* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Handout 4
Survey
Document related concepts
Transcript
BME 502 / handout #4
BME 502 Handout on Synaptic Transmission (#1)
Synaptic Transmission I.
two basic classes of synaptic tranmission: electrical and chemical
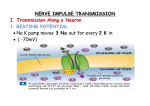
electrical synaptic transmission
initial conception of primary mode of communication between neurons was that transmission
resulted from electrotonic coupling through the extracellular space, i.e., synaptic transmission
is simply a continuation of the events that provide for propogation of the action potential down
the axon; jumping the gap
this assumes that the nervous system was conceived of as a collection of individual elements,
i.e., cells -- the neuron doctrine; recall that even into the early part of the 20th century, there
were many active supporters of the "nerve net" notion, i.e., that the nervous was composed of
a continuous, membrane-bound volume, much like the circulatory system, with nerve cell
bodies constituting "nodes" in the system
if it is assumed that neurons are not continuous, i.e., that there is a physical space between
them, it can easily be seen that electrotonic coupling between neurons is not favored:
assume for neurons 1 and 2 a resistance of the terminal membranes of 1,000 M
(reasonable for a small area of membrane with a resistivity of 104-105 -cm2)
assume input resistance for neuron 2 is 100 M
assume resistance of extracellular space is 1 M
1
BME 502 / handout #4
when an action potential reaches the terminal, the low resistance of the synaptic cleft
relative to the much higher resistance of the postsynaptic membrane shunts the majority
of the electrical current away from postsynaptic neuron and into the extracellular space
results in an attenuation of potential from neuron 1 to neuron 2 by 10-4
even if the gap between neurons is reduced so as to increase resistance of the
extracellular space to 10 M, attenuation remains at 10-3
thus, for every 100 mV in neuron 1, only 100 V would be generated in neuron 2
{analysis assumes steady-state and thus ignores effects of transient signals, which would
be larger, but point is the same -- not efficient mechanism for transfer of charge}
electrical
synapses
have
structural characteristics that
overcome this shunting effect
of the extracellular space
smaller cleft (20 A)
low resistance channels
between pre and postsynaptic
membranes:
gap junctions
2
BME 502 / handout #4
3
BME 502 / handout #4
channels have the effect of decreasing the resistance between pre- and postsynaptic
neuron membranes and thus decreasing the shunting of the presynaptic current to the
extracellular space
4
BME 502 / handout #4
functional characteristics of electrical synapses
bidirectional (but can be rectified)
near identity of pre- and postsynaptic signals (postsynaptic signal depends on time
constant of postsynaptic membrane)
no synaptic delay, thus, speed
high probability of transmission
rectification of electrical synapses
consider two neurons coupled through a gap junction, with R1 representing the input
resistance of neuron 1, and R2 representing the input resistance of neuron 2
5
BME 502 / handout #4
if a voltage, V1, is applied in neuron 1 the voltage that appears in neuron 2 will depend
on R2, the input resistance of neuron 2 and Rc, the coupling resistance:
V2
R2
=
= K 12
V1
R2 + Rc
for a voltage, V2 , applied in neuron 2:
Install Equation Editor and doubleclick here to view equation.
