* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Unit 1 - Introduction
Survey
Document related concepts
Transcript
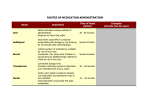
Unit 1 - Introduction Pre-reading task A. Before you read the text, can you tell … 1. What factors delay/prevent the discovery of drugs that could cure diseases that are so far incurable? ………………………………………………………………………………………………..… ………………………………………………………………………………………………..… 2. What features of a substance does a researcher look into when considering the development of a new drug? ………………………………………………………………………………………………..… ………………………………………………………………………………………………..… B. 1. The term ‘lead compound’ is an important one in the process of drug discovery. Can you scan the text to find what it is? ………………………………………………………………………………………………..… ………………………………………………………………………………………………..… 2. The questions of the pre-reading activity are answered by the information contained in the text. You can skim the text to verify or enhance your answers. A1. ………………………………………………………………………………………………….. ………………………………………………………………………………………………….. A2. ………………………………………………………………………………………………….. ………………………………………………………………………………………………….. What have you noticed about the answers? Where are they to be found? How does that help you locate the main ideas of a text? ………………………………………………………………………………………………..… ………………………………………………………………………………………………..… 1 Introduction P. N. Kourounakis and E. A. Rekka Department of Pharmaceutical Chemistry, School of Pharmacy, Aristotelian University of Thessaloniki, Thessaloniki 540 06, Greece The discovery of drugs and drug molecules has always been the aim of pharmaceutical sciences and, in particular, of medicinal chemistry, which evolved from pharmaceutical chemistry. Half a century ago, pharmacochemistry, the modern expression of pharmaceutical chemistry, as a science whose main interest is the design and development of new pharmacomolecules, was at the beginning of its evolution. Drug design in its broad sense and structure-activity relationship studies are essential and at the heart of medicinal chemistry, and it is the progress and development of this field of research that has made medicinal chemistry the modern and enormously productive science it has become in recent decades. Today, studies on structure-activity relationships and their influence on the design of new drugs have rendered them one of the most useful and thus important activities of pharmacochemistry, a modern component science in the group of pharmaceutical sciences. Despite the advances in medical and pharmaceutical sciences, there are still many diseases which are incurable or can only be treated symptomatically, and at a great economic and social cost owing to only moderately effective or even to the lack of appropriate therapeutic agents. Of the 30 000 or so diseases or disorders currently known, only one third can somehow be treated with drugs. Furthermore, there are incurable maladies, like viral diseases (influenza, AIDS), CNS disorders (Alzheimer’s disease), cancer and autoimmune disorders, which can be fatal or cause great suffering and disability. Therefore, there is still a great need for more and better drugs – more active and selective, drugs with fewer undesired or toxic side-effects, agents useful in prophylaxis and drugs which will cause as little as possible harmful contamination in the already polluted environment. In a systematically planned program of drug discovery, several questions have to be answered: - Is the research for the discovery of a certain drug justified by the medical expectation? - How will the expected drug contribute to health? - - What would be the economic or other, more noble benefit that is expected from the drug? Is the state of the art of medicinal chemistry at a satisfactory level so that the risk of investing in the project should be taken? That is, have the coordinated attempts a favorable possibility for solving the problem in a reasonable time period? Does the specific disease affect sufficient people for the economic attempt to be justified? It is tragic that serious diseases, mainly in developing countries, are sometimes uncontrolled because of a lack of effective therapeutic agents due to the non-existent financial profit. 2 Because of the strict prerequisites of national drug authorities, which are becoming even more demanding, the cost of drug discovery is steadily increasing. Thus, rational drug design becomes the main objective of medicinal chemistry today. Based on rational design, new structures can be developed with a high probability of possessing the required properties. The setting of clear rules to help in the access to information hidden in accumulated experimental data is necessary, and this requires studies on the quantitative relationships between (physicochemical) properties and (biological) activity. We are thus led to the selection of a subsystem of compounds originating from an initial structure, the lead compound, the discovery of which is the most decisive step in the process of drug discovery. Methods used in lead compound discovery include: folk/ethnopharmacy and therapeutics; massive pharmacological screening; fortuitous discovery; modification of bioactive natural products; exploitation of secondary or side-effects of drugs; study of the basic processes of life; body biochemistry and the use of metabolic analogues; study and exploitation of differences in molecular biology, differential cytology, biochemistry and endocrinology; study of the biochemistry of diseases; an approach through the molecular mechanism of drug action; analysis of the mechanism of action and multipotent compounds; drug metabolism (hard, soft, pro- drugs); and chemical delivery systems. The pharmacochemical manipulations following the discovery of the lead compound include: attempts aimed at the development of substitutes of existing biologically active molecules; attempts aimed at the alteration of the activity spectrum of biologically active molecules; attempts aimed at the modification of the pharmacokinetics of the compounds used as drugs or as lead compounds; structural changes in natural products; molecular transformations performed by microbes; and other chemical processes that follow the molecular manipulations on the lead compounds (for example, construction of homologous series, application of the rule of bioisosteric groups, resolution of stereoisomers). It is evident that in the process of drug development the molecular structure is the main feature that determines the molecular properties, and thus whether the particular molecule finally reaches the patient. Since, in the majority of drugs, action appears after the interaction of the pharmacomolecule with its receptor, it seems reasonable to study the drug structure in relation to its