* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Guidance - Scottish Medicines Consortium
Survey
Document related concepts
Electronic prescribing wikipedia , lookup
Drug design wikipedia , lookup
Drug interaction wikipedia , lookup
Compounding wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
National Institute for Health and Care Excellence wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Transcript
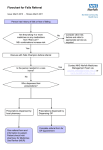
Scottish Medicines Consortium Guidance on Horizon Scanning Process October 2013 Contents Page 1. Background 1 2. Outputs 2.1 Forward Look reports 2 2.2 Forward Look addendum 4 2.3 Forward Look interim updates 4 2.4 Ad hoc advice 4 3. Horizon scanning processes 3.1 Overview and timelines 5 3.2 Company contacts 8 3.3 Collation of intelligence 8 3.4 Forward Look report content 10 3.5 Product monographs 10 3.6 Clinical expert advice 10 3.7 Pharmaceutical industry input 11 3.8 Financial spreadsheets 11 4. Confidentiality 12 5. Using the Forward Look report in practice 13 Appendices Appendix A Example of a completed horizon scanning update form 14 Appendix B Sample monograph and monograph template 17 Appendix C Example of financial spreadsheet 25 1. Background In 2003 the (then) Scottish Executive requested that the Scottish Medicines Consortium (SMC) establish a horizon scanning initiative to provide Scottish Health Boards with advance intelligence on new medicines to support financial and service planning for their managed introduction into practice. The main focus of the horizon scanning initiative is to provide financial planners with reliable information to support resource planning for the managed introduction of new medicines. In accordance with SMC’s remit, the horizon scanning initiative encompasses new medicines as well as new indications, licence extensions and new formulations of existing medicines. The budget implications of new medicines are subject to a high degree of uncertainty. However, if financial planning processes are supported by robust intelligence about medicines in the pipeline and their potential cost impact, this should facilitate patient access to cost-effective new medicines at the earliest opportunity after SMC approval. Horizon scanning intelligence also assists SMC in workload planning in relation to new product assessments. As part of the current new product assessment process, pharmaceutical companies provide SMC with an estimate of the potential budget impact of the new medicine for NHS Scotland within the new product assessment form (‘submission’). Companies estimate the net resource implications over the five years following a medicine’s introduction, taking into account the acquisition cost as well as any direct effect on the use of other resources. These budget impact estimates are intended to give Health Boards an indication of the financial implications if the medicine is accepted for use in NHS Scotland. However, they are not a component of SMC decision making on cost-effectiveness. Since 2005 SMC has produced an annual horizon scanning report, entitled Forward Look, to provide NHS Boards with information on potentially ‘high impact’ medicines that may come to the UK market within the next calendar year. A new medicine is regarded as ‘high impact’ if it: (i) has a predicted net drug budget impact (relative to comparators) for NHS Scotland of >£500K per annum within the first five years of its introduction in the UK; or (ii) may be associated with major service implications. The Forward Look report provides an insight into the potential costs associated with the adoption of a new medicine and hence provides a tool for illustrating the pressures on the budget allocation process. The purpose of this guidance document is to give stakeholders a detailed overview of SMC horizon scanning processes and outputs, including standard documentation. The Association of the British Pharmaceutical Industry (ABPI) has contributed to the development and maintenance of these processes through the SMC User Group Forum. It is hoped that the document will foster a better understanding of the horizon scanning processes and lead to more efficient and effective interactions between SMC and the pharmaceutical industry to produce higher quality horizon scanning information for NHS Scotland. The aims, processes, timelines and outputs of the horizon scanning initiative are described, with an emphasis on how pharmaceutical companies can optimise their contribution. The document also outlines the type and sources of information that are of value to SMC in providing Health Boards with a realistic forecast of the potential budget impact of new medicines. 1 2. Outputs 2.1 Forward Look Reports Horizon scanning information is detailed in an annual report (Forward Look) issued in confidence each October to key Health Board personnel, including Chief Executive Officers and Directors of Medicine, Finance, Pharmacy and Public Health. The Directors of Finance and Pharmacy also receive a set of financial spreadsheets that summarise the net budget impact of ‘high impact’ new medicines. The report lists all new medicines expected to reach the UK market within the next calendar year but focuses on those with the potential for ‘high impact’. These include medicines with a predicted moderate to high net drug budget impact for NHS Scotland (i.e. >£500,000 per annum at year five) and those potentially associated with major service implications. Detailed information on these new medicines is provided within the report in a product monograph. In the past, Forward Look also provided detailed information on all medicines being developed for orphan indications. However from 2010 onwards medicines with orphan status have been treated like all other medicines in the report, with detailed information only being included in the form of a monograph if the medicine is predicted to have a moderate to high net drug budget impact for NHS Scotland (i.e. >£500,000 per annum at year five) and / or the potential for major service implications. The report consists of two main sections. The first is arranged by British National Formulary (BNF) chapter and contains tabulated information on all new medicines likely to be launched in the UK in the next calendar year, with a brief description of the key developments. An example is shown in figure 1. Figure 1: Forward Look Section 1 - Example of Format BNF Section 1: Gastro-intestinal system Product Drug A Drug B Drug C Therapeutic Area Peptic ulcers Crohn’s disease Constipation Comments Compete with existing therapy Monograph Compete with existing therapy Summary: A key development is the potential launch of drug B, the first drug in a new pharmacological class for the treatment of Crohn’s disease. The second main section contains detailed product monographs for medicines that are expected to be associated with moderate to high net drug budget impact and / or major service implications. These are arranged alphabetically within BNF chapters, with cancer drugs further sub-divided by tumour type. An example of a product monograph is shown in figure 2. There is also a summary table that includes all new medicines in the report that are expected to be associated with moderate to high net drug budget impact or major service implications. This includes details of their estimated UK launch date, estimated uptake and predicted net drug budget impact at years one and five. 2 Figure 2: Forward Look - Example of Product Monograph Format Product Indication Status Mode of action Estimated eligible population in Scotland Estimated uptake Drug C (Brand C) Intravenous Injection (Company) Treat adults with moderate to severe psoriasis unresponsive to standard therapies. Plan to file with EMA Q3 2013. Estimated UK launch Q3 2014. Biologic. Likely to be first in this new class of biologic drugs. Estimated prevalent population of adults with psoriasis unresponsive to standard therapies of approximately 625 patients. Assumptions Estimated prevalent adult population with psoriasis of approximately 100,000 patients. 