where K12 and K21 are called the coupling coefficients
rectification of electrical synapses can occur whenever there is a significant
difference between coupling coefficients
6
BME 502 / handout #4
7
BME 502 / handout #4
e.g., two neurons of unequal size
neuron 1, R1 = 109
neuron 2, R2 = 107
Rc = 107
for this case,
K12 = 107 / (109 + 107) = 1/101 = 0.01
100 mV in neuron 1 results in 1 mV in neuron 2
K21 = 109 / (109 + 109) = 0.5
100 mV in neuron 2 results in 50 mV in neuron1
increasing evidence that electrical synapses are widespread in the mammalian brain
dye coupling in hippocampus; at soma level
dye coupling in neocortex; at dendritic level
notion that electrical synapses are particularly important for synchronization of activity
among populations of neurons; potential importance of gap junctions in epilepsy
evidence that gap junction strength varies as a function of intracellular pH, and thus, as a
function of past history of activity
8
BME 502 / handout #4
ephaptic coupling
any time a synaptic potential or action potential is generated in a neuron, there is an
extracellular current flux that is equal and opposite to that of the intracellular current flux
though it has been assumed for the cases considered to date that the extracellular space
has a zero resistance, in fact it has a finite resistance which leads to the generation of an
extracellular voltage
recording extracellular potentials was the first experimental means for monitoring the
activity of neurons, prior to the development of intracellular, whole-cell, and patch-clamp
recording methods
in brain structures which have a geometrically simplified organization, i.e., those in which
the neurons, their dendritic processes, and the axons/terminals of afferents are
"laminated", the relation between intracellular and extracellular potentials is interpretable
9
BME 502 / handout #4
dendritic region
intracellular: inward current; depolarization
extracellular: corresponding current sink
** note sharp rise/fall of extracellular potential -due to capacitive properties of membrane, i.e.,
differentiation of intracellular potential
somatic region
intracellular: attenuated and slowed
depolarization
extracellular: corresponding current source, i.e.,
opposite polarity of current sink
** note lower magnitude extracellular field
response in somatic region due to lower density
of extracellular current (many paths for return of
current, so source current more diffusely distributed;
current density is major determinant of extracellular potential
dendritic region
intracellular: outward current; hyperpolarization
extracellular: corresponding current source
somatic region
intracellular: attenuated and slowed
hyperpolarization
extracellular: corresponding current sink, i.e.,
opposite polarity of current source
10
BME 502 / handout #4
chemical synaptic transmission
basic mechanisms common to almost all chemical synapses:
narrowing of the extracellular space between nerve cell membranes
vesicles; uniform amount of neurotransmitter (quanta)
presynaptic voltage-dependent Ca2+ channels
presynaptic specializations/involved in release process
postsynaptic specialization/receptors
mechanisms to limit transmitter action
degradative enzymes
uptake sites presynaptically
uptake sites on glial cells
11
BME 502 / handout #4
12
BME 502 / handout #4
important functional constraints of this method of synaptic communication
unidirectional: rectification
probability of postsynaptic action potential low (for each synapse)
synaptic delay
variation in characteristics that distinguish the synapses associated with different
neurotransmitters
types of vesicles (flattened, spherical, dense-core)
thus, vesicles also have morphology (distinguish terminal vs. synapse)
different vesicles linked to specific neurotransmitter types:
elliptical, GABA, dense core: amines
types of receptors
location of receptors (pre/post)
mechanisms by which receptor leads to change in membrane potential
type of channel to which it is linked (can be linked directly to a channel but to channels
with different properties, e.g., pass different ion species)
may be linked indirectly to a channel, i.e., through a second messenger molecule
number of regulatory mechanisms that control channel conductance
predominant mechanism for limiting duration of neurotransmitter action, i.e., degradative
enzymes or uptake mechanisms
13
BME 502 / handout #4
neuromuscular junction
typically used as example because have the most information about this particular
synaptic junction; first one examined experimentally in great detail
morphological characteristics
active zones
rows of particles (calcium channels)
postsynaptic specializations by edges of junctional folds
total region of postsynaptic receptors associated with terminals: end-plate
postsynaptic potential: not EPSP but EPP, or end-plate potential
14
BME 502 / handout #4
15
BME 502 / handout #4
neurotransmitter for neuromuscular junction (striated muscle) is acetylcholine, ACh
postsynaptic specialization = ACh receptor
nicotinic subtype: leads to excitation of postsynaptic element through increased
conductance to Na+ and K+ (for striated muscle)
conductance to both ion species identified because of reversal potential, zero
muscarinic subtype: leads to excitation of postsynaptic element through closing
of K+ channels, thus decreasing leakage current
action of ACh terminated primarily by degradative enzyme, AChE, or acetylcholinesterase;
typically located within the synapse
so can prolong action of ACh with treatment with AChE inhibitors
uptake mechanisms are primarily involved in uptake of precursor, choline