receptor site (the dynamic aspect). For a productive drug-receptor interaction a good fit, determined by physicochemical properties, is required. Besides solving the basic synthetic problems, studies on the geometry and shape, conformational analysis and investigation of the influence of electronic and hydrophobic effects on drug-receptor interaction are performed. In quantitative structure-activity relationship studies attempts have been made, with success, to correlate quantitatively biological activity with molecular properties (electronic, hydrophobic, steric). This relationship has been based on the 3 assumption that the relative importance of physicochemical properties for biological activity can be described numerically, for an objective evaluation of drug-receptor interactions. Numerous methods have been invented for the quantification of electronic, hydrophobic and steric effects of functional groups. Statistical methods, mainly Hansch or extrathermodynamic analysis, as well as those of Free and Wilson, pattern recognition/principal components analysis and cluster analysis, can lead to the prediction and optimization of activity, and ultimately to the design of better drugs. The development of powerful, interactive computers and molecular graphics systems helps in the analysis and visualization of biologically active compounds and in a better understanding of drug-receptor interactions. Techniques have been developed for the determination and visual presentation of pharmacophores (receptor mapping), as well as techniques for drug design based on a knowledge of receptor structure (receptor fitting). The pharmacomolecule, before interacting with its receptor (this interaction being direct or indirect, a simple binding –affinity- or a productive interaction yielding a biological effect –efficacy), must reach, intact and in satisfactory concentration, the immediate environment of the receptor site. Access to the receptor is also determined by the physicochemical properties of the molecule. Thus, structure plays a decisive role not only in the dynamics, but also in the kinetics of the drug molecule. Molecular structure is usually altered by the body. Drug metabolism, basically an adaptive process, is a rather useful property of the (liver) cell, as a whole. Drug biotransformation usually leads to more polar compounds, and thus to faster elimination, and to substances with lower or no activity. Only rarely is an increase of activity observed after biotransformation. However, in certain cases, very dangerous highly (chemically) reactive metabolic intermediates are formed. During drug metabolism, and through the catalytic activity of enzymes like the cytochrome P-450 family, prostaglandin synthase and xanthine oxidase, free radicals may be formed, which participate in the initiation and propagation of chain reactions. Oxygen is activated, and the presence of active oxygen species (O 2 - , H2O2, HO) may lead, via lipid peroxidation or other cellular structure damage, to cell injury and necrosis. Numerous pathophysiological conditions are probably due to radical attack and oxidative damage. A knowledge of the pathophysiology of diseases constitutes a decisive step towards the discovery of lead compounds. This could be conducted in various ways, for example by the study either of free radical scavenging activity or of free radical formation. Therefore, drug metabolism and, in particular, relationships between the structure of the drug molecule and the enzyme systems responsible for drug biotransformation, resulting in detoxification, but also in biotoxification, are currently subjects of active pharmacochemical investigation. The ever-increasing number of modern, improved drug molecules, the discovery of which is based upon a knowledge of drug biotransformations and oxygen 4 activation, supports the argument for the prominent position held by drug metabolism and free radical pharmacochemistry in currently used rational drug design techniques. This volume covers topics such as drug discovery and physicochemical properties, structure-activity relationships, structure-interaction with specific receptor subtypes, and in combating serious diseases that cause great financial and social problems, for example Alzheimer’s disease and gastric ulceration. Also discussed is the dependence of the biological properties of a compound on chemical structure, in terms of quantitative structureactivity relationships, the merits and shortcomings of computational chemistry and the techniques applied to gaining insight into the complex molecular phenomena in innovative drug research, the characterization and prediction of drug metabolism in humans and the importance of labeling of bioactive compounds in the study of the dynamics but mainly the kinetics of a prospective drug. These topics are presented by contributors, each one a specialist in his or her own field within the greater subject of pharmacochemistry. We are certain that the following chapters, tackling the subject of drug design from different viewpoints, will stimulate the creativity of those involved or interested in innovative drug research. Young medicinal chemistry investigators could be helped and inspired in their attempts to find new and better drug molecules among the structures waiting to be discovered. Kourounakis, P. N. and E. Rekka, 1994: 1-4 C. After having skimmed the text, you could try to assess the truth of the following statements. 1. National drug authorities place strict restrictions on drug discovery research. 5 2. Modification of the pharmacokinetics of a substance is a step towards the development of a new drug. 3. The molecular structure of a component of a drug is the sole feature that determines its efficacy. 4. Drug biotransformation results in the formation of substances with increased activity. You can read the text to see if your answers are correct. D. The text contains several words that may present difficulty in your effort to understand it. Using your knowledge of the field and the context in which these appear, do you know/can you guess what the following mean? (the number of the paragraph appears in the beginning) 1. (1) render: …………………………………………………………………………………… 2. (1) component science: ……………………………………………………………………... 3. (2) moderately: ……………………………………………………………………………… 4. (2) CNS: …………………………………………………………………………………….. 5. (4) accumulated: …………………………………………………………………………….. 6. (5) fortuitous: ……………………………………………………………………………….. 7. (5) modification: ……………………………………………………………………………. 8. (7) feature: …………………………………………………………………………………... 9. (8) site: ……………………………………………………………………………………… 10. (8) fit: ……………………………………………………………………………………… 11. (8) conformational: ………………………………………………………………………... 12. (8) quantification: ………………………………………………………………………….. 