1 An estimated 2.5% of patients with psoriasis have moderate to severe disease,2 equivalent to approximately 2,500 patients. An estimated 25% of these patients fail on standard therapies, 2 equivalent to approximately 625 patients. Uncertainties The proportion of patients failing on standard therapies may alter over time with the introduction and greater use of biologic drugs as standard therapies. Estimated uptake in years 1 and 5 by approximately 30 and 95 eligible patients, respectively. Assumptions Drug C would be given to approximately 5% and 15% of eligible patients in years 1 and 5, respectively. Estimated incremental drug acquisition cost Estimated net drug budget impact Service Implications Key references Uncertainties Uptake may be influenced by cost-effectiveness relative to alternatives and this is unknown. Uptake may be influenced by the publication of new guidelines for psoriasis and the availability of other new therapies. Estimated incremental drug acquisition cost of up to approximately £10,000 per annum. Assumptions In the absence of advice from the pharmaceutical company, the estimated drug acquisition cost of Drug C is approximately £10,000 to £12,000 per annum (30mg intravenously once a month). Drug C would be used in place of Drug X (25mg orally per day) and Drug Y (100mg subcutaneously per week). The alternative treatments (Drug X and Drug Y) cost approximately £1,000 and £3,000 per annum, respectively. The average of these has been assumed as the cost of alternative treatments. Uncertainties Drug X is likely to be out of patent protection in the next few years and generic versions are expected to cost less. Thus the incremental cost of Drug C could increase. Year 1: 30 x £10,000 = £300,000 Year 5: 95 x £10,000 = £950,000 Additional healthcare resources will be required to administer Drug C by intravenous injection once a month. However, this is not expected to be associated with significant service implications. 1. Bloggs J et al. Ann Intern Med 2013 2. Smith J et al. N Engl J Med 2013 3 2.2. Forward Look Addendum It is common for SMC to receive late notification of medicines that are predicted to reach the UK market within the next calendar year. Depending on when notification is received, it might be feasible to list the medicine in the main report but there may be insufficient time to develop a detailed monograph if the medicine is predicted to have significant budget or service impact. In such circumstances, it is possible to develop an addendum to the main report that includes the monographs. Such an addendum must be received by the January prior to the beginning of the new financial year to allow Health Boards to consider the predicted impact of the medicine for financial planning purposes. If it is not possible to produce a monograph within this timeframe, it will be necessary to list the medicine in the next interim update (see section 2.3) without the detailed information being presented in a monograph. This will therefore limit the information available to Health Boards for financial planning purposes, which could be problematic for high impact medicines. Late notification from pharmaceutical companies is not desirable as it means that financial planning information provided to Health Boards is fragmented. It also means that SMC’s resource is diverted onto monograph production for medicines in the late notification category, rather than focusing on the pipeline updates and early intelligence on medicines that will feature in the next year’s report. 2.3 Forward Look Interim Updates In addition to the annual Forward Look report that is made available in October each year, SMC produces an interim update to the main report in the April of the following year. The interim update includes. Details of new medicines that may reach the UK market in the timelines for the previous Forward Look report but that have not been included in any earlier Forward Look reports. Updates on medicines featured in previous Forward Look reports where the regulatory timeframe has changed and UK launch is now expected in the timeframe for the previously published Forward Look report or some other aspect of the information in the previous Forward Look report relating to the product has changed. Information on medicines featured in previous Forward Look reports where clinical development has since been delayed or discontinued. Information on scheduled or ongoing re-submissions for medicines in the SMC work programme. Work is ongoing to refine the content of the main Forward Look reports and the interim updates. Consideration is being given to whether information on delayed and withdrawn products and resubmissions for medicines in the SMC work plan could be extracted from the reports and made available to Forward Look recipients via the secure SMC website. This would allow this information to be updated more frequently than is currently possible. 2.4 Ad Hoc Advice Horizon scanning intelligence may be used to answer ad hoc enquires from staff within NHS Scotland. Such enquiries can range from simple enquires by clinicians about an expected UK launch date of a new medicine to requests for more complex information, for example provision of input to ten-year plans for service development within a clinical speciality. In answering enquires SMC maintains the confidentiality of any information provided in confidence by the Pharmaceutical Industry and would not disclose sensitive UK launch or cost data provided in confidence. 4 3. Horizon Scanning Processes 3.1 Overview and Timelines Horizon scanning work is carried out by a team including principal and senior pharmacists, finance experts and an administrator. The figure below provides an overview of horizon scanning processes and timelines. The timelines for the production of the annual Forward Look report are driven by financial timelines in Health Boards. In October each year financial planners require estimates of the potential budget and service impact of new medicines likely to be launched in the UK in the next financial year to inform decisions in annual budget setting processes. The Forward Look report covers a calendar year but the availability of products to prescribe in the UK will align with the financial year as it is considered that SMC advice on a product will be available three months after UK launch (i.e. Q4 2014 UK launch equates to Q1 2015 SMC advice). Figure 3: Overview of Processes and Timelines for Production of the Forward Look Report Interactions with the pharmaceutical industry are shown in italics. January Extract pipeline updates for medicines expected to be launched in the UK in the designated Forward Look timeframe from UK PharmaScan for companies registered to use the system. Receive annual horizon scanning update from companies via SMC proformas for companies not registered to use UK PharmaScan. Review all pipeline updates and request further information from companies where necessary. February Draft a list of all new medicines, new indications of existing medicines and new formulations expected in the following calendar year (i.e. provisional