presynaptic control of neurotransmitter release
depolarization of presynaptic terminal necessary
Katz and Miledi (1957) using squid giant axon synapse with stellate cell recorded
intracellularly both presynaptically and postsynaptically:
through hyperpolarization of presynaptic terminal, found that depolarization was necessary
for neurotransmitter release to occur, i.e., for a postsynaptic epp to be generated
relationship between presynaptic membrane potential change and postsynaptic epsp
amplitude was logarithmic; for every 10 mV increase in presynaptic depolarization, was a
10-fold increase in amplitude of epp
increase in presynaptic intracellular calcium necessary for release
Katz and Miledi (1967) using frog neuromuscular junction
eliminated Ca2+ from extracellular space
used a CaCl2 electrode to eject Ca2+ locally next to presynaptic terminal
if Ca2+ ejected just prior to depolarization of presynaptic terminal, then recorded epp in
muscle; if after depolarization, no epp
16
BME 502 / handout #4
quantal release of neurotransmitter
spontaneous mini's: amplitude of miniature epp is unimodal
Castillo and Katz (1954) decreased Ca2+ concentration so that less Ca2+ entered
presynaptically
examined amplitude of responses to stimulation
found that distribution of resulting epp not continuous: was a minimum epp amplitude of
approx 0.5 to 1.0 mV
also observed for spontaneously occurring epp's (or mepp's) Fatt and Katz (1952)
conclusion that all epp's are some multiple of the minimum epp, or mepp, and that Ca2+
increases the probability of vesicle release without influencing the amount of
neurotransmitter released per vesicle, or the quantal size
has led to a probabilistic model for neurotransmitter release that, in its simplist form, is
reflected in the relationship:
m = np
where m represents the mean number of quanta released following an action potential,
n represents the number release sites, and p represents the probability of release (assuming
that all sites have the same probability of release
17
BME 502 / handout #4
panel A: total ionic current
panel B: after TTX and general K+ channel blocker (3-aminopyridine); thus, trace a in both
panels presumably represents Ca2+ current
note slow time to peak for residual current; if Ca2+ current, then the mechanisms that lead
to channel opening are very slow
note difference in calibration bars, i.e., difference in amplitude of current
cooperativity of Ca2+ in the release process
Dodge and Rahamimoff (1957): assumed that Ca2+ must bind to some presynaptic protein
critical for the release process; thus the following kinetic equation:
18
BME 502 / handout #4
Install Equation Editor and doubleclick here to view equation.
with dissociation constant, K1
assuming the law of mass action:
Install Equation Editor and doubleclick here to view equation.
where W is a constant
19
BME 502 / handout #4
predictions:
if release is directly proportional to [CaX], then amplitude of the EPP should be directly
proportional to [CaX]
if release is dependent on the formation of 2 CaX molecules, then amplitude of the EPP
should be directly proportional to [CaX]2
so more generally:
Install Equation Editor and doubleclick here to view equation.
where k is a proportionality constant and n is a positive integer
if allow n to vary between 1 and 5, get curves shown in figure below
Dodge and Rahamimoff performed experiments in which they varied the external
concentration of Ca2+ and measured the amplitude of the EPP
20
BME 502 / handout #4
21
BME 502 / handout #4
best fit was for n=4, i.e., transmitter release dependent on the 4th power of external Ca2+
concentration
consequences: (i) release of a single quantum requires cooperative action of Ca2+, and (ii)
relationship between EPP amplitude and external concentration is highly nonlinear
22
BME 502 / handout #4
voltage-clamp studies: presynaptic Ca2+ currents
voltage-clamp studies
amplitude of current increases with increasing depolarization, reaching a maximum at
approximately 0 mV, and decreasing with further depolarization (typical for voltagedependent channel with a positive equilibrium potential -- see I-V plot on right)
very little activation of the current during step commands
onset of current is slow and delayed compared to the onset of the step command
large "tail current" -- driving force is maximal when command step is turned off
23
BME 502 / handout #4
voltage-clamp studies: relationship between Ica and release
24
BME 502 / handout #4
delay in the onset of ICa relative to onset of step depolarization
rise in ICa very slow -- relfected in the p.s.c., which does not begin until nearly half way through
command step
peak of the postsynaptic current doesn't occur until after end of the command step, i.e., the
ONSET of the presynaptic depolarization does little; the turning OFF of the voltage causes
most of the release
explanation:
onset of the current is slow (channel kinetics) and delayed, so that release -- which is
nonlinearly related to Ca2+ concentration and thus is slow to begin with -- is slowed further
large offset response is due to large tail current
**large tail current and resulting postsynaptic response should allow determination of minimum
time between Ca2+ entry and a postsynaptic response: 200 sec
25
BME 502 / handout #4
additional observation is that latency for depolarization following termination of voltage step is
shorter (~1 msec) than latency for depolarization following onset of voltage step (~3 msec)
implication:
much of the synaptic delay is associated with the time required to open the
Ca2+ channels
