* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download just slime
Cheating (biology) wikipedia , lookup
History of biology wikipedia , lookup
Hologenome theory of evolution wikipedia , lookup
Genetic engineering wikipedia , lookup
Developmental biology wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Antimicrobial resistance wikipedia , lookup
Horizontal gene transfer wikipedia , lookup
Antibiotic use in livestock wikipedia , lookup
Dictyostelium discoideum wikipedia , lookup
Human microbiota wikipedia , lookup
Disinfectant wikipedia , lookup
Marine microorganism wikipedia , lookup
Microbial cooperation wikipedia , lookup
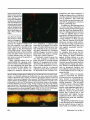
Features Not just slime Beneath the slipperyexteriorof a microbialbiofilm lies a remarkably organizedcommunityof organisms do the slippery,slimy Whatcoating on rocks in a stream, the scum that on shower curtains and toilet grows bowls, and dental plaque have in common? All are examples of biofilms-structured communities of microorganisms that adhere to surfaces. Wherever a suitable surface and some water and nutrients are available, biofilms are likely to grow, their clusters of cells bound together by a matrix of self-produced polysaccharides that gives these structures their stickiness and sliminess. The biofilm lifestyle provides many advantages to the bacteria and other microorganisms, including fungi, archaea, and protozoans, that may populate these sticky structures. Organisms in a biofilm can get nutrients more readily than when they live on their own, and they are protected from many of the insults of daily life. And by sticking to a surface and forming biofilms, microorganisms can keep from being washed away to a place where conditions may be less hospitable-be it down a stream or into the stomach. Biologists and biology students accustomed to seeing bacteria grow in liquid-filled laboratory flasks may wonder why bacteria form biofilms, but biofilm researchers say that a more appropriate question may be "why not?" Indeed, mounting evidence suggests that, in the majority of cases, a bacterium's natural tendency is to grow on a surface. "In the real world, the vast majority of microbial growth is not occurring in batch cultures, it is not occurring in liquid-it is occurring on surfaces," by Elia T. Ben-Ari September1999 says Roberto Kolter, a molecular geneticist at Harvard Medical School who studies biofilms. The remarkable ability of bacteria to sense and rapidly respond to their environment allows them to switch back and forth between the individual, "free-floating," or planktonic, state and the stationary biofilm state. Whereas most microbiology research has focused on planktonic bacteria, "the more I investigate this, the more I'm convinced that bacterial cells are naturally at surfaces...and [that] microbiologists have been looking in the wrong place for 200 years," says James Bryers, a chemical engineer at the University of Connecticut Health Center. Although some early microbiologists did recognize that bacteria can adhere to and grow on surfaces, the concept of the biofilm as a biological unit of organization did not emerge until the 1970s. Rapid advances in biofilm research have come in the 1980s and 1990s with the development of new technologies such as confocal scanning laser microscopy, which lets researchers view biofilms in three dimensions (and in the fourth dimension of real time), and new molecular biology techniques, including specific probes that help researchers visualize individual cells or bacterial species within a biofilm. Researchers now know that a biofilm is not just a bunch of cells stuck haphazardly to a surface but rather an organized, cooperative community with a distinct architecture. In a typical bacterial biofilm, the cells are clustered together in mushroom- and pillar-shaped structures with water channels between them that let nutrients flow in and metabolic waste products flow out. The entire structure is enclosed in a sticky mesh of polysaccharides and proteins. For better or worse The ubiquity of these sticky communities of bacteria in the environment and the many roles-both beneficial and harmful to other organismsthat biofilms can play were not appreciated until relatively recently. Chemical and environmental engineers and industrial microbiologists were among the first to recognize the importance of biofilms. For decades, engineers have been deliberately cultivating particular bacterial biofilms to break down contaminants in waste water. More recent interest has focused on using biofilms for pollution prevention and control, including bioremediation of toxic materials in soil and water. In nature, some biofilms provide benefits to other organisms-for example, by