13. (8) optimization: …………………………………………………………………………… 14. (9) yield: …………………………………………………………………………………… 15. (10) intact: …………………………………………………………………………………. 16. (10) via: ……………………………………………………………………………………. 17. (11) shortcomings: ………………………………………………………………………… E. Can you now guess which words mean the following? (2) disorders or diseases of the body: …………………………… (3) the latest and most sophisticated or advanced stage of a technology, art, or science: ……………………………………… (4) required beforehand: ……………………………. 6 (5) act of selecting, rejecting, considering, or grouping by examining systematically: ………………………………. (5) one of a group of chemical compounds similar in structure but different in respect to elemental composition: …………………………… (5) the study of the microscopic appearance of cells, esp. for the diagnosis of abnormalities and malignancies: ……………………………. (5) having power to produce or influence several effects or results: ……………………….. (8) assignment of a set of observations into subsets so that observations in the same set are similar in some sense: …………………………….. (9) act of uniting: …………………………….. (10) multiplication by natural reproduction; transmission or dissemination: ……………………... F. Can you match the terms on the right with their definitions on the left? 1. an atom or molecule that bears an unpaired electron and is extremely reactive, capable of engaging in rapid chain reactions that destabilize other molecules and generate a. correlate many more 2. the force by which atoms are held together in chemical b. steric c. affinity compounds 3. act of converting into an oxide of an element that d. peroxidation contains an unusually large amount of oxygen e. radical 4. bring into mutual or reciprocal relation 5. purify by introducing a substance that will combine chemically with impurities or undesired elements 6. pertaining to the spatial relationships of atoms in a molecule 7 f. scavenge Unit 2 - Routes of drug administration Pre-reading task: A. Which routes of drug administration do you know? Can you think of cases in which each one should be selected? Are you aware of how they affect the formulation of medicines? ...................................................................................................................................................... ...................................................................................................................................................... ...................................................................................................................................................... Pre-reading task: Oral route A. Do you know what kind of medicinal formulations are administered orally? Can you think of any advantages or disadvantages in the choice of this route of administration? ...................................................................................................................................................... ...................................................................................................................................................... B. Do you know or can you guess if the following statements are true or false? 1. The action of a drug in a tablet begins minutes after its administration. 2. A tablet is more suitable for pediatric use than other formulations administered orally. 3. Other medication that the patient may have taken affects the solubility of the drug administered in tablet form. 4. Enteric coating of tablets reduces the possibility of inactivation of the drug by the acidity of the stomach. 5. Tablets disintegrate faster than capsules in the body. 6. Emulsion formulations may take the form of capsules. 7. Suspensions are unsuitable for patients suffering from tonsillitis. You can check your answers by reading the text. Routes of drug administration The absorption pattern of drugs varies considerably between individual drug substances as well as between the different administration routes. Dosage forms are designed 8 to provide the drug in a suitable form for absorption from each selected route of administration. The following discussion considers briefly the routes of drug administration and whilst dosage forms are mentioned, this is intended only as an introduction since they will be dealt with in greater detail later in this book. Oral route The oral route is the most frequently used route for drug administration. Oral dosage forms are intended usually for systemic effects resulting from drug absorption through the various epithelia and mucosa of the gastrointestinal tract. A few drugs, however, are intended to dissolve in the mouth for rapid absorption or for local effect in the tract due to poor absorption by this route or low aqueous solubility. Compared with other routes, the oral route is the simplest, most convenient and safest means of drug administration. However, disadvantages include relatively slow onset of action, possibilities of irregular absorption and destruction of certain drugs by the enzymes and secretions of the gastrointestinal tract. For example, insulin-containing preparations are inactivated by the action of stomach fluids. Table 1.2 Variation in time of onset of action for different dosage forms Time of onset of action Dosage forms Seconds i.v. injections Minutes i.m. and s.c. injections, buccal tablets, aerosols, gases Minutes to hours Short-term depot injections, solutions, suspensions, powders, granules, capsules, tablets, modified-release tablets Several hours Enteric-coated formulations Days to weeks Depot injections, implants Varies Topical preparations Whilst drug absorption from the gastrointestinal tract follows the general principles described later in this book, several specific features should be emphasized. Changes in drug solubility can result from reactions with other materials present in the gastrointestinal tract, as for example the interference of absorption of tetracyclines through the formation of insoluble complexes with calcium, which can be available from foodstuffs or formulation additives. Gastric emptying time is an important factor for effective drug absorption from the intestine. Slow gastric emptying can be detrimental to drugs inactivated by the gastric juices and can delay absorption of drugs more effectively absorbed from the intestine. In addition, since environmental pH can influence the ionization and lipid solubility of drugs, the pH change occurring along the gastrointestinal tract, from a pH of about 1 in the stomach to approximately 7 or 8 in the large intestine, is important to both degree and site of drug absorption. Since membranes are more permeable to unionized rather than ionized forms and 9 since most drugs are weak acids or bases, it can be shown that weak acids, being largely unionized, are well