content of next Forward Look report). March to April Start the filtration process to identify medicines with the potential for high budget and / or service impact. Seek views on potential impact of new medicines from the Horizon Scanning Steering Group and Clinical Review Group and NHS clinical experts as required. Inform companies by email of all new medicines for inclusion in the next Forward Look report and detail which products are anticipated to have high impact. If company views are required on likely impact, seek feedback at this time. Review company feedback on provisional content of the Forward Look report. March to June June to August Prepare draft product monographs and forward to NHS clinical experts for comment. August to September Review company feedback on monographs and prepare final versions. Collate report in the appropriate format and draft the narrative for section 1 of the report that includes all medicines expected in the designated time horizon. Quality assure final content of report. Prepare the financial spreadsheets that accompany the Forward Look report for high impact medicines. October Make Forward Look report and financial spreadsheets available to NHS Health Boards. Review clinical expert comments and incorporate into monographs as appropriate. Prepare a revised draft of monographs for company review. Forward revised version of monographs to companies for comment. 5 November to December Inform companies by email of the content of the Forward Look report relating to their new medicines. Request annual horizon scanning update from companies not registered to use UK PharmaScan and contact companies registered to use UK PharmaScan to request that they confirm that their UK PharmaScan records are up to date. 6 Figure 4: SMC Forward Look Production Process KEY INTELLIGENCE AND EXPERTISE PROCESS AND TIMELINES January Gather intelligence on pipeline medicines and populate SMC’s horizon scanning database Annual updates from Pharmaceutical companies on pipeline drugs Updates from UK PharmaScan for registered companies on pipeline drugs Continuous routine scanning of information sources by Horizon Scanning Team February March to April Draft list of all new medicines expected in the next financial year Analysis and interpretation of intelligence, and filtration / selection of medicines for report based on predicted UK launch and budget impact Horizon Scanning Team Horizon Scanning Steering Group and Clinical Review Group Horizon Scanning Team NHS Clinical Experts Verification of Forward Look content Pharmaceutical Industry Development of monographs for high impact medicines in Forward Look report Horizon Scanning Team Verification of monograph content NHS Clinical Experts Verification of monograph content Pharmaceutical Industry Production of Forward Look report and financial spreadsheets Horizon Scanning Team March to June June to August August to September October Make Forward Look report and financial spreadsheet available on the SMC secure website NHS Chief Executive Officers, Directors of Medicine, Pharmacy, Finance and Public Health and other key personnel Local Health Boards use for financial planning purposes Inform Pharmaceutical Industry of Forward Look report content Pharmaceutical Industry November to December Commence annual horizon scanning update 7 Direct contact with Pharmaceutical Industry UK PharmaScan 3.2 Company Contacts When a medicine in clinical development is identified and added to SMC’s tracking lists, the pharmaceutical company developing the medicine is identified. If SMC has not had any previous contact with the company, the Horizon Scanning Team will attempt to identify the most appropriate contact person within the company with responsibility for horizon scanning intelligence. Companies planning to launch medicines in the UK that have not had previous contact with SMC are requested to make contact to ensure that SMC is aware of their developments and is planning to include their medicines in future Forward Look publications. SMC maintains a database of named company contacts. It will include details of the primary company contact(s) in relation to the new product assessment process and also contacts for the horizon scanning function. It is possible that in some companies the same person may be responsible for both aspects of communication with SMC. Contact is routinely made by email. It is extremely helpful if companies can advise SMC of any personnel changes or any updated information to their contact details. 3.3 Collation of Intelligence The Horizon Scanning Team reviews a wide range of sources of information on new medicines in clinical development on an ongoing basis and maintains details of these within a customised database. These include: UK PharmaScan Confidential NHS publications Public domain information from regulatory authorities, pharmaceutical and commercial analyst companies Other NHS organisations involved in horizon scanning work e.g. the National Institute for Health Research Horizon Scanning Centre (NIHR NHSC) and the UK Medicines Information (UKMI) network. UK PharmaScan is a confidential national horizon scanning database populated by the pharmaceutical industry that provides intelligence to horizon scanning organisations of company pipeline medicines. Information in UK PharmaScan can be invaluable if it is kept up to date and it is comprehensive. However, if information is missing or out of date, SMC will either need to source the information from other sources or contact the pharmaceutical company directly. This can be time consuming and if the information is not confirmed, it could result in information relating to a company’s pipeline being missed from SMC Forward Look publications and therefore missed from NHS Scotland’s financial and service planning cycles. Pipeline updates are received by SMC either from UK PharmaScan or directly from the company if the company is not registered to use UK PharmaScan. If no UK PharmaScan record is available the company will be asked to complete a standard proforma issued by SMC in November or December each year (see Appendix A for an example of a completed proforma). Note that information is requested on all new indications, licence extensions and new formulations of existing products as well as new medicines (chemical or biological entities) and biosimilars. If SMC considers that a product listed in the proforma may have a significant financial or service impact for NHS Scotland, a subsequent proforma is sent to the company that includes a monograph template (see Appendix B for an example of a blank proforma and a proforma with guidance notes for completion). The company will be asked to complete as much of the proforma as is possible at the time of the communication. Companies that are registered to use UK PharmaScan and whose records are complete and comprehensive will not be asked to complete a monograph proforma and UK PharmaScan will be used to source the additional information required. SMC acknowledges that companies may have difficulty providing all the information, particularly in relation to the provision of uptake and costing data up to a year or more prior to UK launch when studies may be incomplete. For some companies these difficulties may be compounded by the 8 need to have information sourced from or authorised by company divisions based outside the UK. As this advice is a critical component of the information used by NHS financial planners to facilitate the managed introduction of cost effective new medicines, SMC is committed to collaborative working with