preventing the growth of harmful fungi on a plant root or blocking the growth of disease-causing microorganisms on the surface of some human tissues. But many biofilms have detrimental effects that are made much worse because of their relatively high resistance to antibiotics, disinfectants, and other antimicrobial agents. In industrial processes, for example, biofilms can clog and corrode pipes and filters, contaminate food-processing equipment, and foul the surfaces of computer chips. Dental researchers were the first to recognize that bacteria that stick to and grow on surfaces can cause health problems; the biofilms that form dental plaque can cause not only cavities but also gum and periodontal disease. More and more evidence suggests 689 University of California Press is collaborating with JSTOR to digitize, preserve, and extend access to BioScience ® www.jstor.org lar to the circulatorysystemof higher organisms. And because one organism's waste productmay be another's favoritefood, a biofilm consisting of more than one species provides bacteria and other microorganismswith an added advantage by facilitating the exchange of nutrients. Forminga biofilmcommunityalso lets organisms establish a firm foothold near a good source of nutrients. "For example, if you're stuck to a tooth in the mouth,you areassuredof an intermittentbut reliablesource of nutrientsthat will be flowing past," says PhilStewart,a chemicalengineer and deputy directorof the Centerfor Biofilm Engineering, at Montana State University. Biofilm formation tends to be promoted when a particular nutrient is available in limited quantitiesor is diluted in a large volume of water, Bryersnotes. The structureandcompositionof a biofilm, including its sticky coating, help to source of some more severe, short- trap and concentrateessential nutriterm bacterial infections. "Bacteria ents for its microbial residents. The safe harbor that a biofilm in a biofilm existence can spawn off non-biofilm bacteria...that can, when provides, shielding microorganisms the conditions are right, cause an from the vagaries of the environacute infection," Greenberg explains. ment and from potential predators or pathogens, such as amoebae or viruses, is yet another benefit of life Life in a biofilm in such a community.Organismsin a biofilm are also protected against microordo bacteria and other Why biofilm the ganisms adopt lifestyle attack by a host animal's immune on human tissues and elsewhere? system. The disease-fighting white "Why is it better for groups of humans blood cells that can engulf and deto live in a village as compared to stroy an individual bacterium can[living as] scattered, wandering no- not tacklethe bulkymassof a biofilm, mads?" Kolter asks in reply. The and antibodies tend to get stuck on answer lies in the many benefits, the surfaceratherthan penetratinga such as increased efficiency of services biofilm's interior. Another noteworthy difference and shelter from the outside world, that life in a biofilm-like life in a between planktonic and biofilm bacteria is that cells within a biofilm are human community-can provide. The advantagesof life in a biofilm heterogeneous-for example,in their also lead inevitably to analogies be- growth rates and metabolic status. that biofilms are also responsiblefor many chronic infections that are difficult, if not impossible, to eradicate-particularly in people with weakened immune systems. A host of medical problems are caused by biofilms that flourish on nonliving surfacesimplantedin the body, such as catheters,contact lenses, artificial joints, and other medical devices. Biofilms can also grow on human tissues, causing such chronic infections as endocarditis (inflammation of the heart), the recurringlung infections that ultimately devastate most people with cystic fibrosis, and the middle ear infections that plague many youngsters. "Abiofilminfectionquiteoftencan be a low-gradeinfection, an ongoing aggravation that can be undetected for prolongedperiods of time," says Peter Greenberg,a microbiologist at the University of Iowa. However, biofilms may also be the indirect tween biofilms and multicellular organisms or the tissues of higher organisms. For one thing, the organized architecture of a biofilm, with its network of channels, allows nutrients to be delivered to all the cells in the community and provides for efficient removal of waste products, simi690 This heterogeneity makes bacteria in biofilms more challenging to study than planktonic bacteria. "Whereas in a test tube that's mixed and being shaken or a flask that's being stirred we can say that this population of bacteria is in a certain state, in the biofilm we have to live with the fact that we're dealing with a heterogeneous