absorbed from the stomach. In the small intestine (pH about 6.5), with its extremely large absorbing surface, both weak acids and weak bases are well absorbed. The most popular oral dosage forms are tablets, capsules, suspensions, solutions and emulsions. Tablets are prepared by compaction and contain drugs and formulation additives which are included for specific functions, such as disintegrants which promote tablet breakup into granules and powder particles in the gastrointestinal tract, facilitating drug dissolution and absorption. Tablets are often coated, either to provide a protective barrier to environmental factors for drug stability purposes or to mask unpleasant drug taste, as well as to protect drugs from the acid conditions of the stomach (enteric coating). Increasing use is being made of modified-release tablet products such as fast dissolving systems and controlled, delayed or sustained-release formulations. Benefits of controlled-release tablet formulations, achieved for example by the use of polymeric-based tablet cores or coating membranes, include reduced frequency of drug-related side-effects and maintaining steady drug-plasma levels for extended periods, important when medications are delivered for chronic conditions or where constant levels are required to achieve optimal efficacy, as in treating angina and hypertension. Capsules are solid dosage forms containing drug and, usually, appropriate filler(s), enclosed in a hard or soft shell composed of gelatin. As with tablets, uniformity of dose can be readily achieved and various sizes, shapes and colors of shell are commercially available. The gelatin shell readily ruptures and dissolves following oral administration and in most cases drugs are released from capsules faster than from tablets. Recently, renewed interest has been shown in filling semi-solid and microemulsion formulations into hard gelatin capsules to provide rapidly dispersing dosage forms for poorly soluble drugs. Suspensions, which contain finely divided drugs suspended in a suitable vehicle, are a useful means of administering large amounts of drugs that would be inconvenient if taken in tablet or capsule form. They are also useful for patients who experience difficulty in swallowing tablets and capsules and for pediatric use. Whilst dissolution of drugs is required prior to absorption, fine particles with a large surface area are presented to dissolving fluids which facilitate drug dissolution in the gastrointestinal tract, absorption and thereby the onset of drug action. Not all oral suspensions, however, are formulated for systemic effects and several are designed for local effects in the gastrointestinal tract. On the other hand, solutions, including formulations such as syrups and linctuses, are absorbed more rapidly than solid dosage forms or suspensions since drug dissolution is not required. Aulton, M., 2007: 7-8 10 C. Can you answer the following questions using the information in the extract you have read? 1. What are the disadvantages of the oral route of administration? ...................................................................................................................................................... ...................................................................................................................................................... 2. What are the factors that affect drug absorption when this route is selected? ...................................................................................................................................................... ...................................................................................................................................................... 3. What do tablets consist of? ...................................................................................................................................................... ...................................................................................................................................................... 4. Why are tablets coated? ...................................................................................................................................................... ...................................................................................................................................................... 5. In which cases can suspensions prove useful? ...................................................................................................................................................... ...................................................................................................................................................... 11 D. Can you explain the meaning of the following words as these appear in the extract? (the number of the paragraph they appear in is given in the brackets) (1) onset: ………………………………………………………………………………………. (2) detrimental: ………………………………………………………………………………… (2) permeable: …………………………………………………………………………………. (3) side-effects: ………………………………………………………………………………... (3) optimal: …………………………………………………………………………………….. (4) readily: ……………………………………………………………………………………... (4) ruptures: ……………………………………………………………………………………. (5) vehicles: ……………………………………………………………………………………. E. Can you match the following words with the definitions given? (the number of the paragraph they appear in is given in brackets) mucosa (1), gastrointestinal (1), secretion (1), lipid (2), angina (3), capsule (4), linctus (5) 1. any of a group of organic compounds that are greasy to the touch, insoluble in water, and soluble in alcohol and ether; together with proteins and carbohydrates, they constitute the chief structural components of living cells: …………………………….. 2. lubricating membrane lining an internal surface or an organ, as the alimentary, respiratory, and genitourinary canals: ……………………………… 3. syrupy preparation containing medicaments exerting a local action on the mucous membrane of the throat: ……………………………… 4. (in a cell or gland) the act or process of separating, elaborating, and releasing a substance that fulfills some function within the organism or undergoes excretion: ……………………………. 5. gelatinous case enclosing a dose of medicine: ………………………….. 6. pertaining to, or affecting the stomach and intestines: ……………………………. 7. any attack of painful spasms characterized by sensations of choking or suffocating: ………………………………… 12 Rectal route Pre-reading task: A. What do you know about this route? 1. Do you know what drug formulations are administered by this route? ...................................................................................................................................................... 