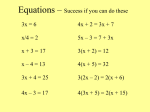* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download + __ O 2
Survey
Document related concepts
Transcript
Balancing chemical equations Balancing chemical equations Balancing Equations __ B4H10(g) + __ O2(g) H2O(g) ----> __ B2O3(g) + __ 1. There should be just as many of each kind of atom on the reactant side as on the product side 2. Start with coefficients of “1” 3. Do not change subscripts 4. Find lowest whole number coefficients 5. Find coefficients for elements last 6. “temporary” fractional coefficients are okay Balancing Equations __ B4H10(g) + __ O2(g) ----> __ B2O3(g) + __ H2O(g) B H O B H O Balancing Equations Fe(s) Fe + O O2 (g) ----> Fe2O3(s) Fe O Balancing Equations __ B4H10(g) + __ O2(g) B H O ----> __ B2O3(g) + __ H2O(g) B H O Write the balanced chemical equation for the combustion of ethane (C2H6) C2H6 + O2 C H O -----> H2O + CO2 C H O Write the balanced chemical equation for the combustion of methane CH4 + O2 C H O -----> H2O + CO2 C H O Write the balanced chemical equation for the combustion of octane (C8H18) C8H18 + O2 C H O -----> H2O + CO2 C H O Write the balanced chemical equation for the combustion of butane (C4H10) C4H10 + O2 C H O -----> H2O + CO2 C H O Word Equations to Formula Equations Exercise 6.3- 6.4 notes page 2 Part A and Part B Word Equations to Formula Equations A) Translate the word equations into chemical equations and balance them. E.g. 1) Word equation: solid sodium reacts with chlorine gas to produce solid sodium chloride. Chemical equation: ________________________________________________________ Species Reactant Side Product Side Balanced? Word Equations to Formula Equations Exercise Workbook Page 111 Question Carbon reacts with Oxygen to make carbon dioxide Word Equations to Formula Equations Carbon reacts with Oxygen to make carbon dioxide Word Equations to Formula Equations THE GROUP OF SEVEN: There are seven atoms that exist as diatomic molecules in nature They form a number 7 on the periodic table: Memorize them! H2, N2, O2, F2, Cl2, Br2, I2 H NO F Cl Br I Word Equations to Formula Equations Don’t forget about the diatomic elements! (Br I N Cl H O F) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a Word Equations to Formula Equations Exercise 6.3- 6.4 notes page 1 and Page 2 Part A 2 Word Equations to Formula Equations Nitrogen reacts with aluminum to make aluminum nitride Word Equations to Formula Equations N2 + 2Al 2AlN Word Equations to Formula Equations Hydrogen racts with nitrogen to make ammonia(NH3) Word Equations to Formula Equations Work book page 111 Question 40-48 Page 114 Question 60- 64