principal coordinate analysis of the similarity based on ten traits between 179 species of spiders (Table 1). Vectors represent the Spearman correlation of traits, with the length and direction indicating the relationship with PCoA axes. Abbreviations: Phen= Phenology, AbScute= Abdominal Scutes, Hunt= Hunting strategy, Cephalothorax= Heavy sclerotisation of cephalothorax. Six dominant families display unique symbols with the remaining species pooled into one symbol for clarity. The third PCoA axis explained 21.9% of the total variation (91.7% for all three axes) and correlates strongly with Phenology (Appendix S4). 214 Group III Mean abundance 15 c abc 10 ac abc ab b 5 0 0 5 10 15 20 Group IV 15 Mean abundance c a 10 a abc ab b 5 0 0 5 15 20 Group V 8 Mean abundance 10 a 6 ab 4 bc 2 bc bc c 0 0 5 10 15 20 Post-fire age (years) Fig. 3. Mean abundance (4th root) ± SE for emergent groups which had a significant response with post-fire age (years). For the traits and species of each group see Table 3. Unlike letters indicate significant differences in pair-wise tests. 215 Appendix S1 Study area The study was conducted in central Western Australia at the proposed Lorna Glen (Matuwa) Conservation Park, located approximately 150 km NE of Wiluna. The former sheep and cattle station receives a median annual rainfall of 235 mm (range 86–654 mm) and experiences mean monthly temperatures from 21–38 ˚C in summer and 5–21 ˚C in winter (Australian Bureau of Meteorology). Lorna Glen contains a range of habitat types with the most widespread of these being the fire-prone hummock grasslands (Bullimore). This is a vegetation assemblage dominated by spinifex Triodia basedowii E. Pritz, 1918, an over storey of marble gums (Eucalyptus gongylocarpa Blakely, 1936) with scattered mulga (Acacia aneura Benth, 1855) and mallee (E. kingsmillii Maiden and Blakely, 1929 and E. lucasii Blakely, 1934). Spinifex is highly flammable, but fires are unlikely to spread within ages under 10-15 years unless preceded by periods of high rainfall, which increase biomass and promote the growth of short-lived soft grasses (Allan and Southgate 2002). Sites and fires Our study was conducted at the landscape scale with 27 sites selected to represent the majority of fire ages present at Lorna Glen. Prior to 2001 Lorna Glen had a recent history of fire suppression by pastoralists; however, infrequent and large scale (>100 km2) fires still occurred. We used Landsat satellite images from 1985 to 2005 to establish the recent fire history of the study area. From these natural fire histories and some experimental fires, we selected six treatments for post-fire ages: 0, 0.5, 3, 5, 8 and 20+ years since the last fire. The patch size in which each site was located was at least nine hectares (90 000 m2) and there were three to five sites per post-fire age. At each site, a 50 x 50 m (0.25 ha) quadrat 216 was established a minimum of 150 m from the site boundary (change in fire age) or road. We installed five pitfall traps in each quadrat, one at each corner and one in the centre. Many fire ecology studies conducted at the landscape scale have difficulties finding multiple fires of the same age within a study area that can act as true replicates (Brennan et al. 2009). Our study design was similarly constrained by the pyrodiversity present at Lorna Glen with very few sites burnt recently (< 1 year-old). For some post-fire ages (3, 5 and 8 years) multiple sites were located within a single fire scar. However, in these instances, sites were interspersed with other ages and located as far apart as possible (the minimum distance between two sites within the same fire scar was 1.9 km and was more often in the order of 5–10 km). To resolve the problem of a lack of very recent burns we burnt seven patches of habitat not burnt for at least twenty years. We burnt three patches in October 2005 and four patches in April 2006 to examine two additional post-fire ages, 0 and 0.5 respectively. Each patch had a single sampling site (quadrat). References Brennan, K. E. C., Christie, F. J. & York, A. (2009) Global climate change and litter decomposition: more frequent fire slows decomposition and increases the functional importance of invertebrates. Global Change Biology, 15, 2958-2971. 217 Species Cheiracanthium sp.05 Clubiona sp.01 Clubiona sp.02 Clubiona sp.03 Clubiona sp.04 Battalus sp.04 Genus 1 sp.01 Poecilipta sp.02 Poecilipta sp.03 Supunna funerea Simon, 1896 Supunna picta (L. Koch, 1873) Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.04 Phryganoporus sp.01 Genus 1 sp.01 Genus 1 sp.02 Ceryerda cursitans Simon, 1909 Eilica sp.07 Eilica sp.11 Encoptarthria sp.01 Encoptarthria sp.08 Encoptarthria sp.15 Family Clubionidae Clubionidae Clubionidae Clubionidae Clubionidae Corinnidae Corinnidae Corinnidae Corinnidae Corinnidae Corinnidae Desidae Desidae Desidae Desidae Dictynidae Dictynidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae No No No No No No No No No No No No No No No No No No No No No No 218 2.9 3.5 3.4 1.1 0.9 1.2 0.5 0.9 1.2 4.6 2.2 1.4 3.0 3.6 2.7 2.0 1.5 2.9 2.7 2.0 2.1 1.8 2.7 length (mm) behaviour No Carapace Burrowing Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Heavy Normal Normal Normal Normal Normal Normal carapace Sclerotisation of Some Some Some None None None None None None None None None Some Some Some Full Full Some None None None None None scutes Abdominal Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Ballooning