population,"Stewartsays. The averagebacteriumin a biofilm grows more slowly than a bacteriumliving on its own. But within a biofilm, Stewart says, "there are liable to be bacteria that are growing rapidly and...bacteriain zones in the biofilm wherethey are growingslowly or not at all, and those zones may be separated by only tens of micrometers." Studies led by Zbigniew Lewandowski, at the Center for Biofilm Engineering, use microelectrodesfine, needle-shaped chemical sensors-to probethe depthsof a biofilm and measure the concentrations of variouschemicals,includingoxygen, sulfide, and hydrogen peroxide, in differentareaswithin the film. These experiments have revealed chemical heterogeneity among different regions of a biofilm. The existence of microenvironmentswithin a biofilm may help explain phenomena such as the variation in growth rates among bacteria in the film and suggests that individual cells or species within a biofilm may perform distinct functions. Whatexactlyis a biofilm? Although most researchersagree on the general definition of a biofilm as an organized community of microorganisms growing on a surface and coated with polysaccharides, all biofilms are not created equal. A biofilm can be a single thin layer of bacteria attached to the inside surface of a water pipe, or a "complex, macroscopicallythick mat of organisms analogous to a forest ecosystem on the microscopic level, with aerobic bacteria, fermenters,and sulfate reducersall coexisting,"Stewartsays. The nature of a biofilm may also differ depending on whether it forms on a living tissue or on nonliving material such as a catheter, rock, or mollusk shell. And whereas some biofilms-for example, most that grow on implanted medical devicesconsist of only one or perhaps two species of bacteria, others are comBioScience Vol. 49 No. 9 Bacterialbiofilms grow on both living and nonlivingsurfacesand arecomposedof mushroom-and pillar-shapedmicrocolonies, as depicted in this model. Fluid flows through water channels between the microcolonies, carryingnutrients into the biofilm and waste products out. Pieces of a mature biofilm may break off, float away, and establish new biofilms on other surfaces. Illustration:Peg Dirckx, Center for Biofilm Engineering,Montana State University. posed of many different bacterial species as well as other microorganisms. Biofilms in the oral cavity are notorious for their ability to harbor many different species and genera of microorganisms, and most biofilms in the environment consist of multiple species. Biofilm experts are hard pressed to name a bacterial species that cannot form a biofilm. "Bacteria of all stripes and all types, and also some fungi, will form biofilms," Stewart says. "There have been descriptions in the literature of biofilms in environments ranging from very oligotrophic [low-nutrient] environments to very eutrophic [nutrient-rich] environments, from deep sea hydrothermal vents at the bottom of the ocean...to alpine streams," he says. Indeed, even some bacteria that scientists thought could not grow as biofilms, such as some common laboratory strains of Escherichia coli, are able to form biofilms when grown under the right conditions. Nevertheless, some microorgan- September1999 isms may be better than others at forming biofilms. "Some people in the biofilm community would argue that almost all bacteria are capable of forming biofilms and that's the end of the story, but in fact we know that some bacteria form much more elaborate and much tougher biofilms than other bacteria," Greenberg says. For example, Pseudomonas aeruginosa forms a tenacious, difficult-totreat biofilm in the lungs of people with cystic fibrosis, whereas another troublesome bacterial pathogen, Burkholderia cepacia, forms looser, less organized biofilms and colonizes the lungs of a smaller number of cystic fibrosis patients, Greenberg says. He and others hypothesize that B. cepacia may be better at colonizing lungs that have already been colonized with P. aeruginosa because it can take advantage of the existing infrastructure of the P. aeruginosa biofilm to form a dual-species biofilm. The interactions that take place among organisms in multi-species biofilms are probably best understood in the case of dental plaque. The species composition of these complex biofilm communities changes over time, and increasing species complexity is associated with development of disease in the oral cavity, notes Paul Kolenbrander, a microbiologist at the National Institute of Dental and Craniofacial Research, part of the National Institutes of Health. "Presumably it's a succession of species [or] genera of bacteria, and each one contributes to that site to make it available [and] advantageous to another group" in a