the pharmaceutical industry to achieve effective input in these challenging situations. When companies cannot provide a detailed cost analysis and point data for estimates of uptake and costs then best ‘ball-park’ range estimates can be useful. These can be provided with caveats indicating that they are early estimates. This will be taken into account when these are used together with other information (e.g. clinical expert advice) to produce the final draft budget impact estimates that are then shared with the company for further comment. General advice about proposed costing strategies or estimated uptake can also be very useful. The table below contains examples of information that SMC would find useful in the absence of more precise estimates. Information useful to SMC Horizon Scanning Team Acquisition cost An indication of potential cost range or upper or lower levels of range Cost relative to comparators An indication of potential cost relative to existing treatments Examples Estimated uptake An indication of uptake range or upper or lower levels of range Estimated uptake An indication of estimated uptake relative to existing treatments Likely to be given to at least 90% of eligible population Likely to be given to less than 5% of eligible population Likely to be given to between 40% to 60% of eligible population Likely to replace existing drugs within the same class, but not increase numbers of patients prescribed this class Likely to be given to up to 10% of patients already receiving this class of drug Likely to increase the proportion of the eligible population receiving drug therapy for this condition by up to 90% Expected to cost between £5,000 to £10,000 per patient per annum Expected to cost more than £80,000 per patient per annum Expected to cost less than £500 per patient per annum Will be priced in the same range as other drugs in class Likely to cost less than alternative treatments Likely to cost 10% to 20% more than alternative treatments UK PharmaScan contains fields to document anticipated uptake of medicines, costing data and indicative budget impact information. Given the difficulties that companies often experience in providing costing information, broader cost ranges are being added to UK PharmaScan. When reviewing information from UK PharmaScan and the completed proformas at the beginning of each calendar year (from companies not registered to use UK PharmaScan) SMC staff aim to compile a comprehensive list of all new medicines expected to be launched in the UK in the next calendar year and to gain an understanding of those likely to have significant budget and / or service implications. Companies may also be contacted on an ad hoc basis to clarify or provide additional information on particular products. Companies are encouraged to contact SMC via email or telephone at any time to update the information held about particular products, for example a change in the estimated UK launch date. Companies registered to use UK PharmaScan should ensure that their product pipeline information is kept up-to-date on an ongoing basis and that changes to regulatory information are updated immediately the information becomes available to the company, During production of a Forward Look report additional information and advice supporting the interpretation and application of horizon scanning data is obtained from a variety of sources, for example, epidemiological and prescription data from NHS National Services Scotland (NHS NSS) or Health Protection Scotland (HPS), plus advice from clinical experts and pharmaceutical companies. SMC takes appropriate steps to safeguard the security of horizon scanning information provided by pharmaceutical companies. Information provided is not copied. All horizon scanning intelligence is 9 maintained in strict confidence and stored securely. The in-house horizon scanning database is accessible by SMC staff only. 3.4 Forward Look Report Content At the beginning of each year a systematic review and assessment of horizon scanning intelligence is performed to identify new medicines with a potential UK launch date within the timeframe covered in the next annual Forward Look report and to determine those likely to require a product monograph. Clinical expert advice is obtained to inform this process when necessary. After the initial list of products has been collated for the next Forward Look report, SMC notifies individual pharmaceutical companies by email in March or April each year about their products to be included in the report. The communication specifies those products that are expected to have a significant incremental net drug budget impact (relative to comparators) or a significant service impact for NHS Scotland. It is these products that will require a product monograph. Companies are asked to give careful consideration to the proposed content at this stage, particularly in relation to the estimated timelines for product availability in the UK, judgements that have been made about a product’s potential for financial impact and any important omissions (i.e. products that SMC has not identified for inclusion in the report may be highlighted). Company feedback at this stage is extremely helpful in ensuring that Forward Look features the relevant products and that the Horizon Scanning Team’s preliminary estimates of a product’s anticipated incremental net drug budget impact appear reasonable. 3.5 Product Monographs A systematic search is performed for information on the following: How does the new product differ from existing products (e.g. pharmacology, route of administration)? What is the likely indication for the new product? How many people in Scotland would be eligible for treatment with the new product? What proportion of the eligible population is likely to receive the new product? What is the incremental drug acquisition cost of the new product relative to existing products? Would the new medicine be associated with any major service implications? After identifying and assessing all relevant data, a summary statement for each point is included in a draft product monograph together with any assumptions and uncertainties associated with the statements. The research methodology and data supporting the draft monograph are peer reviewed within the Horizon Scanning Team. 3.6 Clinical Expert Advice The SMC Horizon Scanning Team works closely with expert clinicians practising within NHS Scotland. These are identified from the SMC clinical expert panel. Advice is usually sought from around five or six clinical experts from a variety of NHS Boards throughout Scotland whenever possible for each product. However, for rare conditions the number of relevant clinicians in the SMC expert panel may be less than this. In these circumstances, additional efforts are made to identify further relevant clinical experts, for example via requests to Scottish Area Drug and Therapeutics Committees (ADTCs) or identification of relevant clinicians practising within the NHS in England, Wales or Northern Ireland. Clinical experts may be contacted for advice on a new clinical development prior to monograph production if the likely financial and / or service impact is uncertain. Help from clinical experts can be invaluable at this stage as they can provide specific intelligence from a Scottish perspective on current treatment options, current treatment protocols and guidelines, patients eligible for treatment with the condition being considered and likely uptake. 