short short short short short short short short short short short short short short short short short short short short short short short maturity Time to A list of all species with scored traits and emergent group allocation (note: due to size, the table has been split into two sections) Appendix S2 Species Encoptarthria sp.33 Encoptarthria sp.34 Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.04 Genus 1 sp.05 Genus 1 sp.06 Genus 1 sp.09 Genus 1 sp.10 Genus 1 sp.12 Genus 1 sp.13 Genus 1 sp.16 Genus 1 sp.17 Genus 1 sp.18 Genus 1 sp.21 Genus 1 sp.22 Genus 1 sp.24 Genus 1 sp.25 Genus 1 sp.27 Genus 1 sp.28 Genus 1 sp.29 Genus 1 sp.30 Genus 1 sp.31 Genus 1 sp.32 Hemicloea sp.20 Family Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Appendix S2 cont. No No No No No No No No No No No No No No No No No No No No No No No No 219 1.4 1.3 1.5 2.0 1.6 1.9 2.3 1.3 1.9 1.1 1.8 1.3 1.3 1.7 1.6 1.2 1.7 1.6 0.9 0.8 1.9 1.1 1.4 2.0 4.1 length (mm) behaviour No Carapace Burrowing Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal carapace Sclerotisation of None Full None None Some None None None Some None None None Some Some Some Some Some Some None None Some Some Some Some Some scutes Abdominal Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Ballooning short short short short short short short short short short short short short short short short short short short short short short short short short maturity Time to Yes Yes No No Hemicloea sp.26 Laronius sp.19 Asadipus banjiwarn Platnick, 2000 Lamponina asperrima (Hickman, 1950) Lamponata daviesae Platnick, 2000 Lamponina elongata Platnick, 2000 Lampona quinqueplagiata Simon, 1908 Lamponina scutata (Strand, 1913) Erigone prominens Bösenberg & Strand, 1906 Genus 1 sp.01 Hoggicosa alfi Langlands & Framenau, 2010 Hoggicosa bicolor (Hogg, 1905) Knoelle clara (L. Koch, 1877) ‘Pexa grp.' sp.04 ‘Yalkara grp.' sp.02 ‘Yalkara grp.' sp.03 Genus 1 sp.01 Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.04 Genus 1 sp.06 Genus 1 sp.08 Miturga sp.05 Miturga sp.07 Gnaphosidae Gnaphosidae Lamponidae Lamponidae Lamponidae Lamponidae Lamponidae Lamponidae Linyphiidae Liocranidae Lycosidae Lycosidae Lycosidae Lycosidae Lycosidae Lycosidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae No No No No No No No No Yes Yes No No No No No No No No No No No Hemicloea sp.23 220 9.0 5.0 4.1 3.8 3.9 4.6 3.5 3.4 5.9 6.8 4.5 7.9 7.4 9.4 1.0 0.8 3.8 2.2 2.8 1.2 2.4 1.9 2.5 1.8 2.0 length (mm) behaviour Gnaphosidae Carapace Burrowing Species Family Appendix S2 cont. Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Heavy Normal Heavy Heavy Heavy Heavy Heavy Heavy Normal Normal Normal carapace Sclerotisation of None None None None None None None None None None None None None None Full None Some Some Some Some Some Full None None None scutes Abdominal No No No No No No No No Yes Yes Yes Yes Yes Yes Yes Yes No No No No No No Yes Yes Yes Ballooning short short short short short short short short short short short short short short short short short short short short short short short short short maturity Time to Species Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.05 Kwonkan sp.01 Kwonkan sp.06 Yilgarnia sp.04 Cavisternum sp.07 Grymeus sp.04 Grymeus sp.09 Myrmopopaea sp.01 Myrmopopaea sp.05 Myrmopopaea sp.08 Myrmopopaea sp.14 Myrmopopaea sp.15 Opopaea sp.02 Opopaea sp.03 Opopaea sp.06 Opopaea sp.12 Opopaea sp.13 Opopaea sp.16 Xestaspis sp.10 Xestaspis sp.11 Oxyopes sp.01 Oxyopes sp.02 Oxyopes dingo Strand, 1913 Family Nemesiidae Nemesiidae Nemesiidae Nemesiidae Nemesiidae Nemesiidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oxyopidae Oxyopidae Oxyopidae Appendix S2 cont. No No No No No No No No No No No No No No No No No No No Yes Yes Yes Yes Yes 221 3.0 2.6 2.0 1.1 1.3 0.5 0.6 0.5 0.9 0.6 0.6 0.8 0.6 0.5 0.8 0.7 0.9 1.0 0.5 4.5 4.5 5.2 6.3 5.4 3.4 length (mm) behaviour Yes Carapace Burrowing Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Heavy Heavy Normal Normal Normal Normal Normal Normal Normal carapace Sclerotisation of None None None Full Full Full Full Full Full Full Full Full Full Full Full Full Full Full Full None None None None None None scutes Abdominal Yes Yes Yes No No No No No No No No No No No No No No No No No No No No No No Ballooning short short short short short short short short short short short short short short short short short short short long long long long long long maturity Time to No No Genus 1 sp.01 Genus 1 sp.02 Trichocyclus arabana Huber, 2001 Trichocyclus nigropunctatus Simon, 1908 Cryptoerithus halli Platnick & Baehr, 2006 Cryptoerithus occultus Rainbow, 1915 Molycria vokes Platnick & Baehr, 2006 Nomindra sp.01 (cf. cooma) Nomindra sp.02 Nomindra leeuweni Platnick & Baehr, 2006 Wesmaldra learmonth Platnick & Baehr, 2006 Wydundra kennedy Platnick & Baehr, 2006 Wydundra sp.03 Wydundra sp.04 ‘Clynotis albobarbatus grp.' sp.10 Damoetas sp.13 Damoetas sp.17 Genus 1 sp.23 Genus 1 sp.02 'big embolus grp.' Genus 1 sp.27 'big embolus grp.' Grayenulla sp.01 Holoplatys sp.07 (cf. planissima) Holoplatys sp.14 Lycidas chlorophthalmus (Simon, 1909) Philodromidae Philodromidae Pholcidae Pholcidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae No No No No No No No No No No No No No No No No No No No No No No No Oxyopes rubicundus L. Koch, 1878 222 3.2 1.9 1.9 1.7 1.9 1.5 1.6 1.9 2.2 2.7 2.0 2.8 1.7 1.4 0.9 0.9 0.8 1.8 1.2 1.4 1.0 1.0 1.3 2.5 2.0 length (mm) behaviour Oxyopidae Carapace Burrowing Species Family Appendix S2 cont. Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal carapace Sclerotisation of Some Some Some Some Some Some Some Some Full Some None Some Some None Some Full None None None Some Some Some None None None scutes Abdominal Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes No No No No No No No No No No Yes Yes Yes Yes Yes Ballooning short short short short short short short short short short short short short short short short short short short short short short short short short maturity Time to No No No No No Lycidas grp. sp.11 Lycidas grp. sp.12 ‘Neon' sp.15 Ocrisiona yakatunyae Żabka, 1990 Paraplatoides sp.03 Paraplatoides sp.06 Pellenes bitaeniata (Keyserling, 1882) Zebraplatys keyserlingi Żabka, 1992 Genus 1 sp.01 Genus 1 sp.02 Genus 1 sp.01 Genus 1 sp.02 Genus 1 sp.07 Genus 1 sp.08 Genus 1 sp.12 Genus 1 sp.13 Hadrotarsus sp.10 Hadrotarsus sp.11 Latrodectus sp.09 Steatoda sp.01 Steatoda sp.02 Steatoda sp.03 Steatoda sp.04 Steatoda sp.05 Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Segestriidae Segestriidae Sparassidae Sparassidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae No No No No No No No No No No No No No No Yes Yes No No No No Lycidas sp.08 223 1.1 1.0 1.3 1.2 0.9 1.1 0.6 0.5 0.6 0.7 0.9 0.9 4.9 3.1 2.9 2.5 1.8 1.5 2.9 2.8 3.7 0.7 1.7 3.2 1.5 length (mm) behaviour Salticidae Carapace Burrowing Species Family Appendix S2 cont. Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal carapace Sclerotisation of Some Some Some Some Some Some Full Full Some Some Some None None None None None Some None Some Some Some Full Some Some Full scutes Abdominal Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes No No No No Yes Yes Yes Yes Yes Yes Yes Yes Yes Ballooning short short short short short short short short short short short short short short short short short short short short short short short short short maturity Time to Species Genus 1 sp.01 Genus 1 sp.02 Genus 1 sp.04 Genus 1 sp.05 Genus 1 sp.06 Stephanopis sp.03 Fissarena castanea (Simon, 1908) Australutica quaerens Jocqué, 1995 Australutica sp.01 (cf. quaerens) Cavasteron sp.32 (cf. crassicalcar) Genus 1 sp.31 Genus 1 sp.33 Genus 1 sp.34 Habronestes bicornis Baehr, 2003 Habronestes sp.13 (cf. longiconductor) Habronestes sp.16 (cf. grahami) Habronestes sp.18 (cf. hamatus) Habronestes sp.21 Habronestes sp.30 Masasteron piankai Baehr, 2004 Neostorena sp.02 Neostorena sp.03 Neostorena sp.05 Spinasteron cavasteroides Baehr & Churchill, 2003 Family Thomisidae Thomisidae Thomisidae Thomisidae Thomisidae Thomisidae Trochanteriidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Appendix S2 cont. No No No No No No No No No No No No No No No No No No No No No No No 224 2.3 4.3 6.4 2.5 3.3 3.0 2.6 3.3 2.1 3.0 2.6 1.8 2.6 2.6 2.6 2.3 2.4 4.2 4.2 2.3 3.7 1.5 1.8 1.2 length (mm) behaviour No Carapace Burrowing Normal Heavy Heavy Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal Normal carapace Sclerotisation of Some Some Some Some Some Some Some Some Some Some Some Some Some Some None Some Some None Some Some None None None None scutes Abdominal No No No No No No No No No No No No No No No No No No Yes Yes Yes Yes Yes Yes Ballooning short short short short short short short short short short short short short short short short short short short short short short short short maturity Time to Species Argoctenus sp.01 Argoctenus sp.02 Argoctenus sp.03 Argoctenus sp.04 Argoctenus sp.05 Argoctenus sp.08 Genus 1 sp.10 Family Zoridae Zoridae Zoridae Zoridae Zoridae Zoridae Zoridae Appendix S2 cont. No No No No No No 225 2.7 3.0 1.8 2.0 2.5 2.8 2.6 length (mm) behaviour No Carapace Burrowing Normal Normal Normal Normal Normal Normal Normal carapace Sclerotisation of None None None None None None None scutes Abdominal Yes Yes Yes Yes Yes Yes Yes Ballooning short short short short short short short maturity Time to Species Cheiracanthium sp.05 Clubiona sp.01 Clubiona sp.02 Clubiona sp.03 Clubiona sp.04 Battalus sp.04 Genus 1 sp.01 Poecilipta sp.02 Poecilipta sp.03 Supunna funerea Simon, 1896 Supunna picta (L. Koch, 1873) Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.04 Phryganoporus sp.01 Genus 1 sp.01 Genus 1 sp.02 Ceryerda cursitans Simon, 1909 Eilica sp.07 Eilica sp.11 Encoptarthria sp.01 Encoptarthria sp.08 Encoptarthria sp.15 Encoptarthria sp.33 Family Clubionidae Clubionidae Clubionidae Clubionidae Clubionidae Corinnidae Corinnidae Corinnidae Corinnidae Corinnidae Corinnidae Desidae Desidae Desidae Desidae Dictynidae Dictynidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Appendix S2 cont. 226 Three Three Six Three Nine Nine Nine Three Six Nine Three Three Three Nine Three Nine Six Three Six Six Three Three Nine Three Phenology Active Active Active Active Active Active Active TerrWeb TerrWeb TerrWeb TerrWeb TerrWeb TerrWeb Active Active Active Active Active Active Active Active Active Active No No No No No No No No No No No No No No No No No No Yes No No No No No diet on ants strategy Active Specialized Hunting NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat body Flattened II II II II I I I I I I I I I II II II IV IV III I I I I I group Emergent Gnap_Enco_33 Gnap_Enco_15 Gnap_Enco_08 Gnap_Enco_01 Gnap_Eili_11 Gnap_Eili_07 Gnap_Cery_curs Dict_Gen01_02 Dict_Gen01_01 Desi_Phry_01 Desi_Gen01_04 Desi_Gen01_03 Desi_Gen01_02 Cori_Supu_pict Cori_Supu_fune Cori_Poec_03 Cori_Poec_02 Cori_Gen01_01 Cori_Batt_04 Club_Club_04 Club_Club_03 Club_Club_02 Club_Club_01 