* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download AINR
Survey
Document related concepts
Transcript
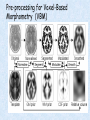
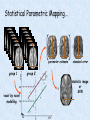
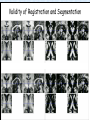
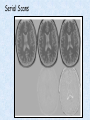
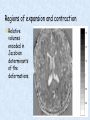
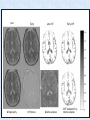
Voxel-Based Morphometry John Ashburner [email protected] Functional Imaging Lab, 12 Queen Square, London, UK. Voxel-Based Morphometry Produce a map of statistically significant differences among populations of subjects. e.g. compare a patient group with a control group. or identify correlations with age, test-score etc. The data are pre-processed to sensitise the tests to regional tissue volumes. Usually grey or white matter. Can be done with SPM software package, or other analysis tools. Pre-processing for Voxel-Based Morphometry (VBM) Smoothing Before convolution Convolved with a circle Convolved with a Gaussian 3 3 Units are mm of original grey matter per mm of spatially normalised space Pre-processed data for four subjects Warped, Modulated Grey Matter 12mm FWHM Smoothed Version Statistical Parametric Mapping… – group 1 voxel by voxel modelling parameter estimate standard error = statistic image or SPM group 2 SPM results... Validity of Registration and Segmentation Comparison with Manually Drawn ROIs. Good et al (2002). NeuroImage 17:29-46. Not quite comparing like-with-like. Validity of the statistical tests Residuals are not normally distributed. Little impact on uncorrected statistics for experiments comparing groups. Invalidates experiments that compare one subject with a group. Corrections for multiple comparisons. Mostly valid for corrections based on peak heights. Not valid for corrections based on cluster extents. SPM makes the inappropriate assumption that the smoothness of the residuals is stationary. • Bigger blobs expected in smoother regions. Alzheimer’s Disease Karas GB, Burton EJ, Rombouts SA, van Schijndel RA, O'Brien JT, Scheltens P, McKeith IG, Williams D, Ballard C & Barkhof F. A comprehensive study of gray matter loss in patients with Alzheimer's disease using optimized voxel-based morphometry. Neuroimage 18:895-907 (2003). Our results confirm earlier findings, but additionally we demonstrate global cortical atrophy with sparing of the sensorimotor cortex, occipital poles, and cerebellum. Moreover, we show atrophy of the caudate head nuclei and medial thalami. Our findings are in full agreement with the established neuropathological descriptions, offering a comprehensive view of atrophy patterns in AD. Gee J, Ding L, Xie Z, Lin M, DeVita C & Grossman M. Alzheimer's disease and frontotemporal dementia exhibit distinct atrophy-behavior correlates. Academic Radiology 10:1392-1401 (2003). VBM provides an important first step in analyzing brain-behavior relations in vivo in patients with neurodegenerative diseases. More refined analyses of brain morphology via high-dimensional normalization methods that are capable of modeling local as well as global variability in neuroanatomical structure promise to be even more informative. Busatto GF, Garrido GE, Almeida OP, Castro CC, Camargo CH, Cid CG, Buchpiguel CA, Furuie S & Bottino CM. A voxel-based morphometry study of temporal lobe gray matter reductions in Alzheimer's disease. Neurobiol Aging 24:221-31 (2003). These VBM results confirm previous findings of temporal lobe atrophic changes in AD, and suggest that these abnormalities may be confined to specific sites within that lobe, rather than showing a widespread distribution Testa C, Laakso MP, Sabattoli F, Rossi R, Beltramello A, Soininen H & Frisoni GB. A comparison between the accuracy of voxel-based morphometry and hippocampal volumetry in Alzheimer's disease. J Magn Reson Imaging 19:274-82 (2004). These results indicate that VBM is more accurate, but the combination of both methods provides the highest accuracy for detection of hippocampal atrophy in AD. Degeneration Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA & Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage 16:23-31 (2002). This work demonstrates methodological consistency between automated VBM and manual stereological analysis of the hippocampus in group comparisons Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, Crum WR, Rossor MN & Frackowiak RS. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage 17:29-46 (2002). We compared voxel-based morphometry (VBM) with independent accurate region-of-interest (ROI) measurements of temporal lobe structures in order to validate the usefulness of this fully automated and unbiased technique in Alzheimer's disease (AD) and semantic dementia (SD). In AD, ROI analyses appear more sensitive to volume loss in the amygdalae, whereas VBM analyses appear more sensitive to right middle temporal gyrus and regional hippocampal volume loss. In SD, ROI analyses appear more sensitive to left middle and inferior temporal gyrus volume loss, whereas VBM appears more sensitive to regional hippocampal volume loss. In addition the significance of volume reductions was generally less in VBM owing to more stringent corrections for multiple comparisons. In conclusion, the automated technique detects a general trend of atrophy similar to that of expertly labeled ROI measurements in AD and SD, although there are discrepancies in the ranking of severity and in the significance of volume reductions that are more marked in AD Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, McCusker E & Frackowiak RS. The distribution of structural neuropathology in pre-clinical Huntington's disease. Brain 125:181528 (2002). This suggests that VBM may be useful in monitoring cross-sectional and longitudinal changes in brain structure in pre-clinical Huntington's disease and for determining the efficacy of neuroprotective agents. Schizophrenia & Bipolar Disorder Moorhead TW, Job DE, Whalley HC, Sanderson TL, Johnstone EC & Lawrie SM. Voxel-based morphometry of comorbid schizophrenia and learning disability: analyses in normalized and native spaces using parametric and nonparametric statistical methods. Neuroimage 22:188-202 (2004). Overall, these VBM results replicate previous ROI findings and are compatible with the view that comorbid learning disability with schizophrenia is a severe form of schizophrenia, rather than a consequence of learning disability. VBM has the facility to compare grey matter distributions in this structurally diverse cohort. Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA & McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 17:1711-9 (2002). These findings suggest both the promise and utility of VBM in evaluating gray matter abnormalities. They further suggest the importance of comparing VBM findings with more traditional ROI analyses until the reasons for the differences between methods are determined. Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC & Lawrie SM. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage 17:880-9 (2002). These results are compatible with and extend the relevant findings of the previous volumetric ROI analysis, when allowing for the differences between the methods and interpretation of their results. Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, Friedman SD, Dunner DL, Renshaw PF. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry 55:648-51 (2004). The observation of a gray matter density decrease in the left anterior cingulate, which processes emotions, in bipolar subjects is consistent with prior reports that used region-of-interest analytic methods. Other... Luders E, Gaser C, Jancke L & Schlaug G. A voxel-based approach to gray matter asymmetries. NeuroImage 22:656-664 (2004). Since we were able to validate gender- and AP-related brain asymmetries previously described using traditional ROI-based morphometric techniques, the results of our analyses support the use of VBM for examinations of GM asymmetries. White NS, Alkire MT& Haier RJ. A voxel-based morphometric study of nondemented adults with Down Syndrome. Neuroimage 20:393-403 (2003). While these results are generally consistent with prior ROI-based imaging studies of nondemented DS individuals, the present findings provide additional understanding of the three-dimensional topography of DS morphology throughout the brain. The consistency of these findings with prior imaging reports demonstrates the utility of the VBM technique for investigating the neuroanatomy of DS. Ettinger U, Kumari V, Chitnis XA, Corr PJ, Sumich AL, Rabe-Hesketh S, Crawford TJ & Sharma T. Relationship between brain structure and saccadic eye movements in healthy humans. Neuroscience Letters 328: 225-228 (2003). VBM replicated this finding: gain was correlated with grey matter in the left cerebellar hemisphere and vermis. These findings agree with previous studies on the role of the cerebellar vermis in saccadic gain and support the validity of structural neuroimaging methods in elucidating the neural correlates of saccadic eye movements. Lesions & Malformations Mehta S, Grabowski TJ, Trivedi Y & Damasio H. Evaluation of voxel-based morphometry for focal lesion detection in individuals. Neuroimage 20:1438-54 (2003). Performance metrics revealed that (1) for this application, VBM had low sensitivity; (2) detection sensitivity was altered by model parameterization; (3) underperformance was due to the adverse influence of lesions on the preprocessing steps and to insufficient statistical power; and (4) VBM could not satisfactorily delineate the spatial extent of lesions, even in simulations that VBM is not a suitable stand-alone technique for detecting or