10 After the first draft of the product monograph has been prepared by the SMC Horizon Scanning Team, it is circulated for comment to clinical expert for review. Clinical experts are asked to comment on each section of the draft monograph. Advice is specifically sought on: (1) the types of patients or clinical circumstances in which the new product is likely to be used; and (2) the number of patients in Scotland likely to receive the new product. The clinical expert comments are collated and reviewed by a member of the SMC Horizon Scanning Team then used to modify the draft monograph as appropriate. This revised monograph undergoes further peer review within the Horizon Scanning Team. 3.7 Pharmaceutical Industry Input After clinical expert advice has been incorporated into the draft product monograph it is sent to the sponsor pharmaceutical company for review and comment. Pharmaceutical companies are invited to comment on each section of the monograph and any points on which input is specifically sought are highlighted. However, if preferred, companies can make general comments on the monograph and / or on the main issues where their advice is valuable. These usually relate to (1) the estimated uptake of the new product within the eligible population and (2) the estimated acquisition cost of the new product. Comments from the company are considered carefully by the SMC Horizon Scanning Team and the draft monograph is modified further if required. If substantial changes to content are made then the company is sent the revised draft for further review and comment. In November each year, after the Forward Look report has been made available to Health Boards, companies are sent an email with details of their products that have been included in the report including any monographs that have been drafted for the companies’ products. Copies of the context statement that forms the introduction of the report are also sent to companies. 3.8 Financial Spreadsheets The purpose of the financial spreadsheets is to summarise the estimated incremental net drug budget impact of each significant new medicine by geographical area (West of Scotland, East of Scotland and North of Scotland) and by individual NHS Board. The spreadsheets summarise the financial information identified in product monographs within the Forward Look reports and also include new medicines identified in previous Forward Look reports that have not yet had a budget impact (e.g. due to delayed UK launch). This ensures that the spreadsheets include the potential budget impact of all medicines that may be initially used in the NHS in the following calendar year. The spreadsheets categorise data into two main types: (1) New cancer medicines, listed by tumour type and indication (2) New non-cancer medicines listed by BNF category and indication. The spreadsheets include data on the following: Annual net cost of treatment per patient or net cost per patient per course (i.e. net of the cost of substituted medicines). Non-recoverable VAT is added where believed appropriate. Estimated patient numbers in the East, West and North of Scotland in years 1 and 5. Patient numbers for each of the above geographical areas and Board are calculated based on mid-year population estimates from the National Records of Scotland. National Resource Allocation Committee (NRAC) shares are not used. Estimated total cost of each new medicine in years 1 and 5 (assumed to be the maximum financial cost) for East, West and North of Scotland and individual Board. In year 1 the estimated cost takes into account the expected approval date of the new medicine by SMC. 11 Therefore for a medicine that is estimated to be reviewed by SMC in October the year one cost will be reduced by half. Whether there are any service implications. This warns the financial teams to be aware of potential additional costs that may occur or savings that may be available (e.g. medicine administration may be more or less complex requiring changes to resources). The financial spreadsheets are prepared in Microsoft Excel format to allow NHS Boards to modify factors (e.g. to adjust patient numbers for local epidemiology, or different rates of uptake based on local clinical expert opinion [see section 5]). The spreadsheets are emailed in confidence to Directors of Finance and Directors of Pharmacy. The spreadsheets are distributed to those that require them for budget preparation, subject to the requirement to maintain the confidentiality of the information provided. An example of a spreadsheet on non-cancer medicines is set out at Appendix C. 4. Confidentiality The provision by the pharmaceutical industry of intelligence, that is often commercially sensitive, is central to the success of SMC’s horizon scanning initiative. The risks to a pharmaceutical company associated with the release of this confidential information are comprehensively appreciated by SMC. Thus SMC staff are particularly aware of the necessity to maintain its confidentiality and various processes have been established to store the data securely, to restrict access to it and to ensure NHS Board recipients understand the precautions associated with its use. The report is issued in confidence to key named individuals within NHS Scotland, including Chief Executive Officers and Directors of Finance, Pharmacy, Medicine and Public Health. These NHS Board recipients are made aware that information included within the Forward Look report is privileged, confidential and intended for those specifically authorised to receive it for planning of resource and estimating budgets. In this regard NHS Scotland personnel who have access to the Forward Look reports sign a confidentiality agreement each year that is accompanied by a code of practice on the appropriate use of the intelligence in the reports. If Health Boards receive requests under the Freedom of Information (FOI) Act relating to information within the report it is strongly recommended that they consult with partners at SMC prior to the release of any information. If information is extracted from the report and incorporated into local documents, these must make reference to the commercially sensitive nature of the information and the recommendation to refer any FOI requests relating to it to SMC. When NHS clinicians are included in the SMC clinical expert panel they agree to maintain the confidentiality of any information they receive in relation to their work for SMC. When these clinicians are approached to comment on draft product monographs the confidential nature of the information is highlighted and the need to maintain the confidentiality of this information is reiterated. 