Club_Chei_01 for cluster analysis Abbreviated names Species Encoptarthria sp.34 Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.04 Genus 1 sp.05 Genus 1 sp.06 Genus 1 sp.09 Genus 1 sp.10 Genus 1 sp.12 Genus 1 sp.13 Genus 1 sp.16 Genus 1 sp.17 Genus 1 sp.18 Genus 1 sp.21 Genus 1 sp.22 Genus 1 sp.24 Genus 1 sp.25 Genus 1 sp.27 Genus 1 sp.28 Genus 1 sp.29 Genus 1 sp.30 Genus 1 sp.31 Genus 1 sp.32 Hemicloea sp.20 Hemicloea sp.23 Family Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Gnaphosidae Appendix S2 cont. Three Three Three Three Three Three Three Three Six Six Six Three Six Three Three Three Three Nine Six Six Nine Six Nine Nine Three 227 Phenology Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active No No No No No No No No No No No No No No No No No No No No No No No No No diet on ants strategy Active Specialized Hunting Flat Flat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat body Flattened II II IV I I II I I I II I I I II II II II II II I I II II II II group Emergent Gnap_Hemi_23 Gnap_Hemi_20 Gnap_Gen01_32 Gnap_Gen01_31 Gnap_Gen01_30 Gnap_Gen01_29 Gnap_Gen01_28 Gnap_Gen01_27 Gnap_Gen01_25 Gnap_Gen01_24 Gnap_Gen01_22 Gnap_Gen01_21 Gnap_Gen01_18 Gnap_Gen01_17 Gnap_Gen01_16 Gnap_Gen01_13 Gnap_Gen01_12 Gnap_Gen01_10 Gnap_Gen01_09 Gnap_Gen01_06 Gnap_Gen01_05 Gnap_Gen01_04 Gnap_Gen01_03 Gnap_Gen01_02 Gnap_Enco_34 for cluster analysis Abbreviated names Three Three Three Three Laronius sp.19 Asadipus banjiwarn Platnick, 2000 Lamponina asperrima (Hickman, 1950) Lamponata daviesae Platnick, 2000 Lamponina elongata Platnick, 2000 Lampona quinqueplagiata Simon, 1908 Lamponina scutata (Strand, 1913) Erigone prominens Bösenberg & Strand, 1906 Genus 1 sp.01 Hoggicosa alfi Langlands & Framenau, 2010 Hoggicosa bicolor (Hogg, 1905) Knoelle clara (L. Koch, 1877) ‘Pexa grp.' sp.04 ‘Yalkara grp.' sp.02 ‘Yalkara grp.' sp.03 Genus 1 sp.01 Genus 1 sp.02 Genus 1 sp.03 Genus 1 sp.04 Genus 1 sp.06 Genus 1 sp.08 Miturga sp.05 Miturga sp.07 Genus 1 sp.02 Gnaphosidae Lamponidae Lamponidae Lamponidae Lamponidae Lamponidae Lamponidae Linyphiidae Liocranidae Lycosidae Lycosidae Lycosidae Lycosidae Lycosidae Lycosidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Miturgidae Nemesiidae Six Six Six Six Three Three Three Nine Six Six Six Three Six Six Nine Three Nine Three Three Three Three Hemicloea sp.26 Gnaphosidae 228 Phenology Species Family Appendix S2 cont. Burrow Active Active Active Active Active Active Active Active Active Active Burrow Burrow Burrow Burrow Active TerrWeb Active Active Active Active Active Active Active No No No No No No No No No No No No No No No No No No No No No No No No No diet on ants strategy Active Specialized Hunting NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat Flat body Flattened V I I I I I I I I I I V V V V IV I III III III III III IV I II group Emergent Neme_Gen01_02 Mitu_Mitu_07 Mitu_Mitu_05 Mitu_Mitu_08 Mitu_Mitu_06 Mitu_Mitu_04 Mitu_Mitu_03 Mitu_Mitu_02 Mitu_Mitu_01 Lyco_Yalk_03 Lyco_Yalk_02 Lyco_Pexa_04 Lyco_Knoe_clar Lyco_Hogg_bico Lyco_Hogg_alfi Lioc_Gen01_01 Liny_Erig_prom Lamp_Lamp_scut Lamp_Lamp_quin Lamp_Lamp_elon Lamp_Lamp_davi Lamp_Lamp_aspe Lamp_Asad_banj Gnap_Laro_19 Gnap_Hemi_26 for cluster analysis Abbreviated names Species Genus 1 sp.03 Genus 1 sp.05 Kwonkan sp.01 Kwonkan sp.06 Yilgarnia sp.04 Cavisternum sp.07 Grymeus sp.04 Grymeus sp.09 Myrmopopaea sp.01 Myrmopopaea sp.05 Myrmopopaea sp.08 Myrmopopaea sp.14 Myrmopopaea sp.15 Opopaea sp.02 Opopaea sp.03 Opopaea sp.06 Opopaea sp.12 Opopaea sp.13 Opopaea sp.16 Xestaspis sp.10 Xestaspis sp.11 Oxyopes sp.01 Oxyopes sp.02 Oxyopes dingo Strand, 1913 Oxyopes rubicundus L. Koch, 1878 Family Nemesiidae Nemesiidae Nemesiidae Nemesiidae Nemesiidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oonopidae Oxyopidae Oxyopidae Oxyopidae Oxyopidae Appendix S2 cont. Nine Nine Three Six Three Nine Nine Nine Three Nine Nine Three Three Six Three Nine Nine Nine Six Nine Three Three Three Three Three 229 Phenology Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Burrow Burrow Burrow Burrow No No No No No No No No No No No No No No No No No No No No No No No No No diet on ants strategy Burrow Specialized Hunting NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat body Flattened I I I I IV IV IV IV IV IV IV IV IV IV IV IV IV IV IV IV V V V V V group Emergent Oxyo_Oxyo_rubi Oxyo_Oxyo_ding Oxyo_Oxyo_02 Oxyo_Oxyo_dess Oono_Xest_11 Oono_Xest_10 Oono_Opop_16 Oono_Opop_13 Oono_Opop_12 Oono_Opop_06 Oono_Opop_03 Oono_Opop_02 Oono_Myrm_15 Oono_Myrm_14 Oono_Myrm_08 Oono_Myrm_05 Oono_Myrm_01 Oono_Grym_09 Oono_Grym_04 Oono_Cavi_07 Neme_Yilg_04 Neme_Kwon_06 Neme_Kwon_01 Neme_Gen01_05 Neme_Gen01_03 for cluster analysis Abbreviated names Three Three Genus 1 sp.02 Trichocyclus arabana Huber, 2001 Trichocyclus nigropunctatus Simon, 1908 Cryptoerithus halli Platnick & Baehr, 2006 Cryptoerithus occultus Rainbow, 1915 Molycria vokes Platnick & Baehr, 2006 Nomindra sp.01 (cf. cooma) Nomindra sp.02 Nomindra leeuweni Platnick & Baehr, 2006 Wesmaldra learmonth Platnick & Baehr, 2006 Wydundra kennedy Platnick & Baehr, 2006 Wydundra sp.03 Wydundra sp.04 ‘Clynotis albobarbatus grp.' sp.10 Damoetas sp.13 Damoetas sp.17 Genus 1 sp.23 Genus 1 sp.02 'big embolus grp.' Genus 1 sp.27 'big embolus grp.' Grayenulla sp.01 Holoplatys sp.07 (cf. planissima) Holoplatys sp.14 Lycidas chlorophthalmus (Simon, 1909) Lycidas sp.08 Philodromidae Pholcidae Pholcidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Prodidomidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Nine Three Three Three Nine Nine Nine Three Three Three Three Six Three Nine Six Nine Three Three Six Nine Nine Three Three Genus 1 sp.01 Philodromidae 230 Phenology Species Family Appendix S2 cont. Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active TerrWeb TerrWeb Sit&Wait No No No No No No No No No No No No No No No No No No No No No No No No No diet on ants strategy Sit&Wait Specialized Hunting NotFlat NotFlat Flat Flat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat body Flattened IV II II II II II II II II IV II I III III I III IV I I I III II II I I group Emergent Salt_Lyci_08 Salt_Lyci_chlo Salt_Holo_14 Salt_Holo_07 Salt_Gray_01 Salt_Gen01_27 Salt_Gen01_02 Salt_Gen01_23 Salt_Damo_17 Salt_Damo_13 Salt_Clyn_10 Prod_Wydu_04 Prod_Wydu_03 Prod_Wydu_kenn Prod_Wesm_lear Prod_Nomi_leeu Prod_Nomi_02 Prod_Nomi_01 Prod_Moly_voke Prod_Cryp_occu Prod_Cryp_hall Phol_Tric_nigr Phol_Tric_arab Phil_Gen01_02 Phil_Gen01_01 for cluster analysis Abbreviated names Six Three Six Six Three Lycidas grp. sp.12 ‘Neon' sp.15 Ocrisiona yakatunyae Żabka, 1990 Paraplatoides sp.03 Paraplatoides sp.06 Pellenes bitaeniata (Keyserling, 1882) Zebraplatys keyserlingi Żabka, 1992 Genus 1 sp.01 Genus 1 sp.02 Genus 1 sp.01 Genus 1 sp.02 Genus 1 sp.07 Genus 1 sp.08 Genus 1 sp.12 Genus 1 sp.13 Hadrotarsus sp.10 Hadrotarsus sp.11 Latrodectus sp.09 Steatoda sp.01 Steatoda sp.02 Steatoda sp.03 Steatoda sp.04 Steatoda sp.05 Genus 1 sp.01 Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Salticidae Segestriidae Segestriidae Sparassidae Sparassidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Theridiidae Thomisidae Three Nine Six Nine Nine Nine Three Three Nine Six Nine Six Three Nine Three Six Six Three Three Six Lycidas grp. sp.11 Salticidae 231 Phenology Species Family Appendix S2 cont. Sit&Wait TerrWeb TerrWeb TerrWeb TerrWeb TerrWeb TerrWeb Active Active TerrWeb TerrWeb TerrWeb TerrWeb Sit&Wait Sit&Wait Burrow Burrow Active Active Active Active Active Active Active No No No No No No No Yes Yes No No No No No No No No No No No No No No No No diet on ants strategy Active Specialized Hunting NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat Flat NotFlat NotFlat Flat NotFlat Flat Flat Flat NotFlat NotFlat NotFlat body Flattened I II II II II II II IV IV II II II I I I V V II I II II II IV II II group Emergent Thom_Gen01_01 Ther_Stea_05 Ther_Stea_04 Ther_Stea_03 Ther_Stea_02 Ther_Stea_01 Ther_Latr_09 Ther_Hadr_11 Ther_Hadr_10 Ther_Gen01_13 Ther_Gen01_12 Ther_Gen01_08 Ther_Gen01_07 Spar_Gen01_02 Spar_Gen01_01 Sege_Gen01_02 Sege_Gen01_01 Salt_Zebr_keys Salt_Pell_bita Salt_Para_06 Salt_Para_03 Salt_Ocri_yaka Salt_Gen01_15 Salt_Lyci_12 Salt_Lyci_11 for cluster analysis Abbreviated names Species Genus 1 sp.02 Genus 1 sp.04 Genus 1 sp.05 Genus 1 sp.06 Stephanopis sp.03 Fissarena castanea (Simon, 1908) Australutica quaerens Jocqué, 1995 Australutica sp.01 (cf. quaerens) Cavasteron sp.32 (cf. crassicalcar) Genus 1 sp.31 Genus 1 sp.33 Genus 1 sp.34 Habronestes bicornis Baehr, 2003 Habronestes sp.13 (cf. longiconductor) Habronestes sp.16 (cf. grahami) Habronestes sp.18 (cf. hamatus) Habronestes sp.21 Habronestes sp.30 Masasteron piankai Baehr, 2004 Neostorena sp.02 Neostorena sp.03 Neostorena sp.05 Spinasteron cavasteroides Baehr & Churchill, 2003 Argoctenus sp.01 Family Thomisidae Thomisidae Thomisidae Thomisidae Thomisidae Trochanteriidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zodariidae Zoridae Appendix S2 cont. Six Six Three Three Three Three Six Three Three Three Three Three Three Three Three Three Three Three Six Three Three Three Three Three 232 Phenology Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Active Sit&Wait Sit&Wait Sit&Wait Sit&Wait No Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes No No No No No No diet on ants strategy Sit&Wait Specialized Hunting NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat Flat NotFlat NotFlat NotFlat NotFlat NotFlat body Flattened I III III III III III III III III III III III III III III III III III I I I I I I group Emergent Zori_Argo_01 Zoda_Spin_cava Zoda_Neos_05 Zoda_Neos_03 Zoda_Neos_02 Zoda_Masa_pian Zoda_Habr_30 Zoda_Habr_21 Zoda_Habr_18 Zoda_Habr_16 Zoda_Habr_13 Zoda_Habr_bico Zoda_Gen01_34 Zoda_Gen01_33 Zoda_Gen01_31 Zoda_Cava_32 Zoda_Aust_01 Zoda_Aust_quae Troc_Fiss_cast Thom_Step_03 Thom_Gen01_06 Thom_Gen01_05 Thom_Gen01_04 Thom_Gen01_02 for cluster analysis Abbreviated names Species Argoctenus sp.02 Argoctenus sp.03 Argoctenus sp.04 Argoctenus sp.05 Argoctenus sp.08 Genus 1 sp.10 Family Zoridae Zoridae Zoridae Zoridae Zoridae Zoridae Appendix S2 cont. Three Three Three Three Three Three 233 Phenology Active Active Active Active Active No No No No No No diet on ants strategy Active Specialized Hunting NotFlat NotFlat NotFlat NotFlat NotFlat NotFlat body Flattened I I I I I I group Emergent Zori_Gen01_10 Zori_Argo_08 Zori_Argo_05 Zori_Argo_04 Zori_Argo_03 Zori_Argo_02 for cluster analysis Abbreviated names Appendix S3 Flow diagram of statistical analyses. Squares represent rows by columns matrices, and triangles are pair-wise similarity half-matrices. Fourth-corner see a) Fourth-corner see c) Principal coordinate Analysis (PCoA) see b) Emergent groups see d) a) Fourth-corner on individual traits sites traits species L Q post-fire age R two stage fourth-corner analysis (permutation model 2 followed by model 4) b) Linked traits traits species Q PCoA axes species Gower coefficient species s species Principal coordinate analysis (PCoA) Q2 c) Combined traits (PCoA) in fourth-corner sites traits L species post-fire age Q2 two stage fourth-corner analysis (permutation model 2 followed by model 4) R d) Emergent groups (EGs) dendrogram sites species species S Cluster analysis, Ward’s method Select EGs EGs Euclidean distance PERMANOVAs on post-fire age for each EG 234 sites sites Appendix S4 Non-metric multidimensional scaling (nMDS) of species composition of spiders based on Bray-Curtis similarity from fourth root transformed data. Each number represents a site and indicates the post-fire age in years. 