2. When would this route be selected? ...................................................................................................................................................... ...................................................................................................................................................... 3. What are the disadvantages of this route? ...................................................................................................................................................... ...................................................................................................................................................... If you cannot answer these questions, you can read the extract to get the information. Rectal route Drugs given rectally in solution, suppository or emulsion form are generally administered for local rather than systemic effects. Suppositories are solid forms intended for introduction into body cavities (usually rectal but also vaginal and urethral) where they melt, releasing the drug, and the choice of suppository base or drug carrier can greatly influence the degree and rate of drug release. This route of drug administration is also indicated for drugs inactivated by the gastrointestinal fluids when given orally or when the oral route is precluded, as for example when a patient is vomiting or unconscious. Drugs administered rectally enter the systemic circulation without passing through the liver, an advantage for drugs significantly inactivated by the liver following oral route absorption. Disadvantageously, the rectal route is inconvenient and drug absorption is often irregular and difficult to predict. Aulton, M., 2007: 8 B. Can you find which words in the extract mean the following? 1. a hollow space within the body, an organ, a bone, etc.: ……………………….. 2. pertaining to the passage leading from the uterus to the vulva in certain female mammals: …………………………. 3. pertaining to the membranous tube that extends from the urinary bladder to the exterior and that in the male conveys semen as well as urine: ………………………… 13 4. excluded: ……………………………. Parenteral route Pre-reading task: A. What do you know about this route of administration concerning the formulations used, the advantages and disadvantages of its use and the cases in which it is preferred? ...................................................................................................................................................... ...................................................................................................................................................... B. Would you assess these statements as true or false? 1. The amount of drug absorbed by the organism is more predictable when it is administered parenterally. 2. Depot preparations can be formulated by solution injections. 3. Local anesthetics make use of vasodilators. You can check your guesses by referring to the extract. Parenteral route A drug administered parenterally is one injected via a hollow needle into the body at various sites and to varying depths. The three main parenteral routes are subcutaneous (s.c.), intramuscular (i.m.) and intravenous (i.v.). Other routes such as intracardiac and intrathecal are used less frequently. The parenteral route is preferred when rapid absorption is essential, as in emergency situations or when patients are unconscious or unable to accept oral medication, and in cases when drugs are destroyed, inactivated or poorly absorbed following oral administration. Absorption after parenteral drug delivery is rapid and, in general, blood levels attained are more predictable than those achieved by oral dosage forms. Injectable preparations are usually sterile solutions or suspensions of drugs in water or other suitable physiologically acceptable vehicles. As referred to previously, drugs in solution are rapidly absorbed and thus injection suspensions are slower acting than solution injections. In addition, since body fluids are aqueous, by using suspended drugs in oily vehicles, a preparation exhibiting slower absorption characteristics can be formulated to give a depot preparation, providing a reservoir of drug which is slowly released into the systemic circulation. Such preparations are administered by intramuscular injection deep into skeletal 14 muscles (e.g. several penicillin-containing injections). Alternatively, depot preparations can be achieved by subcutaneous implants or pellets, which are compacted or molded discs of drug placed in loose subcutaneous tissue under the outer layers of the skin. Such systems include solid microspheres (e.g. polylactide co-glycollic acid homo- and copolymers) containing proteins or peptides (e.g. human growth hormone and leuprolide). More generally, subcutaneous injections are aqueous solutions or suspensions which allow the drug to be placed in the immediate vicinity of blood capillaries. The drug then diffuses into the capillaries. Inclusion of vasoconstrictors or vasodilators in subcutaneous injections will clearly influence blood flow through the capillaries, thereby modifying the capacity for absorption. This principle is often used in the administration of local anesthetics with the vasoconstrictor adrenaline, which delays drug absorption. Conversely, improved drug absorption can result when vasodilators are included. Intravenous administration involves injection of sterile aqueous solutions directly into a vein at an appropriate rate. Volumes delivered can range from a few milliliters, as in emergency treatment or for hypnotics, up to liter quantities, as in replacement fluid treatment or nutrient feeding. Given the generally negative patient acceptance of this important route of drug delivery, primarily associated with pain and inconvenience, recent developments have focused on ‘needle-free’ injection systems and devices which propel drug in aqueous solution or powder form at high velocity directly through the external layers of the skin. Aulton, M., 2007: 8 C. If you wish to know more about this route of administration, consider reading the extract. Then you find answers to the questions that follow. 1. When would this route of administration be preferred? ...................................................................................................................................................... ...................................................................................................................................................... 2. What are the advantages of this route compared to the others? ...................................................................................................................................................... ...................................................................................................................................................... 