manner analogous to the succession of species that occurs in larger-scale ecosystems, he says. Microbiological studies by Kolenbrander and other dental researchers have shown that a bacterial cell of one species or genus will recognize and attach only to cells of certain other species. "Our data support [the idea] that any bacterium that we isolate from your mouth...would have certain partnerships, and it's likely that those partnerships are 691 beneficial in forming dental plaque," Kolenbrander says. Using the knowledge of these specific coaggregation partnerships, Kolenbrander and his colleagues plan to assemble dual-species biofilms in an experimental system that uses saliva-coated glass plates exposed to a constant flow of saliva to mimic the environment of the oral cavity. The goal of these experiments is to use molecular biology approaches to learn how different types of bacteria within a biofilm communicate with one another and to identify specific genes that are activated as a result of this communication. Ultimately, dental researchers hope, such knowledge will lead to new ways to control plaque development and prevent disease. Perhaps, Kolenbrander says, "we could develop some way of doing a little swish with some kind of liquid in the morning and in the evening...that would contain something that would tell these bacteria to be quiet and [that] it's not necessary to grow anymore," or that would even entirely eliminate disease-causing bacteria from the biofilm. The life cycle of a biofilm Whether they consist of a single species or multiple species, bacterial biofilms go through distinct stages of development. "Biofilm formation is not the simple act of bacteria bouncing at random on a surface, getting stuck there, and just growing there without differentiation," Kolter says. "It's a very specific process that is initiated by the bacteria sensing environmental cues," including nutrient levels and temperature. The first step in biofilm development is the attachment of a cell to a surface. Different bacteria use different mechanisms to stick to surfaces, and recent studies of the cholera bacterium (Vibrio cholerae) by Kolter and his colleagues have shown that the specific molecules and pathways that this species uses for adhesion differ depending on whether the 692 surface is living or nonliving. Studies of mutants of various Gram-negative bacterial species in Kolter's laboratory have also demonstrated that, for motile species of bacteria, the propellerlike flagellum is important for the initial interaction with the surface. Kolter speculates that flagellar motility may help to overcome repulsive forces between the surface being colonized and the surface of the bacterium and may also help break the biophysical barrier that exists at the liquid-solid interface. Once the cells have attached to the surface, they begin growing and multiplying and recruiting additional planktonic cells from their surroundings. The cells then start moving across the surface, first forming a monolayer and then aggregating into relatively small groups of bacteria called microcolonies. These microcolonies then differentiate to form the typical three-dimensional biofilm structure with its protective polysaccharide coating. Kolter and his colleagues have evidence that microcolony formation, too, involves flagellar motility in some Gram-negative bacteria. In other motile Gram-negative species, they found that a mechanism known as "twitching motility"-mediated by hairlike appendages called type IV pili-plays a role in microcolony formation. Kolter's lab is currently doing genetic studies to identify the mechanisms that are involved in biofilm formation by several nonmotile species of bacteria. In the final stage of the biofilm life cycle, when a mature community begins to age and get overcrowded, chunks of the biofilm may break off and float away to colonize new surfaces. Individual planktonic cells are also continually detaching from mature biofilms and may establish new biofilm communities on other surfaces. Indeed, Bryers says, the freefloating cells that microbiologists typically find in water samples may be cells that have temporarily and only briefly left their normal, biofilm state. "I think that's why we've gotten this illusion that cells float most of the time-[because] those are the ones that have been sampled," he says. The usual mode of life for bacteria is in a biofilm attached to a surface, "and what we are seeing [when we sample the water] is their children being tossed out of the house and going downstream to set up shop somewhere else," he says. Cell signalingin biofilms At least one stage of biofilm development is triggered by specific bacterial signaling molecules known as quorum-sensing