2D Stress: 0.23 8 20 8 20 20 20 5 8 5 3 5 5 20 0.5 8 8 5 0.5 0 3 0.5 0 3 0 3 3 0 235 Appendix S5 The loadings of ten traits on the first three PCoA axes . Loadings > 0.5 are bolded. PCoA Axes PCoA 1 PCoA 2 PCoA 3 -0.272 -0.841 0.228 0.530 0.259 -0.030 Hunting strategy- Terrestrial Web -0.168 -0.497 -0.081 Hunting strategy- Burrowing -0.433 0.236 -0.050 Hunting strategy- Sit & wait -0.274 -0.064 0.218 0.495 0.377 0.313 Phenology- 3mths -0.152 0.349 0.861 Phenology- 6mths -0.104 -0.106 -0.568 Phenology- 9mths 0.285 -0.311 -0.460 Burrowing -0.433 0.236 -0.050 Body Length -0.238 0.354 0.321 0.368 0.257 -0.031 Time to maturity -0.304 0.311 0.025 Flattened body -0.023 -0.161 0.300 Abdominal scutes- Absent -0.860 0.081 -0.075 Abdominal scutes- Partial 0.671 -0.204 0.288 Abdominal scutes- Full 0.272 0.167 -0.292 Total variation (%) 37.5 32.3 21.9 Cumulative variation (%) 37.5 69.8 91.7 Ballooning Hunting strategy- Active Diet specialization (Ants) Heavy sclerotisation of carapace 236 Appendix S6 Cluster dendrogram used to define the five emergent groups (Group I – V). A similarity matrix of ten traits based on the Gower coefficient was used to create the dendrogram with Ward‟s method. For electronic copy see enclosed CD. 237 Synthesis of Ecological Results and Future Research I was interested in examining the long-term pattern of response of spiders to fire in spinifex habitat in the arid zone of Australia. Consistent with previous studies in other habitats, I found a clear successional pattern. With increasing post-fire age there were considerable differences in the number of species, evenness and the composition of species in the spider assemblage (Chapter 2). Based on these parameters, the spider assemblage seems strongly deterministic, converging on long-unburnt conditions over time. Also consistent with our current understanding of spider assemblages, post-fire changes in the species composition and habitat structure of the vegetation explained the greatest proportions of the variation in richness and composition of the spiders (Chapter 3). Given these results, I anticipated that there would be strong links between the traits of species and environmental conditions. However, when I tested this, the results were equivocal (Chapter 4). This incongruence is a little puzzling. Perhaps the traits that I used are not functional for survival in the post-fire environment, or perhaps they are divergent traits (traits that allow species to coexist under similar environmental conditions). Clearly, this requires further investigation, and one potential avenue is to look at changes in trait space. This approach has recently been applied to post-fire changes in bee and beetle assemblages using functional diversity (FD) or community weighted mean (CWM) trait values (e.g. Moretti et al. 2009, Moretti et al. in press). Another possible avenue to explore these results, particularly the importance of habitat structure, might be to conduct experimental manipulations of the habitat. The amount of ground cover, specifically spinifex, could be reduced manually, to see how this influences the spider assemblage. Does it cause a retrogression of succession as shown for mammals in heath habitat (Fox et al. 2003)? Masters et al. (2003) conducted a study in central Australia, where they manually reduced spinifex cover to study the impact on a carnivorous small marsupial (Dasycercus cristicauda). They found no change in the total biomass of the invertebrates; however, they did not analyse the species composition of the invertebrate fauna (Masters et al. 2003). Although changes in vegetation best explained the changes in the spider assemblage, rainfall and the presence of predators also explained a significant amount of variation. Although technically and practically more difficult, the mechanisms behind how these variables might influence spiders in arid Australia could also be investigated by 238 manipulative experiments. The influence of rainfall via the addition of water could be studied using the numerous bores on Lorna Glen and most stations. I am unaware of any study on fauna that has manipulated rainfall in the arid zone of Australia. Variation in predation pressure by vertebrate predators (lizards and small mammals) could be examined by exclusion experiments using cages or barriers (see for example Spiller and Schoener 1998, Manicom et al. 2008). The spatial location of sites and spatial processes also clearly play a part in structuring the spider assemblage (Chapter 3). Unfortunately, with the available fire histories, it was not possible to intersperse completely all fire ages. To account for this, I included a spatial variable into the permutational analysis of variance as a covariate. Although the results of pair-wise comparisons were slightly different, this did not