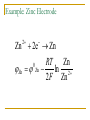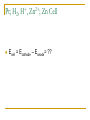* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 03.Thermodynamics in Corrosion Engineering
Survey
Document related concepts
Transition state theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Chemical potential wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
History of electrochemistry wikipedia , lookup
Transcript
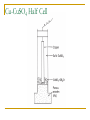
Thermodynamics in Corrosion Engineering Lecture#03-04 Utility of Thermodynamics in Electrochemistry Thermodynamic considerations allow the determination of whether a reaction can occur spontaneously If metal dissolution is unfavorable thermodynamically in a given set of circumstances – the job of the corrosion engineer is done Example: Copper in pure deoxygenated water Objectives To relate your thermodynamic knowledge with the thermodynamics of corrosion-related electrochemistry To describe the need for and characteristics of reference electrodes To describe the origin, use, and limitations of electrochemical phase diagrams (such as Pourbaix diagram) Free Energy: Driving Force of a Chemical Reaction Spontaneous Spontaneous Relation of ΔG and emf G EnF ΔG is in Joules E is emf in volts n is the number of electrons involved in the reaction F is the Faraday (96500 C/equivalent) The larger the value of E for any cell – more is the tendency for the overall cell reaction to proceed Ecell = Ecathode - Eanode The Nernst Equation General Reaction for a Galvanic Cell lL mM qQ rR Nernst Equation: q r RT aQ .aR ... EE ln l m nF aL .aM ... 0 Half Cell Potential When a metal M is immersed in an aqueous electrolyte, it acquires a certain potential. If the activity of the metal ions M++ in the aqueous environment is unity, then the acquired potential is known as standard potential φ0 Potential of each electrode can be calculated using Nernst equation Example: Zinc Electrode Zn 2 2e Zn Zn 0 Zn RT Zn ln 2 2 F Zn Hydrogen Electrode It is assumed arbitrarily that the standard potential for the following reaction is equal to zero at all temperatures So Standard Hydrogen Electrode (SHE) The potential of the electrode equals zero if the hydrogen ion activity and the pressure of hydrogen gas in atmospheres are both unity. This is the standard hydrogen potential The half - cell potential for any electrode is equal to the emf of a cell with the standard hydrogen electrode as the other electrode. The half - cell potential for any electrode expressed on this basis is said to be on the normal hydrogen scale or on the standard hydrogen scale , sometimes expressed as φH or φ ( S.H.E. ) Convention of Signs and Calculation of EMF Zn 2 2e Zn 0 0.763 V Zn - 2e Zn 2 0 0.763 V It was agreed at the 1953 meeting of the International Union of Pure and Applied Chemistry that the reduction potential for any half - cell electrode reaction would be called the potential Pt; H2, + H , 2+ Zn ; Zn Cell Ecell = Ecathode – Eanode= ?? Reference Half Cells It is not always convenient to have a hydrogen electrode in the laboratory Other reference half-cells (reference electrodes) have been introduced. Calomel reference electrode Ag-AgCl half cell The Saturated Copper-Copper Sulfate half cell Calomel Reference Electrode Ag-AgCl Reference Electrode Cu-CuSO4 Half Cell Number Line for Potential Conversion Among Different Reference Electrode Scales Oxygen Electrode Oxygen Electrode and Differential Aeration Cell Consider two O2 electrodes: one in contact with O2 at 1 atm other in contact with O2 at 0.2 atm Oxygen Electrode and Differential Aeration Cell The reaction is not thermodynamically possible as written Thus, the electrode 1 is cathode electrode 2 the anode. In a differential aeration cell, the electrode in lower O2 pressure acts as the anode and the one in higher O2 pressure acts as the cathode EMF Series All metals have been arranged in a series according to their standard potential (φ0) values. The more positive value corresponds to noble metals and the more negative value corresponds to more reactive metals (when arranged according to reduction potential) Of the EMF series – if two metals make up a cell, the more active metal acts as the anode and the more noble metal of the two will act as cathode EMF Series Problems with EMF Series In real situation, the activities of the metal ions in equilibrium with the respective metals usually do not equal unity The position of a metal in the EMF series with respect to another metal may change because of complex formation as is the case with tin (Sn) and steel (Fe) Alloys are not included in the EMF series In oxidizing environment, some metals undergo passivation and are known as active-passive metals. Transition metals usually show passive behaviour in aerated aqueous environment. This dual position of some metals is not reflected in the EMF series. Galvanic Series Galvanic series is an arrangement of both metals and alloys according to their actual measured potentials in a particular environment. There would be one Galvanic series for each environment Metals and alloys showing active-passive behaviour are listed in both active and passive states. Galvanic Series in Seawater Pourbaix Diagram Marcel Pourbaix developed potential-pH diagrams to show the thermodynamic state of most metals in dilute aqueous solutions With pH as abscissa and potential as ordinate, these diagrams have curves representing chemical and electrochemical equilibria between metal and aqueous environment These diagrams ultimately show the conditions for immunity, corrosion or passivation. Simplified Pourbaix Diagram for Iron Pourbaix Diagram for Iron Pourbaix Diagram for Iron at 25°C Benefits of Pourbaix Diagram Pourbaix diagrams offer a large volume of thermodynamic information in a very efficient and compact format. The information in the diagrams can be beneficially used to control corrosion of pure metals in the aqueous environment By altering the pH and potential to the regions of immunity and passivation, corrosion can be controlled. For example, on increasing the pH of environment in moving to slightly alkaline regions, the corrosion of iron can be controlled Changing the potential of iron to more negative values eliminate corrosion, this technique is called cathodic protection. Raising the potentials to more positive values reduces the corrosion by formation of stable films of oxides on the surface of transition metals Limitations of Pourbaix Diagrams These diagrams are purely based on thermodynamic data and do not provide any information on the reaction rates Consideration is given only to equilibrium conditions in specified environment and factors, such as temperature and velocity are not considered which may seriously affect the corrosion rate Pourbaix diagrams deal with pure metals which are not of much interest to the engineers