12 5. Using the Forward Look Report in Practice Health Boards have established processes, mainly through ADTCs and prescribing advisory structures, to utilise the intelligence in the Forward Look report for financial and service planning purposes. The information in relation to specific new medicines is often shared in confidence with local clinical specialists or groups to seek their opinion on the assumptions and estimates used in the Forward Look report. This may provide reassurance, for example, with regard to the estimated number of patients to be treated, or may prompt some reworking of the figures to reflect local epidemiology, treatment of patients within clinical studies, or other aspects of how care is provided. After consideration has been given to the application of the Forward Look report in the Health Board (or region, particularly for new cancer medicines), further internal reports may be prepared and provided to Health Boards or regional advisory groups. If any part of the report is shared with individuals who are not named recipients of the report, they should be made aware of the confidentiality issues and they will be asked to sign and return a confidentiality agreement to SMC. SMC and Health Boards recognise that the figures in the Forward Look report may represent a ‘worst case scenario’ given that some of the new medicines listed may not reach the UK market within the predicted timeframe or at all. Of those that do reach the UK market within the timeframe, a proportion will not be accepted by SMC for use in NHS Scotland, and in these cases the predicted cost impact will not be realised. To account for this, Health Boards may choose to apply a ‘modifying factor’ to generate a more realistic figure for the costs that may be realised in practice. There are inherent risks in this approach, due to uncertainty around what constitutes an appropriate figure, and Health Boards understand the need to regularly review local projections to reflect SMC advice and its forthcoming work programme. The Forward Look financial spreadsheets have been developed to allow this ongoing, dynamic inyear adjustment by Health Boards. For example, if a new medicine is considered by SMC but not recommended for use in Scotland, then it may be removed from the cost projections for the remainder of the current year. If the UK launch of a new medicine is delayed by approximately six months, then it is a straightforward step to adjust the projected costs accordingly. For medicines predicted to have a moderate or high cost impact that are accepted by SMC, a detailed local budget impact model may be prepared. This detailed model is based on epidemiology, literature estimates and information from the SMC Detailed Advice Document. Assumptions in the model are confirmed with local specialists and phased uptake of the product is taken into account (for example the number of patients initiated per month or quarter). A detailed model of this type may be used to inform a business case for the use of the medicine prepared by the relevant clinical team, if appropriate. For some high cost new medicines the actual expenditure in practice can then be tracked alongside the local detailed budget impact model, although for various reasons this can be problematic. When variance between predicted and actual budget impact is identified, clinical expertise may be sought in an attempt to understand or justify the variance, although in practice this is challenging. October 2013 13 Appendix A: Example of a Completed Horizon Scanning Update Form (for companies not registered with UK PharmaScan) Section 1: New Chemical / Biological Entities and Biosimilars Please list new chemical / biological entities and biosimilar medicines that (1) have been filed for European or UK marketing approval (i.e. preregistration); (2) are in phase III clinical development; or (3) are in phase II clinical development and likely to be launched in the UK within two years. We are particularly interested in those that are likely to be launched in the UK during 2014 (i.e. between calendar Q1 2014 and Q4 2014), as these would be included in the next SMC report (Forward Look 9). To allow these products to be identified, please estimate the UK launch date by calendar quarter, if possible. Product generic (Brand) names and formulation Pharmacology or therapeutic class Indication or therapeutic area European development phase Drug A (Brand A) tablet NSAID Pain Phase III Drug B (Brand B) inhaler B-agonist Asthma Drug C (Brand C) sc injection New biologic Psoriasis Preregistration Phase III Estimated European filing date (and route e.g. EMA/ MHRA) Q3 or Q4 2013 Filed in UK Q2 2013 Q3 2013 via EMA Estimated UK launch date Considered to be a ‘significant’ development Q3 or Q4 2014 Q2 2014 No Q3 2014 Yes No * A new medicine is considered to be significant if (1) it is expected to have a moderate to high potential net drug budget impact for NHS Scotland (i.e. >£500,000 per annum at year five); (2) it is expected to have a major service implication. 14 Section 2: New Indications for Existing Products Please list new indications for existing products that (1) have been filed for European or UK marketing approval (i.e. pre-registration); (2) are in phase III clinical development; or (3) are in phase II clinical development and likely to be launched in the UK within two years. We are particularly interested in those that are likely to be launched in the UK during 2014 (i.e. between calendar Q1 2014 and Q4 2014), as these would be included in the next SMC report (Forward Look 9). To allow these products to be identified, please estimate the UK launch date by calendar quarter, if possible. Product generic (Brand) names and formulation Pharmacology or therapeutic class Indication or therapeutic area European development phase Drug D (Brand D) tablet NSAID Arthritis Phase III Estimated European filing date (and route e.g. EMA/ MHRA) Q4 2013 in France (reference member state for mutual recognition procedure) Estimated UK launch date Considered to be a ‘significant’ developme nt Q4 2014 No * A new indication is considered to be significant if (1) it is expected to have a moderate to high potential net drug budget impact for NHS Scotland (i.e. >£500,000 per annum at year five); (2) it is expected to have a major service implication. 15 Section 3: New Formulations of Existing Medicines Please list new formulations of existing medicines that (1) have been filed for European or UK marketing approval (i.e. pre-registration); (2) are in phase III clinical development; or (3) are in phase II clinical development and likely to be launched in the UK within two years. We are particularly interested in those that are likely to be launched in the UK during 2014 (i.e. between calendar Q1 2014 and Q4 2014), as these would be included in the next SMC report (Forward Look 9). To allow these products to be identified, please estimate the UK launch date by calendar quarter, if possible. Product generic (Brand) names and formulation Pharmacology or therapeutic class Indication or therapeutic area European development phase Drug D (Brand D) sc injection Biological therapy Subcutaneous formulation for the treatment of rheumatoid arthritis (to replace existing intravenous formulation) Phase III Estimated European filing date (and route e.g. EMA/ MHRA) Q4 2013 Estimated UK launch date Considered to be a ‘significant’ developme nt Q3 2014 No * A new formulation is considered to be significant if (1) it is expected to have a moderate to high potential net drug budget impact for NHS Scotland (i.e. >£500,000 per annum at year five); (2) it is expected to have a major service implication. 16 Appendix B: Sample Monograph and Monograph Template Monograph Production for Significant New Medicines and Indications Please complete a product monograph template for each significant new medicine or indication likely to be marketed in the UK during 2014 (i.e. between January 2014 and December 2014). A new medicine or indication is considered to be significant if it has a moderate to high net drug budget impact (i.e. >£500,000 per annum at year five) for NHS Scotland and / or major service implication for NHS Scotland. A sample product monograph template with guidance notes in blue text is provided below. This details the information that should be included in the monograph. 