result in any major change (Chapter 2 Appendix B vs Chapter 3 Table 1 and 3). From six to ten of the 15 comparisons were still significant, something which is extremely unlikely to occur by chance alone (Moran 2003). Therefore, the interpretation of results was not altered. The available fire histories also restricted the number of true replicates (sensu Hurlbert 1984). I spent many weeks examining the fire histories via satellite images with the aid of GIS researchers and then „ground truthing‟ potential sites to confirm the fire age, correct vegetation type and the patchiness or completeness of the historic fires. In addition to satellite images, I aged the sites by dendrochronology of mallee (Eucalyptus kingsmillii and E. lucasii). Mallee is a common term given to species that regrow from underground lignotubers following disturbance. This subsequently proved to be an unreliable method, so I relied on the satellite data to come up with the best possible design given the fire histories available on the station. Unfortunately, there were not enough individual fires to avoid nesting sites in a single fire. However, when this was the case, I selected sites several kilometres apart, with roads between, in the hope of collecting independent samples of spiders. The limitations of my experimental design must also be acknowledged. While some studies have demonstrated the validity of the chronosequence or space-for-time approach for certain situations (Twigg et al. 1989, Knops and Tilman 2000), there have also been several critical assessments over the years (Collins and Adams 1983, Pickett 1989, Johnson and Miyanishi 2008). It can be difficult to assess the assumptions of the chronosequence approach; that all sites had similar initial conditions, experienced a 239 similar disturbance, and have received similar conditions since disturbance. In the case of my study, with current techniques, I had little chance of testing these assumptions. Indeed, with the benefit of hindsight, it seems that the characteristics of the arid zone, including extreme variability in rainfall in both time and space (Stafford-Smith and Morton 1990), might make meeting the assumptions required for a chronosequence difficult. Additionally, the inclusion of sites that received experimental burns that were smaller than natural fires is a clear violation of these assumptions. However, a space for time approach provides a snapshot, and one that is achievable within the period of a Ph.D. program. For this reason, chronosequence studies are still used commonly for ecological research (Jeffries et al. 2006, Shipley et al. 2006, Wardle et al. 2008, Aubin et al. 2009), and it is considered a useful approach for fire studies (Clarke 2008). Ideally, to test the patterns I have found, a long-term study of the spiders is required, particularly monitoring over the first three years post-fire. Ultimately, our overall understanding of how the flora and fauna of arid Australia, including spiders, respond to fire will be advanced most rapidly through a long-term, large scale, replicated, multidisciplinary study, along the lines of the Kalpaga fire experiment (Andersen et al. 1998). This however, requires a considerable long-term input of resources and a commitment to maintain experimental fire regimes. In addition, to examine aspects of the fire regime that are difficult to replicate, such as mosaics, another approach would be to conduct a large scale, multidisciplinary chronosequence study, similar to that currently underway in the mallee region of Eastern Australia (Clarke et al. 2006). Along with the need to manage biodiversity, the challenge of anthropogenic climate change adds urgency to such research. Understanding how increased temperatures and changes in rainfall will further alter the fire regimes of inland Australia and the consequences for biodiversity and carbon fluxes will be vital for conservation in the future. 240 References Andersen, A. N., Braithwaite, R. W., Cook, G. D., Corbett, L. K., Williams, R. J., Douglas, M. M., Gill, A. M., Setterfield, S. A. & Muller, W. J. (1998) Fire research for conservation management in tropical savannas: Introducing the Kapalga fire experiment. Australian Journal of Ecology, 23, 95-110. Aubin, I., Ouellette, M.-H., Legendre, P., Messier, C. & Bouchard, A. (2009) Comparison of two plant functional approaches to evaluate natural restoration along an old-field - deciduous forest chronosequence. Journal of Vegetation Science, 20, 185-198. Clarke, M. F. (2008) Catering for the needs of fauna in fire management: science or just wishful thinking? Wildlife Research, 35, 385-394. Clarke, M. F., Bennett, A., Callister, K., Ferguson, S., Kelly, L., Kenny, S., Nimmo, D., Spence-Bailey, L., Taylor, R. & Watson, S. (2006) Mallee Fire and Biodiversity Project: Determining appropriate fire regimes in the Murray Mallee. Collins, S. L. & Adams, D. E. (1983) Succession in grasslands: Thirty-two years of change in a central Oklahoma tallgrass prairie. Plant Ecology, 51, 181-190. Fox, B. J., Taylor, J. E. & Thompson, P. T. (2003) Experimental manipulation of habitat structure: a retrogression of the small mammal succession. Journal of Animal Ecology, 72, 927-940. Hurlbert, S. H. (1984) Pseudoreplication and the design of ecological experiments. Ecological Monographs, 54, 187-211. Jeffries, J. M., Marquis, R. J. & Forkner, R. E. (2006) Forest age influences oak insect herbivore community structure, richness, and density. Ecological Applications, 16, 901-912. Johnson, E. A. & Miyanishi, K. (2008) Testing the assumptions of chronosequences in succession. Ecology Letters, 11, 419-431. Knops, J. M. H. & Tilman, D. (2000) Dynamics of soil nitrogen and carbon accumulation for 61 years after agricultural abandonment. Ecology, 81, 88-98. Manicom, C., Schwarzkopf, L., Alford, R. A. & Schoener, T. W. (2008) Self-made shelters protect spiders from predation. Proceedings of the National Academy of Sciences, 105, 14903-14907. Masters, P., Dickman, C. R. & Crowther, M. (2003) Effects of cover reduction on mulgara Dasycercus cristicauda (Marsupialia: Dasyuridae), rodent and invertebrate populations in central Australia: implications for land management. Austral Ecology, 28, 658-665. 