* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download simple measurements
Fictitious force wikipedia , lookup
Specific impulse wikipedia , lookup
Relativistic mechanics wikipedia , lookup
Fluid dynamics wikipedia , lookup
Classical central-force problem wikipedia , lookup
Biofluid dynamics wikipedia , lookup
Center of mass wikipedia , lookup
Newton's laws of motion wikipedia , lookup
Centripetal force wikipedia , lookup
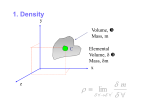
1. SIMPLE MEASUREMENTS An objective of the first experiment in any first year physics lab is to give a student lots of practice in making different kinds of measurements. In addition to measuring one must learn how to estimate the uncertainties in reading the instruments one uses and to calculate the uncertainties that accumulate in derived quantities. In this experiment the actual activity is the study of a marble which is freefalling through a fluid. The final result is the calculation of the property of a fluid called viscosity. Theory Basic and Derived Quantities In physics, a quantity is described as being either basic or derived. A derived quantity is one which can be defined in terms of others, like speed being distance divided by elapsed time. A basic quantity is one which cannot be so defined. The most obvious example is time. Length Length is a basic quantity being dependent only on the unit that is used. You can measure a length with a ruler, a vernier caliper or micrometer caliper and express the result in any unit you like, in centimeters, or even in inches. The uncertainty in reading the ruler is a reading uncertainty. You can’t eliminate a reading uncertainty completely but you can reduce its effect by measuring the same thing a number of times and then taking an average. You learned in the Orientation Workshop that the uncertainty in the average is the standard deviation of the average. This statistical number you have to calculate. Area Area is a derived quantity too. You can’t actually measure an area because there is no instrument for measuring one directly. But you can calculate the area of a circle, for example, by first measuring its diameter and then using a formula. In the same way the volume of an object is also a derived quantity. Mass The mass of an object is a basic quantity whereas weight is derived. These quantities are sometimes confusing to the student because they are treated as synonymous in everyday speech. But mass is a measure of the amount of matter in a body, meaning atoms and molecules, and is therefore a fundamental quantity. Under everyday nonchemical conditions, and nonrelativistic speeds, mass is constant. An astronaut has the same mass whether he or she is floating in space or standing on the earth. The unit of mass in the SI system of units is the kilogram (kg). The mass of an object can be found in terms of a standard mass in an experiment involving accelerated motion.1 Weight The weight of an object is the force of gravity on the object and is therefore different from mass. Unhappily, for purposes of understanding, the magnitude of weight is found by “weighing” the object with an instrument like a balance. But a balance depends for its operation on gravity, and therefore weight is a derived quantity.2 Weight is a force expressed in newtons (abbreviated N). The weight F g of an object of mass m where the acceleration due to gravity is g is therefore 3 r r Fg = mg . …[1] Weight is therefore a derived quantity. At some B1-1 1 Simple Measurements place on the earth an object’s weight is constant (independent of time) because neither the mass of the object nor g change with time. But an object’s weight will vary from one location to another over the surface of the earth because the magnitude of g varies slightly from place to place. More dramatically, the weight of an astronaut on the moon will differ from his/her weight on earth. If an astronaut is “floating” in space where the force of gravity is zero his/her weight is zero. These facts underline the derived nature of weight. Density and Buoyancy The density of an object is a simple scalar ratio— the mass m of the object divided by its volume V. It is commonly denoted ρ ρ= m . V …[2] Density, being dependent on mass and volume, is a derived quantity and has no unit of its own; it is commonly expressed in units kg.m–3. A fluid’s density gives rise to its buoyancy. One form of buoyancy is experienced by all of us every day. If you place an object like a bar of soap on the surface of water in a bathtub it will either sink or float. If it sinks it is most often seen to move downward through the water more slowly than if released in air. It can be shown that if an object is weighed in a fluid with a balance the weight is less than the weight obtained in air. The former is called the apparent weight, the latter the actual weight. 