3. How does the drug reach the blood circulation in the case of subcutaneous injection? ...................................................................................................................................................... ...................................................................................................................................................... 4. What are the more recent developments in this route and what are they trying to achieve? ...................................................................................................................................................... ...................................................................................................................................................... 15 D. Using the context, can you try to explain what these words mean as they appear in the text? (the number of the paragraph appears in brackets) (1) via: …………………………………………………………………………………………. (1) attained: ……………………………………………………………………………………. (2) tissue: ………………………………………………………………………………………. (2) vicinity: …………………………………………………………………………………….. (2) conversely: ………………………………………………………………………………… (3) velocity: ……………………………………………………………………………………. E. The text contains several terms associated with the fields of medicine and biology, both associated with pharmacy. Can you try to match the definitions on the left with the terms on the right? 1. dosage form of a drug that acts over a period of time by controlled-release processes or technology 2. drug that causes narrowing of the blood vessels a. subcutaneous 3. situated or lying under the skin b. intrathecal 4. one of the system of branching vessels or tubes conveying c. depot preparation blood from varying parts of the body to the heart (loosely, any d. biodegradable blood vessel) 5. minute blood vessel between the termination of the arteries and the beginnings of the veins e. capillary f. vasoconstrictor 6. introduced into or occurring in the space under the arachnoid g. vein membrane of the brain or spinal cord 7. agent or drug that produces sleep; sedative h. hypnotic 8. capable of decaying through the action of living organisms F. Two words that you have met in this text are intravenous and intramuscular. They both start with the prefix intra, meaning ‘within’, which is used in the formation of compound words. There are several others. Some prefixes are given below. Can you think of what they mean and think of words that start with them? intra-: ‘within’, ………………………………………………………………………………... bio-: ……………………………………………………………………………………………. 16 trans-: …………………………………………………………………………………………. hydro-: ………………………………………………………………………………………… counter-: ………………………………………………………………………………………. cyto-: …………………………………………………………………………………………... naso-: ………………………………………………………………………………………….. uni-: …………………………………………………………………………………………… G. There are times when information contained in a text or book is not enough to help us comprehend something or new questions come up. In such cases we need to look for further sources that will help us gain better insight into what we are studying or researching. Ability to look for and locate various sources of information is one of the basic skills of university students, scientists, researchers. The present extract contains the phrase ‘sterile solutions’ without giving any further information about them. Supposing you were interested in what these are, how would you go about finding more information on them? Can you try your methods and report back on them? Can you also share the information you may find? ...................................................................................................................................................... ...................................................................................................................................................... ...................................................................................................................................................... Topical route Pre-reading task: A. Do you know what formulations are applied topically? ...................................................................................................................................................... B. Can you tell if the following statements are true or false? 1. The topical route is effective for systemic drug delivery. 2. Formulations intended for application to the nose are viscous. You can see if you were right by crosschecking your answers with the information in the extract. 17 Topical route Drugs are applied topically, that is to the skin, mainly for local action. Whilst this route can also be used for systemic drug delivery, percutaneous absorption is often poor and erratic, although several transdermal patches delivering drug for systemic distribution (e.g. glyceryl trinitrate patches for the prophylaxis and treatment of angina) are available. Drugs applied to the skin for local effect include antiseptics, antifungals, anti-inflammatory agents, as well as skin emollients for protective effects. Pharmaceutical topical formulations – ointments, creams and pastes – are composed of drug in a suitable semi-solid base which is either hydrophobic or hydrophilic in character. The bases play an important role in determining the drug release character from the formulation. Ointments are hydrophobic, oleaginous-based dosage forms whereas creams are semi-solid emulsions. Pastes contain more solids than ointments and thus are stiffer in consistency. For topical application in liquid form other than solution, lotions, suspensions of solids in aqueous solution or emulsions are used. Recently, interest in transdermal electrotransport systems has grown. Here, a low electrical potential maintained across the skin can improve drug transport. Application of drugs to other topical surfaces such as the eye, ear and nose is common and ointments, creams, suspensions and solutions are utilized. Ophthalmic preparations are required, amongst other features, to be sterile. Nasal dosage forms include solutions or suspensions delivered by drops or fine aerosol from a spray. Ear formulations in general are viscous to prolong contact with affected areas. Aulton, M., 2007: 9 18 C. Having read the text, can you answer the following? 1. What formulations are applied topically? ...................................................................................................................................................... ...................................................................................................................................................... 