241 Moran, M. D. (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos, 100, 403-405. Moretti, M., De Bello, F., Roberts, S. & Potts, S. G. (2009) Taxonomical vs. functional responses of bee communities to fire in two contrasting climatic regions. Journal of Animal Ecology, 78, 98-108. Moretti, M., De Cáceres, M., Pradella, C., Obrist, M. K., Wermelinger, B., Legendre, P. & Duelli, P. (in press) Fire-induced taxonomic and functional changes in saproxylic beetle communities in fire sensitive regions. Ecography. Pickett, S. T. A. (1989) Space-for-time substitution as an alternative to long-term studies. Long-Term Studies in Ecology: Approaches and Alternatives (ed G. E. Likens), pp. 110-135. Springer-Verlag, New York. Shipley, B., Vile, D. & Garnier, E. (2006) From plant traits to plant communities: a statistical mechanistic approach to biodiversity. Science, 314, 812-814. Spiller, D. A. & Schoener, T. W. (1998) Lizards reduce spider species richness by excluding rare species. Ecology, 79, 503-516. Stafford Smith, D. M. & Morton, S. R. (1990) A framework for the ecology of arid Australia. Journal of Arid Environments, 18, 255-278. Twigg, L. E., Fox, B. J. & Jia, L. (1989) The modified primary succession following sand mining: a validation of the use of chronosequence analysis. Australian Journal of Ecology, 14, 441-447. Wardle, D. A., Bardgett, R. D., Walker, L. R., Peltzer, D. A. & Lagerstrom, A. (2008) The response of plant diversity to ecosystem retrogression: evidence from contrasting long-term chronosequences. Oikos, 117, 93-103. 242 CONCLUSION Fire is a common disturbance in many ecosystems and an integral part of arid Australia. The displacement of Aboriginal peoples in recent history led to a change in fire regimes in spinifex habitats of arid Australia. To conserve Australia‟s arid biodiversity it is crucial to fill in the many gaps in our current knowledge of how the fauna and flora respond to fire. This is particularly true for spiders, where there are several obstacles to successful biodiversity conservation, including the taxonomic impediment and a better understanding of post-fire response patterns. I set out to improve our knowledge for the conservation of spiders in arid Australia by removing part of this taxonomic impediment and examining the long-term response of spiders to fire. To help remove the taxonomic impediment and facilitate my investigations of the postfire response of spiders, I revised the taxonomy and systematics of a charismatic and common genus of wolf spiders. The Australian wolf spider genus Hoggicosa Roewer, 1960 now includes ten species and a phylogenetic analysis supports the monophyly of Hoggicosa. My analysis found that an unusual sexual dimorphism for wolf spiders (females more colourful than males), evident in four species of Hoggicosa, has evolved multiple times. Hoggicosa are large, burrowing lycosids, which will prove ideal models for further evolutionary and ecological studies. In my ecological studies, using a chronosequence approach, I found significant nonlinear changes in species richness and evenness, as well as marked changes in the species composition of spiders with increasing post-fire age. However, there was no difference in multivariate dispersion within ages. While it appears that all sites converge towards long unburnt sites, a longitudinal study would be required to confirm these findings and shed light on the changes within the first three years following disturbance by fire. This could also test the two alternative hypotheses that I propose to explain the return trajectory of sites burnt experimentally. Sampling regularly over this period could uncover a lag in the full impact of disturbance by fire (inertia) or a rapid recolonization from the surrounding areas. 243 Although only correlative, for the first time, I have quantified the ability of seven different variables (Fire, Rainfall, Soil, Habitat, Floristics, Predators and Space) to explain post-fire changes in a spider assemblage. These results now focus attention and highlight future manipulative experiments that can further develop our understanding of the post-fire response of spiders in the Australian arid zone. Finally, I have attempted to develop a predictive understanding of how spiders respond to fire by adapting an approach from plant ecologists. The development of a traits-based approach, which is highly novel, was less successful than hoped. However, the development of such a mechanistic approach still deserves considerable attention. I therefore consider this thesis makes a substantial contribution of new knowledge to assist with conservation of spiders in the arid zone of Australia. 244