signals. These small molecules were originally found to be involved in triggering certain activities of bacterial cells-such as bioluminescence in some marine bacteria and the production of virulence factors in P. aeruginosa-that occur only when the bacteria reach a certain population density. When enough of a bacterium's compatriots are nearby, secreted quorum-sensing signals reach a critical concentration that is sensed by the other bacteria, leading to a coordinated change in behavior that occurs via the activation of specific genes. Last year, Greenberg and several collaborators reported that acyl homoserine lactone compounds, which were already known to be involved in several quorum-sensing responses, are required for P. aeruginosa bacteria that have attached to a surface and formed a monolayer to differentiate into a mature biofilm. P. aeruginosa mutants that do not make the acyl homoserine lactone signaling molecule form thin biofilms that, unlike normal biofilms, are easily disrupted by a detergent. This finding opens up the intriguing possibility that, by interfering with signaling by acyl homoserine lactones and other small molecules now being found to play a role in bacterial communication, researchers may be able to block biofilm formation or break up biofilms that have already formed. Experiments BioScience Vol. 49 No. 9 with P. aeruginosa, which in addition to infecting the lungs of cystic fibrosis patients can form biofilms on catheters and other implanted medical devices, have shown that "if you knock out quorum sensing...the organism becomes avirulent-it no longer can cause infection," Greenberg says. However, whether the loss of virulence results from an inability to form biofilms is not yet clear. Disrupting a biofilm might also make bacteria more susceptible to antimicrobial agents. Greenberg explains the potential power of this approach by way of analogy: "In any sort of military conflict, when you attack, the first thing you want to do is take out the opponent's communication systems. Once you've done that, you make them vulnerable to everything else you can do." He likens traditional antibiotics to "big bombs" from which bacteria in biofilms are protected. But, Greenberg says, "if we can wipe out the communication system, then maybe the bombs will be effective." Mechanismsof resistance The ability of biofilms to resist attempts to eliminate them-be it by the immune system or by humancontrived means-makes them a huge in a problem. "Microorganisms biofilm are remarkably resistant to all kinds of antimicrobial challenges," Stewart says. "That's true across the spectrum of biofilms, [and] it's also true across the spectrum of antimicrobial agents. We measure biofilm resistance to brute force, sledgehammer biocides like chlorine bleach, and also to antibiotics that have very specific targets." Just how much more resistant to antibiotics biofilm bacteria are than their planktonic counterparts is difficult to determine, Greenberg notes, because there are no standard laboratory methods for growing biofilms or for measuring their antibiotic sensitivity. Estimates suggest that bacteria in biofilms are somewhere between 100 and 1000 times more September 1999 antibiotic resistant than planktonic bacteria. Although the development of antibiotic resistance in planktonic bacteria is a growing concern, most acute infections caused by such free-floating bacteria can still be eliminated with antibiotics. By contrast, doctors are generally unable to eradicate biofilm infections. "We can improve the patient's condition with high levels of long-term antibiotic treatment, but when the antibiotic is stopped, as often as not the bacterial biofilm just grows back," Greenberg says. Researchers hope that if they can understand the mechanisms that account for this resistance, they will be able to devise ways to overcome it. Originally, researchers thought that the polysaccharide slime that surrounds a biofilm acted as a simple physical barrier against antibiotics and other antimicrobial agents. However, recent studies indicate that the mechanisms of resistance are more complex, possibly resulting from both physical and biological differences between biofilm bacteria and planktonic bacteria. The combination of these various mechanisms may make biofilm infections particularly tricky to deal with. One mechanism for resistance results from the ability of the polysaccharides on the biofilm's surface to chemically inactivate some antimicrobials and antibiotics. As a result, these compounds penetrate poorly into biofilms. Resistance also occurs because a significant proportion of the bacteria within a biofilm are growing very slowly or not at all, making them resistant to killing by the many