2. Why is the base of a topical formulation important? ...................................................................................................................................................... ...................................................................................................................................................... D. The words that follow appear in the extract. Can you guess what they mean? (1) erratic: ……………………………………………………………………………………… (2) potential: …………………………………………………………………………………… E. Conversely, can you find what terms the following definitions refer to? 1. medication used to reduce redness, swelling, pain, tenderness and disturbed function of an area of the body, that is caused esp. as a reaction of tissues to injurious agents: ………………………. 2. having the power of softening or relaxing, as a medicinal substance; soothing, esp. to the skin: ………………………………. 3. administered, removed, or absorbed by way of the skin, as an injection, or transdermal drug: ………………………………… 4. having the nature or qualities of oil: ………………………… 5. soft, unctuous preparation, often medicated, for application to the skin: ……………………… Respiratory route Pre-reading task: A. Do you know what kind of formulations would be administered by this route? What medical problems could they be used to treat? ...................................................................................................................................................... ...................................................................................................................................................... You can check your answers with the text. 19 Respiratory route The lungs provide an excellent surface for absorption when the drug is delivered in gaseous, aerosol mist or ultrafine solid particle form. For drug particles presented as an aerosol or solid form, particle size largely determines the extent to which they penetrate the alveolar region, the zone of rapid absorption. Drug particles that are in the region 0.5 – 1 μm diameter reach the alveolar sacs. Particles smaller than this range are either exhaled or, if larger, deposited upon larger bronchial airways. This delivery route is particularly useful for the direct treatment of asthmatic problems, using both powder aerosols (e.g. sodium cromoglycate) and metered aerosols containing the drug in liquefied inert propellant (e.g. salbutamol sulphate aerosol). Importantly, this delivery route is being increasingly recognized as a useful means of administering the therapeutic agents emerging from biotechnology requiring systemic distribution and targeted delivery, such as peptides and proteins. Aulton, M., 2007: 9 B. Do you know? 1. Why is the particle size of a drug important when it is presented as an aerosol form? ...................................................................................................................................................... ...................................................................................................................................................... 2. Why is the presence of an inert propellant important in metered aerosols? ...................................................................................................................................................... ...................................................................................................................................................... C. Certain words of the extract are defined as follows. Can you find which they are? 1. cloudlike aggregation of minute globules of a substance: ……………………………… 20 2. pertaining to the air cells of the lungs, formed by the terminal dilation of tiny air passageways: …………………………………. 3. breathe out: ……………………………… 4. laid down by a natural process; precipitated: ……………………………. 5. pertaining to either of the two main branches of the trachea: …………………………… 6. having no pharmacological action, as the excipient of a pill: …………………………….. 21 Unit 3 - Microorganisms - Viruses Introduction Pre-reading task: A. What do you know about microorganisms? Can you give a definition of the term? …………………………………………………………………………………….……………. ..………………………………………………………………………………………………… B. What do you think of the following statements? Are they true or false? 1. Pharmaceutical microbiology studies the total of microorganisms. 2. Microorganisms are classified into two taxonomies. 3. Only a small part of them cause diseases. 4. Viruses are living agents. 5. Viruses contain protein but lack nucleic acid. You can check your answers with the information contained in the extract. 22 Introduction Microorganisms are ubiquitous in nature and are vital components in the cycle of life. The majority are free-living organisms growing on dead or decaying matter whose prime function is the turnover of organic materials in the environment. Pharmaceutical microbiology, however, is concerned with the relative small group of biological agents that cause human disease, spoil prepared medicines or can be used to produce compounds of medical interest. In order to understand microorganisms more fully, living organisms of similar characteristics have been grouped together into taxonomic units. The most fundamental division is between prokaryotic and eukaryotic cells, which differ in a number of respects (Table 14.1) but particularly in the arrangement of their nuclear material. Eukaryotic cells contain chromosomes, which are separate from the cytoplasm and contained within a limiting nuclear membrane, i.e. they possess a true nucleus. Prokaryotic cells do not possess a true nucleus and their nuclear material is free within the cytoplasm, although it may be aggregated into discrete areas called nuclear bodies. Prokaryotic organisms make up the lower forms of life and include Eubacteria and Archaeobacteria. Eukaryotic cell types embrace all the higher forms of life, of which only the fungi will be dealt with in this chapter. Table 14.1 Differences between prokaryotic and eukaryotic organisms Structure Prokaryotes Eukaryotes Cell wall structure Usually contains peptidoglycan Peptidoglycan absent Nuclear membrane Absent Present. Possess a true nucleus Nucleolus Absent Present Number of chromosome One More than one Mitochondria Absent Present Mesosomes Present Absent Ribosomes 70S 80S One characteristic shared by all microorganisms is the fact that they are small; however, it is a philosophical argument whether all infectious agents can be regarded as living. Some are little more than simple chemical entities incapable of any free-living existence. Viroids, for example, are small circular, single-stranded RNA molecules not complexed with protein. One particular well-studied viroid has only 359 nucleotides (one10th the size of the smallest known virus) and yet causes a disease in potatoes. Prions are small, self-replicating proteins devoid of any nucleic acid. The prion associated with Creutzfeld-Jacob disease in humans, scrapie in sheep and bovine spongiform encephalitis in cattle has only 250 amino acids and is highly resistant to inactivation by normal sterilization procedures. 