spatially characterizing focal lesions. avoided preprocessing artifacts. In its current form, Gitelman DR, Ashburner J, Friston KJ, Tyler LK & Price CJ. Voxel-based morphometry of herpes simplex encephalitis. Neuroimage 13:623-31 (2001). We found that, despite problems in normalizing and segmenting these severely distorted brains, VBM was able to identify correctly a number of the regional gray matter abnormalities in HSE. The results, while consistent with the well-known histopathology of the disease, also demonstrate potential difficulties with this method. Wilke M, Kassubek J, Ziyeh S, Schulze-Bonhage A & Huppertz HJ. Automated detection of gray matter malformations using optimized voxel-based morphometry: a systematic approach. NeuroImage 20:330-343 (2003). However, a number of approaches failed to perform well. The reasons for these failures and the implications this has for other studies are discussed. We conclude that voxel-based morphometry is able to detect cortical malformations with a high degree of accuracy. However, specific problems seem to arise when using an optimized protocol for voxel-based morphometry, indicating that this protocol may not be optimal for all voxel-based studies on brain morphology. Our approach, involving systematic alterations of parameters and evaluation, may be useful for other studies. Words of Caution Bookstein FL. "Voxel-Based Morphometry" Should Not Be Used with Imperfectly Registered Images. NeuroImage 14:1454-1462 (2001). John Ashburner and Karl Friston (2000) introduced a standardized method of "voxel-based morphometry" (VBM) for comparisons of local concentrations of gray matter between two groups of subjects. Segmented images of gray matter from grossly normalized high-resolution images are smoothed and their group differences analyzed by the now-conventional voxelwise Worsley approach to Gaussian random fields of unfortunate interaction between the algorithm's spatial normalization and voxelwise comparison steps, whereby several obvious quantitative differences. This comment concerns an confounds are injected at the core of the inference engine the authors put forward. Specifically, the statistics of the resulting voxelwise comparisons are uninformative about group differences wherever the spatial normalization algorithm has failed to register on any robustly appearing image gradient. The method of Ashburner and Friston is defensible only far from all image gradients. Salmond CH, Ashburner J, Vargha-Khadem F, Connelly A, Gadian DG & Friston KJ. Distributional assumptions in voxel-based morphometry. Neuroimage 17:1027-30 (2002). In brief, our results indicate that nonnormality in the error terms can be an issue in VBM. However, in balanced designs, provided the data are smoothed with a 4-mm FWHM kernel, nonnormality is sufficiently attenuated to render the tests valid. Unbalanced designs appear to be less robust to violations of normality: a significant number of false positives arise at a smoothing of 4 and 8 mm when comparing a single subject to a group. This is despite the fact that conventional group comparisons appear to be robust, remaining valid even with no smoothing. Methodology References I. C. Wright, P. K. McGuire, J.-B. Poline, J. M. Travere, R. M. Murray, C. D. Frith, R. S. J. Frackowiak & K. J. Friston.A Voxel-Based Method for the Statistical Analysis of Gray and White Matter Density Applied to Schizophrenia. NeuroImage 2:244-252 (1995). I. C. Wright, Z. R. Ellison, T. Sharma, K. J. Friston, R. M. Murray & P. K. Mcguire. Mapping of Grey Matter Changes in Schizophrenia. Schizophrenia Research 35:1-14 (1999). J. Ashburner & K. J. Friston. Voxel-Based Morphometry - The Methods. NeuroImage 11:805821 (2000). J. Ashburner & K. J. Friston. Why Voxel-Based Morphometry Should Be Used. NeuroImage 14:1238-1243 (2001). C. D. Good, I. S. Johnsrude, J. Ashburner and R. N. A Henson, K. J. Friston & R. S. J. Frackowiak. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. NeuroImage 14:21-36 (2001). C. D. Good, R. I. Scahill, N. C. Fox, J. Ashburner, K. J. Friston, D. Chan, W. R. Crum, M. N. Rossor & R. S. J. Frackowiak. Automatic Differentiation of Anatomical Patterns in the Human Brain: Validation with Studies of Degenerative Dementias. NeuroImage 17:29-46 (2002). W.R. Crum, L.D. Griffin, D.L.G. Hill & D.J. Hawkes. Zen and the art of medical image registration: correspondence, homology, and quality. NeuroImage 20:1425-1437 (2003). http://www.fil.ion.ucl.ac.uk/spm Serial Scans Early Late Difference Data from the Dementia Research Group, Queen Square. Regions of expansion and contraction Relative volumes encoded in Jacobian determinants of the deformations. Late Warped early Early Difference Late CSF Relative volumes Early CSF CSF “modulated” by relative volumes Late CSF - modulated CSF Late CSF - Early CSF Smoothed