* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download What is Matter
Survey
Document related concepts
Transcript
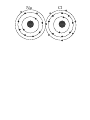
What is Matter? Anything that can be smelled, tasted, touched… Has mass and volume (takes up space) Matter exists in some state or phase Most common are solid, liquid, gas. Others are non-newtonian, & plasma Matter is made up of atoms. The word ATOM comes from the Greek philosopher Democritus. It means “Indivisible” Atomic Theory: It is thought that atoms are made of particles. Most common particles are: PROTON- has a positive Charge NEUTRON- has a neutral charge; has both positive and negative attributes ELECTRON- has a negative charge Atom Smallest part or piece of matter that still has the properties of matter Proton: Positively charged particle; largest of three particles; symbol is P+ Neutron: Neutrally charged particle; has both positive and negative charges; slightly smaller than proton; symbol is N= Electron: Negatively charge particle; 2000 times smaller than proton; symbol is e- Atomic Structure: Proton Nucleus Neutron Electron Electron Cloud Periodic Table First organized using the known properties from other chemists like Stanislao Cannizzaro, Dmitri Mendeleev created the table of elements in 1869 Period of elements Organized horizontally Indicates # of electron levels Family of elements Organized vertically Indicates # of electrons in outer cloud Atomic Number (# of P+) Elemental Symbol (Often from Latin or Greek) (1st letter is upper case, 2nd is lower case) 7 N 2 Nitrogen 5 Elemental Name Atomic Mass (# of P+ & N=) # of e- in each electron cloud Q: How can we calculate the # of Neutrons? Covalent Bonding: Atoms join together to form molecules of a compound through bonding. The most common way to bond is by sharing electrons The first three periods of elements are the easiest to understand. The most eheld in the outer cloud of these elements is 8. In order to become more stable, elements will share e-. EX: Na Cl