4 This effect is buoyancy and is articulated by Archimides’ Principle: “The apparent weight of an object is diminished by an amount equal to the weight of the medium the object displaces.” Clearly, a buoyant force acts in a direction opposite gravity and is directly proportional to the density of the medium. Why Study Fluids? This experiment, besides providing lots of practice in measuring things, involves the study of an important property of a fluid. The study of fluid behaviour is a large component of the environmental sciences. After all, our climate is driven by the large-scale movement of water in rivers and oceans. Rain is one fluid flowing through another (air). Certain aspects of geology cannot be understood without knowing a little of the flow characteristics of molten rock. Also important is the topic of an object moving down through a fluid as will be studied here. This requires a little knowledge of the physics of a freely falling body which we consider next. The Physics of a Body in Free Fall The object you will actually study is a falling sphere, a marble. The free body diagram of such a sphere falling in a vacuum is drawn in Figure 1a. The sphere is subject to the force of gravity F g. If the sphere should be falling in a medium like air then the air will exert a frictional force, say F s, on the sphere (Figure 1b). This force is in some way dependent on the speed of the sphere, and like all frictional forces, acts in a direction opposite to the direction of motion. And finally, if the B1-2 medium is of sufficient density then the sphere may be subject to an appreciable buoyant force as well, here represented in Figure 1c as F b. F b does not depend on speed. Calculating F b is easy; it is just the force exerted on the sphere by the mass of the medium the sphere displaces (ala Archimedes’ Principle). The calculation of F s is rather more difficult; it involves the property of a fluid called viscosity, which we consider next. Simple Measurements 1 How Viscosity was Originally Defined Viscosity was defined in the days of the Industrial Revolution when oil began to be widely used as a lubricant. From numerous experiments it was found that a body which is pulled (or that falls) through a fluid is subject to a resistive or damping force which is not just a function of the body’s size, but is also a function of the body’s speed and by what could be termed the fluid’s “stickiness”. This stickiness is a manifestation of the property called viscosity. Viscosity was first modelled in terms of the force exerted on a unit area of a flat plate being pulled through a fluid (Figure 2). The force per unit area on the plate was modelled as proportional to the velocity gradient ∆v/∆d of the fluid in the region adjacent to the plate, that is, the force per unit area is given by Fs ∆v =η , A ∆d where A is the plate’s cross-sectional area. The proportionality factor η (pronounced “eta”) is the viscosity. Viscosity is found to be a constant for a pure liquid at constant temperature and in the SI system has the unit Poiseuille (abbreviated Pl; 1 Pl = 1 kg.m –1.s–1.) Viscosity (very definitely a derived quantity) is dependent on temperature; it decreases as the temperature increases. dependant on speed Fs (b) (a) …[3] Fs Fb (c) v mg mg mg Figure 1. Freebody diagrams of a sphere moving downwards under the force of gravity: (a) in a vacuum, (b) in air or a fluid which exerts a resistive force on the sphere, and (c) in a fluid whose density is sufficiently large to produce an appreciable buoyant force in addition to a resistive force. velocity vectors of fluid "dragged" along by the plate d v v Fs Figure 2. A body moving through a fluid is subject to a resistive force which depends on the body’s velocity. B1-3 1 Simple Measurements Viscosity Derived in terms of a Falling Sphere As you might imagine it is difficult to measure the velocity gradient of a fluid in the neighbor-hood of a moving plate, making eq[3] of limited use in an actual experiment. A simpler model is a sphere which is falling under gravity at its ter-minal velocity as has already been sketched in Figure 1. The magnitude of the resistive force on such a sphere was found by the British mathemat-ician and physicist G. G. Stokes (1819-1903) to have the following form: r d r Fs = –6πη v , 2 …[4] where d is the sphere’s diameter and v its terminal velocity. (As shown in Figure 1, the –ve sign in eq[4] arises because F s and v point in opposite directions.) In general, as the sphere falls, three forces are exerted on it: the gravitational force F g, the buoyant force F b, and the resistive force F s. The gravitational force is given by r 4 d 3 r Fg = π g ds , 3 2 B1-4 …[5] where ds is the density of the sphere. (F g and g point in the same direction.) The buoyant force is the force exerted by the mass of the displaced fluid. Thus r 4 d 3r Fb = – π g dl , 3 2 …[6] where dl is the density of the fluid. (F b and g point in opposite directions.) When the sphere is moving at its constant, or terminal velocity, Newton’s First Law demands that the sum of the forces on it be zero: r r r Fb + Fs + Fg = 0 . …[7] One can show that by substituting eqs[4], [5] and [6] into eq[7] η has the following form 2 2g d η = (ds – dl ) , 9v 2 …[8] where g = g and v = v . Calculating η from the measurement of v, d, ds and dl is the objective of this experiment. Simple Measurements 1 The Experiment Exercise 0. Preparation Orientation Identify the large glass cylinder of shampoo mounted on a stand. On the surface of the cylinder two marks are inscribed a distance 0.50 meter apart to assist you in measuring average velocity. Identify three marbles of different color and the Prosonic model 1301 stopwatch (precision ± 0.01s). The stopwatch will enable you to measure times of fall between the marks. A vernier caliper has been issued to enable you to measure the diameter of the marbles and therefore to calculate their volumes. There are a number of measurements both basic and derived in this experiment. First Measurements As you can see from eq[8] the expression for viscosity involves a number of factors. The simplest measurements are the diameter d of the marbles and their mass m. (The density of the shampoo is given as 1.013 kg.l–1 @ 20ºC.) You can measure the mass of the marbles with the balance set up in the lab. You will use the diameter of your marble in two places—in eq[8] and in the density ρ (=m/V). =AVERAGE(B2:B7) =STDEV(B2:B7)/SQRT(6) Average: STDM: You are strongly urged to measure a diameter several times and take an average (as your