1. Sample Monograph with Guidance Notes Product Guidance notes: Enter details of the generic name; proprietary name and formulation of the product. Please also enter the dosing schedule if known. Indication Guidance notes: Enter details of the likely indication of the new medicine. If this is not known, then it may be possible to extrapolate this from details of the patient population included in the registration clinical studies. Status Guidance notes: Note if the product has European orphan drug status for the indication under consideration. Enter details of the licence status of the product, including the estimated date of European filing for marketing authorisation that would allow the product to be marketed in the UK and the route through which a marketing authorisation will be sought. Also, enter details of the estimated launch date for the product in the UK. Mode of action Guidance notes: Enter brief details of pharmacology including pharmacological class and if appropriate details of the clinical significance of the new product, for example: likely to be the first drug in a new pharmacological class or likely to be first oral formulation of a drug previously only available as parenteral formulations. Estimated eligible population Estimated prevalent / incident (delete as necessary) population with (insert likely indication or patient group) of approximately (insert number) patients / episodes (delete as necessary). 17 Assumptions Guidance notes: Enter details of assumptions used to estimate the number of patients who would be eligible for treatment with the new product or licence. These are usually presented as bullet points, with one assumption per point. Where a number of assumptions contribute to the estimate, these should flow in sequence from the top. Where possible Scottish data are used e.g. epidemiology data from NHS National Services Scotland (NHS NSS) or Health Protection Scotland (HPS). If Scottish data are not available, English or Welsh data from reliable sources (e.g. guidance from the National Institute for Health and Care Excellence [NICE]) may be extrapolated to produce estimates for Scotland. Information on the epidemiology of the condition obtained through a systematic search of the published literature can be used to check the estimates derived from these epidemiological data or may be used in place of them, if these data are not available. Where the eligible population in Scotland is estimated from an extrapolation of figures in published paper(s), Scottish population data should be taken from the latest mid-year population estimates from the General Register for Scotland. If possible, all assumptions should be clearly referenced using superscript numbers in sequential order throughout the text. Uncertainties Guidance notes: Enter details of any factors or issues that create uncertainty around the estimate of eligible population, for example limitations of data used to estimate mean patient numbers for a rare condition or disease; or potential disparity in the distribution of patients across Health Boards in Scotland for rare diseases with a genetic component. These issues are usually presented as bullet points. If possible, all uncertainties should be clearly referenced using superscript numbers in sequential order throughout the text. Estimated uptake Estimated uptake in years 1 and 5 by approximately (insert number) and (insert number) eligible patients / episodes (delete as necessary), respectively. Assumptions The new product would be given to / used in (delete as necessary) approximately (insert percentage) and (insert percentage) of eligible patients / episodes (delete as necessary) in years 1 and 5, respectively. 18 Guidance notes: Enter details of the proportion of the eligible population who would be likely to receive the new product in the first and fifth full calendar years after it is launched in the UK and any other assumptions used to estimate uptake, for example, an assumption that the new product would only be given to those eligible patients who had a certain severity of disease. Assumptions are usually presented as bullet points, with one assumption per point. If possible, all assumptions should be clearly referenced using superscript numbers in sequential order throughout the text. Uncertainties Guidance notes: The first bullet point should read: ‘Uptake will be influenced by cost-effectiveness relative to alternatives and this is unknown’. Clinical data should be presented in this section. If phase III clinical studies are underway these should be discussed here (ideally comparator studies but if only placebo-controlled studies are available these should be discussed), with brief details of methodology (clinicaltrial.gov reference number, planned or actual patient numbers, treatment strategies, primary endpoints [and secondary endpoints if relevant] and duration of the study or planned completion date if it is still ongoing). If no phase III clinical study results are available but phase II data are available these should be detailed briefly in this section. Ideally use one bullet point per study but if there are multiple relevant studies a judgment should be made about whether the data would best be presented in a different way. If results of clinical studies are available provide details of statistical significance of the treatment under consideration versus comparator(s) or placebo. Briefly describe safety results, primarily focusing on grade III or IV adverse events for the drug under consideration and the comparator(s) or placebo. Finally, enter details of any factors or issues that create uncertainty around the estimate of uptake of the new product, for example lack of data on the costeffectiveness of the new product relative to relevant comparators; the potential for ‘off-label’ use in patients not covered by the indication in the proposed product licence; or proposed clinical guidance that may alter treatment pathways. These are usually presented as bullet points. If possible, all uncertainties should be clearly referenced using superscript numbers 19 in sequential order throughout the text. Estimated incremental drug acquisition cost Estimated incremental drug acquisition cost of approximately (insert cost) per annum / episode (delete as necessary). Guidance notes: This is calculated by subtracting the drug acquisition cost of alternative treatment(s) from the drug acquisition cost of the new product. Assumptions The estimated drug acquisition cost of the new product is approximately (insert cost) per annum / episode (delete as necessary). Guidance notes: Enter details of the estimated drug acquisition cost (or cost range) of the new product and the dosing regimen associated with this cost. This need not be an accurate and definitive estimate; a range or ‘ball-park’ possible estimate is acceptable. If the product is used annually in some patients and per course in others (e.g. seasonally), this should be accounted for in the cost calculations so that the cost per annum and / or per course is clearly stated. If the product is to be used in the hospital setting value added tax (VAT) should be added at the appropriate rate and this should be clearly stated. If the product is to be used in the primary care setting no VAT is added. If the product is initiated in the hospital setting but prescribing is continued in the primary care setting or the product is made available via a homecare arrangement no VAT is added. This also applies to the cost of alternative(s) outlined below. SMC acknowledges that providing a cost estimate up to one year in advance of UK launch can be challenging. However, this is required as financial planners use the monographs to set budgets (one year in advance) for the calendar year in which the new product is likely to be introduced in the UK. Thus, financial planners have advised SMC that they must have a figure for this budget setting process and, in the absence of advice from the company, have requested that SMC provide an estimate of acquisition cost. SMC acknowledges that their estimate of likely drug acquisition cost (or cost range) will be less robust that an estimate made by the relevant company and, therefore, seeks the advice of the company to help with this essential estimate. 