implication:
that not very much of the synaptic delay is associated with the fusion of the
vesicle and release of the neurotransmitter
experiments using Ca2+ chelators verified this possibility
EGTA and BAPTA are two chelators that bind free Ca2+; can be injected
into presynaptic terminal
kinetics of binding with BAPTA much faster than with EGTA (though both
equally potent)
can calculate that Ca2+ reaches binding sites to trigger release within
200 sec of entry
also can calculate that Ca2+ channels then must be 100 nm of binding site,
i.e., very close to binding site; one of the sources of evidence that the
"particles" observed next to release sites of vesicles at neuromuscular
junction are associated, or part of, the Ca2+ channels involved in release
26
BME 502 / handout #4
27
BME 502 / handout #4
when all examined together:
at -33, significant ICa but essentially no p.s.c. -- explained by nonlinear relationship
between Ca2+ concentration and release
largest postsynaptic response is at end of command step -- explained by voltage
dependence of Ca2+ channels
magnitude of response increases to a max at approximately 0 mV and then decreases
with further magnitude depolarization
28
BME 502 / handout #4
presynaptic facilitation
key consideration is that the probability of release depends on the intracellular concentration
of Ca2+ (and is nonlinearly related to Ca2+)
thus, one of the major determinants of release will be mechanisms involved in buffering
calcium in the presynaptic terminal
e.g., consider the case of two action potentials separated by a time interval that exceeds
the time course for buffering calcium in the presynaptic terminal: the amount of
neurotransmitter released should be approx equal to each action potential
e.g., consider the case of two action potentials separated by a time interval that is less
than the time course for buffering calcium in the presynaptic terminal:
there should be residual calcium in the terminal region from the first action potential
the calcium which enters in response to the second action potential will then add with
this residual calcium
given the nonlinear relation between calcium concentration and release:
the residual calcium by itself may be insufficient for triggering release
but may significantly affect the probability of release when it adds (nonlinearly) with
the calcium that enters in response to the second action potential
known as the "residual calcium" hypothesis
facilitation (paired-pulse facilitation)
appear to be two time constants: = 50 msec and = 300 msec
plot log (V-V0) / V0 vs linear time
post-tetanic potentiation (PTP) and augmentation
occur in response to high frequency trains of action potentials
also reveals two time constants: = 7 sec (augmentation)
and = 1 min (PTP)
29
BME 502 / handout #4
facilitation, PTP, and augmentation all believed to reflect an increase in quantal content, m
with these mechanisms in mind, re-examine characteristics of above figure in more detail
for command steps to +27 mV, +37 mV, and +57 mV, the off response decreases with
increasing depolarizations even though Ca2+ conductances are maximally activated and
the resulting tail currents are maximal in amplitude
facilitation of release by small amount of calcium that flows into terminal during command
step
e.g., for +27 mV, calcium current causes little to no release but the calcium that does
enter facilitates release to the tail current
for +37 mV, there is less calcium current (because approaching ECa) and thus less
facilitation of release in response to the tail current
30
BME 502 / handout #4
vesicle-associated proteins
31
BME 502 / handout #4
transport / mobilization to active zone
neurotransmitters are one example of specialized proteins synthesized in the cell body
and/or nerve terminal and that must be transported to a distant site without degradation or
biochemical alteration
solution to need for compartmentalization is transportation in a membrane-bound vesicle
important constraint imposed by vesicular transportation: need for mechanisms of
recognition
(1) by cytoskeleton during transportation
(2) at target site so that vesicle can be deposited
the terminal region contains a matrix of microtubules and actin filaments that are attached
to the plasma membrane, the vesicles and the presynaptic specializations (microtubules,
which are involved in axonal transport, do not extend into the presynaptic specializations of
the release site)
constitutes the structure that guides and anchors the vesicles
synapsins
(1) recognition / attachment molecules that surround vesicle
(2) multiple forms of synapsins
(3) roles in binding to cytoskeleton and mobilization to presynaptic release site
32
BME 502 / handout #4
docking
in the particular case of
neurotransmitters, there is an
additional requirement for positioning
the vesicle relative to the presynaptic
specializations that mediate fusion of
the vesicle membrane with the plasma
membrane
synaptobrevin, or, vesicularassociated membrane protein
(VAMP): embedded in vesicular
membrane
syntaxin: embedded in plasma
membrane
NSF/SNAP complex:
N-ethylmaleimide-sensitive fusion
protein (NSF) and soluble NSF
attachment protein (SNAP) that binds
VAMP and syntaxin, anchoring vesicle
to plasma membrane
fusion with plasma membrane
NSF/SNAP complex forms fusion
pore between vesicle membrane and
plasma membrane
synaptotagmin: inhibits formation of
fusion pore; with Ca2+ binding,
inhibition removed
33
BME 502 / handout #4
role of cytoskeleton
34
BME 502 / handout #4
35
BME 502 / handout #4
co-existence and co-release of neurotransmitters
36