marbles might not be as spherical as you expect). Your table of data might resemble Figure 3. Find an average ds for each marble and, of course, the uncertainty in the average. For completeness note the temperature of the shampoo. First Uncertainties Though you can estimate reading uncertainties, uncertainties in derived quantities you have to calculate. To get some practice in calculating uncertainties using the quadrature formula, first find the uncertainty in density. You may wish to confirm your answer with the following result from the Maple worksheet Quadrature Calculator: the_error := 6 9 ∆( d ) 2 m 2 2 π d8 + ∆( m ) 2 2 π d6 where ∆(d) represents the uncertainty in d, ∆(m) the uncertainty in m. Diameter (cm) 2.458 2.469 2.457 2.473 2.471 2.448 2.46266667 0.00402216 (2.463 ±0.004) cm Figure 3. An example table (taken from an Excel spreadsheet) showing how you might tabulate the data for the diameter of a marble and calculate the average and standard deviation of the mean (STDM). B1-5 1 Simple Measurements Exercise 1. Finding Terminal Velocity The most dramatic activity in this experiment is finding a marble’s terminal velocity. You may be skeptical at first that a marble will indeed move downwards through the shampoo at a uniform velocity. For the sake of an initial demonstration therefore, choose one of the marbles and very carefully release it with the tweezers from a point just beneath the surface of the fluid. (If you do this carefully you should be able to minimize the formation of bubbles which might otherwise influence the marble’s motion.) As you watch the marble descending, does it appear to be moving at a constant velocity (or constant speed)? Do you think the marble is probably moving at a constant speed by the time it reaches the upper mark on the cylinder? (There is more on this in the section “Activities Using Maple”.) When the marble has reached the bottom of the cylinder you can retrieve it using the tool provided. Go ahead and find the terminal speed v of each marble by releasing it in the fluid and measuring its time of fall between the two marks. Take several readings, tabulate your results and estimate the uncertainties. Exercise 2. Calculating Viscosity Now that you have averages for the factors v, ds , dl and d for each marble you can calculate an η for the use of each marble from eq[8]. Keep track of your experimental uncertainties very carefully. There is no “accepted value” for the viscosity of this type of shampoo. The distributor suggests it may be anywhere in the range 12 - 15 Pl. ? ? ? Questions and Problems: ? Is there a systematic change in your calculated value of viscosity as you go to larger marbles? ? What is your average value of viscosity? What is the difference expressed as a percent between your average value and, say, what might be described as a “suggested average” of 13.5 Pl? List those quantities you have measured or calculated in this experiment and describe them as basic or derived. The SI system of units is also described as the MKSA system. What do the letters “M”, “K”, “S”, “A” stand for? Comment on their nature —are they basic or derived? Exercise 3. Calculating the Uncertainty in the Viscosity Calculating the experimental uncertainty in the viscosity is a good test of the method of quadratures. You should be able to show that eq[8] is just a special example of Example 3 in Appendix 1, i.e., ∆η is ∆( r ) 2 g 2 r 2 ( ds −dl ) 2 ∆( ds ) 2 g 2 r 4 ∆( g ) 2 r 4 ( ds −dl ) 2 the_error := sqrt 4 + + 9 2 2 v v v2 2 + ∆( dl ) 2 g 2 r 4 B1-6 v2 ∆( v ) 2 g 2 r 4 ( ds −dl ) 2 + 4 v Simple Measurements 1 Having calculated the uncertainty in the viscosity, ∆η, can you state that your value of η agrees with the “suggested average” value to within ∆η? Physics Demonstrations on LaserDisc from Chapter 31 Viscosity Demo 14-02 Viscous Drag Demo 14-06 Oil Viscosity Activities Using Maple T04Quadrature Calculator Quadrature Calculator is a Maple worksheet that will calculate the uncertainty in any function based on the variables you input. An alias to Quadrature Calculator is inside all experiment folders. To run just double click its icon. E01Simple Measurements Simple Measurements is a Maple worksheet that enables you to model the motion of your marble to determine if it really is moving with terminal velocity by the time it passes the top mark on the cylinder of shampoo. An example of its output is shown in Figure 5. B1-7 1 Simple Measurements Figure 5. Typical Output from the Maple worksheet Simple Measurements. SQ97 EndNotes for Simple Measurements 1 The mass obtained in such an experiment is called inertial mass. Weighing an object with a balance is a process of comparing the gravitational force that is exerted on the object with the force exerted on an object of known mass. (The latter is commonly referred to as a standard mass.) It only works if gravity is present. A balance is usually not calibrated in force units like it should be but rather in mass units of grams or kilograms. The mass obtained is often called the gravitational mass (for obvious reasons, perhaps, and to distinguish it from the inertial mass which is found from an experiment involving accelerated motion). 3 The magnitude of g in Scarborough, rounded to 3 decimal places, is 9.804 m.s–2 . The vector nature of weight is often ignored as the direction of the weight vector is usually understood to be downwards towards the center of the earth. 4 In physics we try to avoid the word “actual” because it implies the existence of some intrinsic reality beyond what is establishable by measurement. By “actual weight” here is only meant the weight measured in the absence of a buoyant force, as might be obtained in a vacuum. 2 B1-8