antibiotics that work only, or much more efficiently, against growing cells. More recent data suggest that-as is the case for planktonic bacteriagenetic mechanisms also play a role in biofilms' resistance to antibiotics. Bryers and his colleagues have evidence that, compared to free-floating bacteria, bacteria in biofilms can much more readily exchange plasmids, circular pieces of DNA that carry genes involved in antibiotic resistance. This exchange of genetic material, Bryers says, is stimulated in biofilms by quorom-sensing signaling molecules "that are being traded back and forth, sort of like Chanel No. 5.... A cell that doesn't have a plasmid can actually seduce one that does to come close to it and trade genes with it." Bryers believes that this rapid exchange of plasmids among cells in a biofilm, which can occur both among bacteria of a single species and across species, can lead to the rapid spread of antibiotic resistance throughout a biofilm community. Yet another possible genetic explanation for biofilm bacteria's increased resistance has recently come to the fore. "It has become increasingly clear that there are whole sets of proteins that bacteria express in biofilms that aren't expressed when they're not in biofilms," Greenberg says. Bacteria "make special things when they are organized in societies that they don't make when they are individual, nomadic bacteria. We believe, although we don't know yet, that some of those things are antibiotic resistance mechanisms." Tackling the biofilm problem This notion that biofilm bacteria are genuinely different from their freefloating brethren in terms of their patterns of gene expression has intrigued biofilm researchers. Preliminary evidence suggests that the early steps of biofilm formation may trigger a new program of gene expression in bacteria, including production of the polysaccharides that form the mucuslike extracellular matrix of the mature biofilm. "A cell that attaches is switched into a different developmental pathway and is in some ways a different creature than one that's free-floating, and that [difference] is genetically orchestrated," Stewart says. This concept may lead to new approaches to fight bacteria in biofilms. "If we recognize biofilm formation as a biologically programmed pro693 Intheearlystagesof biofilmformation,bacteria attach to surfaces and form disperse monolayers.Here,Pseudomonas fluorescensbacteria that were labeledwith a molecularprobe bound to greenfluorescentprotein are attachedto the surfaceof a plant'sroot hairs, which appearorange. Photo: Roberto Kolter. cess,thenallof a suddenwe havea whole newlist of targetsfor interfering with that process. You might not need to kill in P. aeruginosa, a regulator gene the cells anymore, you might just senses the environment at an earlier needto learnhow to interfereat the stage of biofilm differentiation than right stages of the developmental cycleof thebiofilm,"hesays.Understandingthe detailsof how biofilms form and what makes bacteriain biofilmsdifferentmay also lead to new ways to promote the growth of desirablebiofilms. Many ongoing studies focus on understanding the signaling path- waysinvolvedin biofilmdifferentiation and identifyingthe genes that are activatedby these signals. Re- cent work by Kolter and his col- leagues,for example,indicatesthat that mediated by the quorum-sensing signal identified by Greenberg and his colleagues. Activation of this gene, Kolter says, leads to a change in the cells' motility behavior. Greenberg's research group has also been working to identify the genes activated by quorum-sensing signals in P. aeruginosa and to determine which of those genes are critical for biofilm maturation. One of these genes codes for a compound called pyocyanin, which is known to be important for the virulence of P. Bacteriawithin a biofilm grow at differentrates. In this side view of a frozen crosssection of a Klebsiella pneumoniae biofilm that has been stained with acridine orange (a fluorescent dye), regions of relatively rapid growth appear orange and regions of slower growth appearyellow or green. The biofilm was grown on a steel surface (bottom of photograph), under turbulent flow conditions. Growth occurs preferentiallyin the outer 30 micrometersof this biofilm, closest to the nutrientproviding fluid that surroundsit (top). Orange staining at the base of the biofilm may be an artifact.The scale bar is 100 micrometers.Photo: Ching-TsanHuang and Phil Stewart, Centerfor Biofilm Engineering,Montana State University. Reprinted with permission from Biotechnology Progress 1996, 12: 316-321. ? 1996 American Chemical Society and AmericanInstitute of Chemical Engineering. 