* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Review of the Atom
Survey
Document related concepts
Transcript
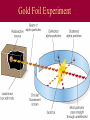
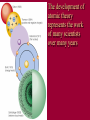
History of Atomic Theory Democritus vs. Aristotle • proposed all matter made of particles (“atoms”) • proposed 4 elements: Earth, Wind, Fire, Water John Dalton & Atomic Theory 1803 Dalton proposed Atomic Theory: 1. All matter composed of atoms ٭2. Atoms are indestructible - can’t be created or destroyed during physical/chemical changes ٭3. All atoms of an element are identical (same mass) 4. Atoms of different elements have different masses 5. Compounds are formed by combining atoms of different elements Dalton’s Model: billiard ball model •Solid, indivisible sphere Thomson Discovered the electron Cathode Ray Tubes Thomson showed that cathode rays were streams of negatively charged particles • rays were much smaller than atoms Thomson’s Model: plum-pudding model (AKA: “chocolate chip cookie”) Solid, positive sphere; negative charges distributed randomly throughout Thomson’s Plum-Pudding Model atom’s (+) charge evenly spread out while (-) charge is in bits – like chocolate chips in cookies Rutherford Rutherford’s Experiment - 1911 Discovered nucleus with his alpha scattering gold foil experiment The experiment showed the atom: Is mostly empty space Contains a dense core called nucleus (-) charged particles surround nucleus Nucleus contains (+) charged particles called protons Gold Foil Experiment Rutherford’s exp’t: animation Rutherford’s Model: nuclear model source • atom has a dense (+) core with (-) electrons orbiting around the nucleus Neils Bohr: •Proposed that electrons travel in fixed, circular pathways around nucleus, held in place by protons (+) •worked only for hydrogen Bohr’s Electron Shell Model: • Electrons travel in distinct, fixed energy levels around a (+) nucleus Ground state: electrons in lowest possible energy levels Excited state: one or more electrons move up to a higher energy level Schrodinger’s Model Orbital Model Electron’s are in a cloud surrounding the (+) nucleus Electrons are located in “probability regions” or orbitals These are not circular orbits! Orbitals differ in size, shape & spacial orientation Explained bright-line spectra for all elements, not just hydrogen The development of atomic theory represents the work of many scientists over many years