20 The new product would be used in place of (insert details of alternative treatments used in current practice). Guidance notes: Enter details of the most common treatment(s) that the new product is likely to replace within Scottish clinical practice. If the new product under consideration will be used in addition to current practice or if there are no treatments currently available for use in NHS Scotland, this should be stated. Consideration should also be given to whether alternative products have been approved for use in NHS Scotland by SMC at the time the monograph is being drafted. The alternative treatment costs approximately (insert cost) per annum / episode (delete as necessary). Guidance notes: Enter details of the drug acquisition cost of the treatment(s) that the new product would replace and their associated dosing regimen(s). Cost should be taken from Part 7 of the most recent version of the Scottish Drug Tariff where possible and for other drugs from the most recent version of the BNF or MIMS. If more than one alternative treatment is used routinely in NHS Scotland, they should all be listed with associated costs and an average cost of alternatives should be presented and subtracted from the cost of the new product being considered, unless it is apparent that one alternative is used preferentially in NHS Scotland, in which case a weighted estimate of proportion of usage should be considered and the alternative costs calculated accordingly. If possible, all assumptions should be clearly referenced using superscript numbers in sequential order throughout the text. Uncertainties Enter details of any factors or issues that create uncertainty around the estimate of incremental drug acquisition cost, for example the pending loss of patent protection of an alternative treatment, or the pending UK launch of a similar new product. If the duration of treatment is uncertain (generally applicable for cancer medicines) this should be stated with reference to the reason the estimated duration of treatment was chosen (e.g. median time to progression in the phase III clinical study used as a guide). All uncertainties should be clearly referenced using superscript numbers in 21 sequential order throughout the text. Estimated net drug budget impact Year 1: (insert number of patients who will receive the new product) x £ (insert incremental drug acquisition cost [i.e. cost of new product – cost of alternative]) = £ Year 5: (insert number of patients who will receive the new product) x £ (insert incremental drug acquisition cost [i.e. cost of new product – cost of alternative]) = £ Guidance notes: Enter the appropriate figures from the above estimated uptake and estimated incremental drug acquisition costs sections. If the product is used annually in some patients and per course in others (e.g. seasonally), this should be accounted for in the net drug budget impact calculation by using the appropriate cost for each treatment duration and the appropriate proportion of the number of patients who will receive each treatment strategy. The statement ‘(including VAT)’ should be included after the budget impact figure for each year above if appropriate. Guidance notes: Enter details of any potential impact (other than drug acquisition Service Implications cost) of the new product, for example cost of testing or new equipment associated with its use; impact on staffing or service provision. Also, note whether the impact is likely to be significant. If a diagnostic / therapeutic test is required to assess whether patients are eligible for the new treatment, details (including estimated cost of the test if available) should be included. References and Additional Information Guidance notes: Enter references in the Vancouver style. Enter any additional information that may be relevant to the medicine / indication being considered. 22 2. Product Monograph Template Product Indication Status Mode of action Estimated eligible population Estimated prevalent / incident (delete as necessary) population with (insert likely indication or patient group) of approximately (insert number) patients / episodes (delete as necessary). Assumptions Uncertainties Estimated uptake Estimated uptake in years 1 and 5 by approximately (insert number) and (insert number) eligible patients / episodes (delete as necessary), respectively. Assumptions The new product would be given to / used in (delete as necessary) approximately (insert percentage) and (insert percentage) of eligible patients / episodes (delete as necessary) in years 1 and 5, respectively. Uncertainties Uptake will be influenced by cost-effectiveness relative to alternatives and this is unknown. Estimated incremental drug acquisition cost Estimated incremental drug acquisition cost of approximately (insert cost) per annum / episode (delete as necessary). Assumptions The estimated drug acquisition cost of the new product is approximately (insert cost) per annum / episode (delete as necessary). The new product would be used in place of (insert details of alternative treatments used in current practice). The alternative treatment costs approximately (insert cost) per annum / episode (delete as necessary). Uncertainties Estimated Year 1: (insert number of patients who will receive the new product) x £ (insert incremental drug acquisition cost [i.e. cost of new 23 net drug budget impact product – cost of alternative])) = £ Year 5: (insert number of patients who will receive the new product) x £ (insert incremental drug acquisition cost [i.e. cost of new product – cost of alternative]) = £ Service Implications References and Additional Information 24 Appendix C: Example of Financial Spreadsheet Predicted Annual Expenditure 2014 Cost per patient per year or course Number of patients or Episodes year 1 Number of patients or episodes year 5 Health Board 1 Health Board 2 Health Board 3 Projected Maximum Annual Expenditure Health Board 1 Generic Name B N F Drug 1 1 849 Drug 2 2 1,011 5 8 0 0 0 702 Drug 3 2 495 201 768 6,477 21,043 47,101 32,998 Drug 4 3 682 106 395 3,137 10,193 22,815 23,383 Drug 5 3 49 1,964 2,648 4,177 13,569 30,372 11,262 Drug 6 4 8,000 4 8 1,389 4,512 10,099 Drug 7 4 5,962 7 18 0 0 Drug 8 7 2,000 5 8 0 Drug 9 9 20,000 1 3 Drug 10 9 638 22 169 Drug 11 1 0 795 10 18 Total £ Number 239 Number 401 Health Board 2 Health Board 3 Service Implication Predicted Launch Date Predicted SMC Date 214,891 No Jan-14 Apr-14 1.00 2,281 5,105 No Jan-14 Apr-14 1.00 107,205 239,957 No Apr-14 Jul-14 0.75 75,968 170,039 No Jul-14 Oct-14 0.50 36,590 81,899 No Jul-14 Oct-14 0.50 5,555 18,048 40,397 No Jul-14 Oct-14 0.50 0 9,315 30,263 67,738 Yes Oct-14 Jan-15 0.25 0 0 1,389 4,512 10,099 No Oct-14 Jan-15 0.25 2,155 7,001 15,671 5,208 16,920 37,872 No Jan-14 Apr-14 1.00 0 0 0 9,359 30,406 68,057 No Jul-14 Oct-14 0.50 518 1,681 3,764 1,242 4,035 9,032 No Apr-14 Jul-14 0.75 35,465 115,221 257,900 129,965 422,235 945,088 £ £ 17,613 £ 57,221 £ 128,077 £ 29,551 25 96,007 £ Proportion of 2014 / 15
































![My_Body[1] - Junior2TopicWiki](http://s1.studyres.com/store/data/008060165_1-be31cd2568d5e2c9fee6ce67732b07b4-150x150.png)