10 694 aeruginosa, and others constitute "a big group of genes that look like they encode a system for synthesis of some sort of antibiotic" that could help fend off other bacteria that may compete with P. aeruginosa to colonize a surface, Greenberg says. In addition to devising new ways to control biofilms, researchers are testing existing antimicrobial agents that may have been dismissed as inferior for killing planktonic bacteria to find out whether they are more successful at killing biofilm bacteria. "We may have good agents for controlling biofilms out there and just not...know it, because all of the disinfectants, biocides, [and] antibiotics that we're using currently have been selected and risen to the top in tests against suspended bacteria," Stewart says. Studies in Stewart's laboratory, for example, have shown that hypochlorite-the active agent in chlorine bleach-is largely ineffective against biofilms because it is neutralized at the film's surface before it can diffuse in. By contrast, a related chemical, monochloramine (made by mixing chlorine bleach and ammonia), which is a weak disinfectant in tests on planktonic bacteria, was more effective than hypochlorite on a thick biofilm because it could penetrate the film. Yet another avenue of research, aimed at controlling the harmful biofilms that form on nonliving surfaces, focuses on developing new materials or surfaces that discourage biofilm formation. "The holy grail of biofilm control is the anti-fouling surface-the Teflon of biofilm formation-that organisms simply don't stick to," Stewart says. "Part of the difficulty," he says, "is that any clean surface that is placed into a real environment will rapidly become conditioned with a film of organic molecules that mask the chemistry of the surface." The greatest prospects for success in this area may be for biomaterials used in medical devices, in part because funds are available to finance BioScience Vol. 49 No. 9 .. the sophisticated chemistry that is needed for such efforts. Bryers and his colleagues, including collaborators at the University of Washington in Seattle, have been working on a variety of "situationally specific" approaches that are designed to fight biofilm formation by specific bacterial species known to cause infections of particular medical devices. These approaches, Bryers says, include changing the surface properties of the biomaterial to make it a "stealth material" that bacteria don't see and that does not absorb the proteins from body fluids that normally coat the surface and allow bacteria to stick; filling the biomaterial with an antibiotic and then releasing the antibiotic in a slow, sustained manner so that it reaches concentrations lethal to bacteria in the proximity of the medical device; and releasing selective anti-adhesion molecules from the biomaterial that prevent bacteria from sticking to the surface. One challenge that some biofilm researchers see for the future is increasing the amount of collaboration between engineers and biologists in a field that is already more interdisciplinary than most. Whereas molecular biologists are interested in looking for differences in gene expression among the individual bacteria within a biofilm, for example, chemical engineers may focus on the gradients of nutrients, metabolic products, and other chemicals in biofilms and the transport processes that underlie those gradients. "It's critical to understand transport phenomena in the biofilm at the same time that we're trying to understand the genetics of what's happening-they can't be divorced," Stewart says. "When we really put the biology and engineering together, 0 we can do a lot." k;K. W.4.2N, Smithsonian Institution Call for The American Institute of Biological Sciences 51st Annual Meeting cosponsored with the Smithsonian Institution BIOLOGY: CHALLENGES FOR THE NEW MILLENNIUM 22-24 March2000 Washington, DC will highlight major discoveries in biology made The meeting during the past century and will attempt to capture a perspective on the coming millennium'schallenges and opportunities, includingthe breakthroughs necessary to continue the advancement of biology. Confirmedspeakers include: Ernst Mayr, Peter Raven, Rita Colwell, Stephen J. Gould, Gene E. Likens, Marvalee Wake, Gordon Orians, Lynn Margulis, Daniel H. Janzen, Edward 0. Wilson Approximately100 abstracts will be accepted for poster presentations at the meeting. Alldates and relevant informationcan be found on the AIBS Web site: http: //www.aibs.org/ meeting2000/ A limited number of posters will be accepted in each of the following areas: Evolution and Ecosystems, Biodiversity, Regulations, Energetics,Integration,Morphologyand Development, and Behavior. For more informationcontact: Mar/lynnMaury,Meetings Manager, e-mail: mmaury@aibs,org September 1999 695