* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Avian flu
Globalization and disease wikipedia , lookup
DNA vaccination wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Henipavirus wikipedia , lookup
Immunocontraception wikipedia , lookup
Common cold wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
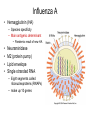
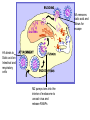
The Avian flu H5N1 The Next Pandemic ? • At the end of every summer million of ducks and wild geese mass on Canadian and Siberian lakes for their annual migration. • Influenza blooms. – In intestinal tract of juveniles – Diverse strains – Shed virus as they migrate south Influenza in Mammals • In humans and pigs influenza is very pathogenic. – Infects the respiratory tract. – Spread by aerosol. • Three genera of Influenza: A, B, and C. – B and C endemic to human population – Type A is mostly found in birds • A, is most lethal to humans. The common symptoms of the flu include: • • • • • • • Fever (usually high) Headache Muscle aches Chills Extreme tiredness Dry cough Runny nose may also occur but is more common in children than adults • Stomach symptoms, such as nausea, vomiting, and diarrhea, may also occur but are more common in children than adults Virulence factors • Portal of entry – Respiratory mucus membrane • Attachment – Hemagglutinin • Evade the immune system – Antigenic drift or shift – Envelope contains host proteins • Tissue Distruction – Inflamation of the respritory system. Transmission • Vehicle transmission through the Air. • Flu season in northern latitudes is from November to March, the coldest months. In southern latitudes, it is from May until September. In the tropics, there is not much flu at all and no real flu season. • In Italian, it was originally named influenza di freddo, or “influence of the cold.” – The virus is much more stable in cold dry wheather. Influenza A • Hemagglutinin (HA) – Species specificity – Main antigenic determinant • Pandemic result of new HA • • • • Neuraminidase M2 (protein pump) Lipid envelope Single stranded RNA – Eight segments called ribonucleoproteins (RNAPs) – make up 10 genes NA removes sialic acid and allows for escape HA binds to Sialic acid on Intestinal and respiratory cells M2 pumps ions into the interior of endosome to uncoat virus and release RNAPs Evolutionary Shape Shifters • Compared to other pathogens, influenza A is evolving at record breaking speed. • From year to year its proteins change amino acids to create modified strains requiring new vaccines (antigenic drift). • About every 20 -30 years influenza A will change drastically enough to jump species (antigenic Shift). Mutation rate of Influenza • The synthesis of RNA is radically error prone – DNA polymerases proof read and auto corrects their mistakes. • 1 mutation every billion nucleotides – RNA polymerases does not proof or correct their copy • Error rate is 1 million times higher than DNA pol. • Progeny often referred to as a “mutant swarm”. – Lives on the edge of error catastrophe. The Making of a Pandemic • Influenza can mutate by great leaps. – RNA is packaged in separate segments. a co-infection of a host by two different subtypes can result in a reassortment of their genes and cause an antigenic shift. • Influenza can trade RNP’s between different strains. This antigenic shift produce new hybrids. • These new hybrids have never been seen by the human population. A pandemic will ensue. History tells us it is not a matter of “IF” but a matter of “When” 1918 Flu • Researchers have Isolated and sequenced the genes from the 1918 flu. • The 1918 flu came from birds. • It killed more people in 2 months than HIV has killed in 20 years. Scenario for a Pandemic What we can expect N1H5 1. 2. Infect as much as a quarter of the world's population and killing at least seven times the number of AIDS deaths, all within a matter of weeks. Travel will be restricted. Food supply will shut down. People won’t travel between countries. Drugs come from other countries. We will run short on pharmaceuticals. Oil shipments are likely to lag as transportation between countries grinds to a halt. Heat in the winter months may be short supply H5N1 • We have very little surge room in our hospitals. • Patients will be in gymnasiums and coliseums. – In Katrina we had 48 other states and other countries that weren’t effected that could help. But in a pandemic no one will be there to help, everyone will be asking for help. N1H5 • Mask (N95 ) will run out. – No one will be allowed to leave their house without a mask. – Church and schools will all close. – Many businesses will shut down – Quarantines will be enforced. – President Bush is talking about Marshal Law. N1H5 • How do we handle the dead. – 1.5 million dead in this country alone. – We need to start planning for this. • How many Body bags does our community have? FDA Approves First U.S. Vaccine for Humans Against the Avian Influenza Virus H5N1 • The vaccine could be used in the event the current H5N1 avian virus were to develop the capability to efficiently spread from human to human, resulting in the rapid spread of the disease across the globe. Should such an influenza pandemic emerge, the vaccine may provide early limited protection in the months before a vaccine tailored to the pandemic strain of the virus could be developed and produced. What vaccines are being tested? • In 2004, NIAID awarded two contracts for production and clinical testing of investigational vaccines against H5N1. Both Sanofi Pasteur (Swiftwater, PA) and Chiron (Emeryville, CA) are producing vaccines made from inactivated H5N1 viruses for NIAID to test in clinical trials. Under these contracts, sanofi pasteur has already delivered more than 8,000 doses to NIAID; Chiron will produce 10,000 doses, which are expected to be delivered to NIAID within the next few months. When did NIAID begin testing the H5N1 vaccines? • The first clinical trial began in April 2005; it is testing the vaccine produced by sanofi pasteur in 451 healthy adults ages 18 to 64. This trial,is investigating the safety of the vaccine and its ability to generate an immune response • So far the vaccine has failed to provide adequate immunity even after a second booster. (For more info, visit www.ClinicalTrials.gov.)