23 Viruses are more complex than viroids or prions, possessing both protein and nucleic acid. Despite being among the most dangerous infectious agents known, they are still regarded as living. Aulton, M., 2007: 183 C. After reading the text, can you tell … What is the main criterion according to which microorganisms can be classified in either of the two taxonomies? ………………………………………………………………………………………………….. ………………………………………………………………………………………………….. D. Using the context of the following words, and your knowledge on the subject, can you explain what they mean? (2) aggregated: ………………………………………………………………………………… (3) entities: …………………………………………………………………………………….. (3) replicating: …………………………………………………………………………………. (3) devoid of: ………………………………………………………………………………….. E. Can you now match the words on the right with their definitions on the left? 1. cell substance between the cell membrane and the nucleus a. ubiquitous 2. composed of two or more elements or ingredients b. compound 3. existing or being everywhere, esp. at the same time; c. cytoplasm omnipresent d. strand 4. one of a number of fibers that are twisted together Viruses Pre-reading task: A. The following statements refer to viruses. Can you assess if they are true or false? 1. Viruses exhibit metabolic activity due to lack of ribosomes. 2. They can be seen with the help of a microscope. 24 3. Membrane filters used for the sterilization of thermolabile liquids are effective in keeping viruses away too. 4. The protein capsid accounts for the 50-90% of the total weight of a virus. 5. Viruses use the metabolic capability of the host cells to reproduce. 6. Chemotherapy can be targeted solely on viruses. If you are not sure of your answers, you can refer to the text. Viruses Viruses are obligate intracellular parasites with no intrinsic metabolic activity, being devoid of ribosomes and energy-producing enzyme systems. They are thus incapable of leading an independent existence and cannot be cultivated on cell-free media, no matter how nutritious. The size of human viruses ranges from the largest poxviruses, measuring about 300nm, to the picornaviruses, such as the poliovirus which is approximately 20nm. When one considers that a bacterial coccus measures 1000 nm in diameter, it can be appreciated that only the very largest virus particles may be seen under the light microscope, and electron microscopy is required for visualizing the majority. It will also be apparent that few of these viruses are large enough to be retained on the (0.2 μm) membrane filters used to sterilize thermolabile liquids. Viruses consist of a core of nucleic acid (either DNA as in vaccinia virus or RNA as in poliovirus) surrounded by a protein shell or capsid. Most DNA viruses have linear, doublestranded DNA but in the case of the parvovirus it is single stranded. The majority of RNAcontaining viruses contain one molecule of single-stranded RNA, although in reoviruses it is double stranded. The protein capsid comprises 50-90% of the weight of the virus and, as nucleic acid can only synthesize approximately 10% its own weight of protein, the capsid must be made up of a number of identical protein molecules. These individual protein units are called capsomeres and are not in themselves symmetrical but are arranged around the nucleic acid core in characteristic symmetrical patterns. Additionally, many of the larger viruses possess a lipoprotein envelope surrounding the capsid arising from the membranes within the host cell. In many instances the membranes are virus modified to produce projections outwards from the envelope, such as haemagglutinins or neurominidase. The envelope viruses are often called ether sensitive, as ether and other organic solvents may dissolve the membrane. The arrangement of the capsomeres can be of a number of types. 25 Helical – the classic example is tobacco mosaic virus (TMV), which resembles a hollow tube with capsomeres arranged in a helix around the central nucleic acid core. Other examples include mumps and influenza virus. Icosahedral – these often resemble spheres on cursory examination but when studied more closely, they are seen to be made up of icosahedra that have 20 triangular faces, each containing an identical number of capsomeres. Examples include the poliovirus and adenovirus. Complex – the poxviruses and bacterial viruses (bacteriophages) make up a group whose members have a geometry that is individual and complex. Reproduction of viruses Because viruses have no intrinsic metabolic capability, they require the functioning of the host cell machinery in order to manufacture and assemble new virus particles. It is this intimate association between the virus and its host that makes the treatment of viral infections so complex. Any chemotherapeutic approach which damages the virus will almost inevitably cause injury to the host cells and hence lead to side-effects. An understanding of the life cycle of the virus is vital in determining suitable target sites for antiviral chemotherapy. The replication of viruses within host cells can be broken down into a number of stages. Aulton, M., 2007: 183-184 B. The extract provides information on viruses. You can use it to answer the questions: 1. What is a capsomere? ………………………………………………………………………………………………….. ………………………………………………………………………………………………….. 26 2. Why are viral infections difficult to treat? ………………………………………………………………………………………………….. ………………………………………………………………………………………………….. C. At the end of the extract there is reference to the stages of virus reproduction but these are not mentioned. Do you know them? If not, how could you find more about them? Share the information on methods and findings with your colleagues. ………………………………………………………………………………………………… ………………………………………………………………………………………………… ………………………………………………………………………………………………… ………………………………………………………………………………………………… D. The crossword that follows includes terms appearing in the extract. Do you think you can solve it? Across 3. the central, innermost, or most essential part of anything 4. infectious disease characterized by inflammatory swelling of the parotid and usually other salivary glands 5. spherical bacterium 7. restricted to a particular condition of life, as certain organisms that can survive only in the 27 absence of oxygen 8. belonging to a thing by its very nature Down 1. subject to destruction or loss of characteristic properties by the action of moderate heat, as certain toxins and enzymes 2. protruding part 6. a living animal or plant from which a parasite obtains nutrition 28