* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chemistry - Lyons USD 405
Survey
Document related concepts
Transcript
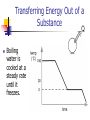
Chemistry Calculating Heat Transfer of Energy into a Substance Solid water (ice) is heated at a steady rate until some has boiled away Transfer of Energy into a Substance At the plateaus, the particles rearrange as the substance changes phase. Energy is stored as Ei. Transfer of Energy into a Substance On the inclines, particles are moving faster as the temperature of the substances increases. Energy is stored as Eth. Transferring Energy Out of a Substance Boiling water is cooled at a steady rate until it freezes. Transferring Energy Out of a Substance At the plateaus, the particles are rearranging as the Ei decreases and it changes phase Transferring Energy Out of a Substance On the declines, the particles are slowing down as the Eth of the substance decreases. Heat Capacity (c) The amount of energy required to raise the temperature of one gram of a substance one degree Celsius. 4.18J/g°C or 1 calorie for liquid water Thus it takes 4.18Joules of energy to raise one gram of liquid water one degree Celsius. Which Requires More Energy? Raise 10 grams of liquid water one degree Celsius? J 4.18 x10 g 41.8 J or g C Raise one gram of liquid water 100 degrees Celsius? 4.18 J x100C 418 J g C Calculating Heat required to change temperature (Eth) The energy required to change the temperature of a substance is equal the mass of the substance X the heat capacity of the substance X the change in Temperature. Q (m)(c)( T) Determining Change in Temperature (ΔT) Change in temperature is the difference from beginning temperature to final temperature T T f Ti Change in temperature will be positive if energy is being added and negative if energy is being lost Energy Transfer during a Phase Change We can’t use the earlier equation because there is no temperature change during a phase change. This new equation is based on the amount of energy required to rearrange the particles during a phase change. Heat of Fusion (ΔHf ) The amount of energy required to rearrange the particles of one gram of solid to one gram of liquid (or one gram of liquid to one gram of solid) Heat of fusion for water is 334J/g. Heat of Fusion (ΔHf ) Heat of fusion can be positive or negative depending on the direction of the energy transfer -Hf Q Substance Substance Q +Hf Heat of Vaporization (ΔHv) The amount of energy required to change one gram of liquid into one gram of gas (or one gram of gas into one gram of liquid) The heat of vaporization of water is 2260J/g Heat of Vaporization (ΔHv) Heat of vaporization can be positive or negative depending on the direction of energy transfer. -Hv Q Substance Substance Q +Hv Calculating Heat during a Phase Change The heat required for a phase change is equal to the mass of the substance X the heat of fusion / heat of vaporization of the substance. Q mHf Q mH v Practice Problems A cup of coffee (140 g) cools from 75˚C down to comfortable room temperature 20˚C. How much energy does it release to the surroundings? Eth Ei Eth Cup of Coffee Q (m)(c)( T) J 20C 75C Q 140 g 4.18 g C Q 32,200 J Ei Practice Problems Suppose during basketball practice, you lost 2.0 lbs of water due to sweating. If all of this water evaporated, how much energy did the water absorb from your body? (1kg = 2.2lbs) 2.0lb 1kg Eth Ei Eth Sweat Q mH v J Q 910 g 2260 g Q 2,060,000 J Q 2,060kJ Ei x 1 2.2lb .91kg 910 g .91kg