* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download IND Exemption Determination
Polysubstance dependence wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Compounding wikipedia , lookup
Drug design wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
List of off-label promotion pharmaceutical settlements wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacognosy wikipedia , lookup
Theralizumab wikipedia , lookup
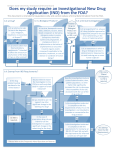
REVIEWER CHECKLIST INVESTIGATIONAL NEW DRUG (IND) APPLICATION DETERMINATION PROTOCOL TITLE: PRINCIPAL INVESTIGATOR: SPONSOR/FUNDING SOURCE: IRB Panel: A B IRB REFERENCE #: Meeting Date: Reviewer Initials: Primary Reviewer When a clinical investigation involves the use of a drug or biologic other than the use of an FDA approved, marketed drug/biologic in the course of medical practice, ONE of the following conditions must be true: The drug or biologic must have an IND issued by the FDA An IND is not required – the investigation meets the criteria for exemption Verification of IND Status Does the drug or biologic have an IND issued by the FDA? Yes No If yes, is the IND valid? (The Investigator’s Brochure may not be used for this purpose). Only one of the following must be “Yes”: The number is imprinted on the sponsor’s protocol. The number is noted in written correspondence from the sponsor. The number is noted in written correspondence from the FDA (required if the Investigator holds the IND) . Yes No Yes No Yes No Assessment of Requirement for an IND For a clinical investigation involving a drug or biologic lawfully marketed in the U.S., ALL of the following items must be found to be “Yes” for an IND to not be required. N/A Is the investigation intended to be reported to FDA as a well-controlled study in support of a new indication for use? Yes No Is the investigation intended to be used to support any other significant change in the labeling for the drug? Yes No (rev. 7/4/11) Is the investigation intended to support a significant change in the advertising for the lawfully marketed drug product undergoing investigation? Yes No Does the investigation involve a route of administration or dosage level or use in a patient population or other factor that significantly increases the risks (or decreases the acceptability of the risks) associated with the use of the drug product? Yes No Will the investigation be conducted in compliance with the requirements for institutional review set forth in part 56 and with the requirements for informed consent set forth in part 50? Yes No Will the investigation be conducted in compliance with the requirements of 21 CFR 312.7? Yes No The investigation is not intended to invoke exception from informed consent requirements for planned emergency use under 21 CFR 50.24. Yes No For a clinical investigation involving a combination of drug products, this item must be found to be “Yes” for an IND to not be required. N/A Each drug in the combination has been approved by the FDA for marketing in the United States. Yes No Note: Consultation with the FDA may be needed at the discretion of the IRB, for example, if the IRB finds that statement 1.d. (above section) applies to the combination of products. For a clinical investigation involving an in vitro diagnostic biological, ALL of the following items must be found to be “Yes” for an IND to not be required. N/A The unlicensed biological product is limited to one or more of the following in vitro diagnostic products: (a) blood grouping serum; (b) reagent red blood cells; and (c) anti-human globulin; Yes No It is intended to be used in a diagnostic procedure that confirms the diagnosis made by another, medically established, diagnostic product or procedure; Yes No It is shipped in compliance with 312.160. Yes No For a clinical investigation involving the use of a placebo: An IND is not required for a clinical investigation involving use of a placebo if the research does not otherwise require submission of an IND. For an in vivo bioavailability or bioequivalence study in humans, ALL of the following 6 items must be answered “No” for an IND to not be required. N/A [If any of the following is answered “Yes”, an IND is required.] Page 2 of 3 (rev. 7/4/11) The test product contains a new chemical entity** as defined in 21 CFR 314.108(a). [**a drug that contains no active moiety that has been approved by FDA in any other application] Yes No The study involves a radioactively labeled drug product; Yes No The study involves a cytotoxic drug product. Yes No The investigator will conduct a bioavailability or bioequivalence study in humans using a drug product that contains an already approved, non-new chemical entity and the study will involve a single dose in normal subjects or patients where either the maximum single or total daily dose exceeds that specified in the labeling of the drug product that is the subject of an approved new drug application or abbreviated new drug application; Yes No The investigator will conduct a bioavailability or bioequivalence study in humans using a drug product that contains an already approved, non-new chemical entity and the study will involve a multiple-dose study in normal subjects or patients where either the single or total daily dose exceeds that specified in the labeling of the drug product that is the subject of an approved new drug application or abbreviated new drug application; Yes No The investigator will conduct a bioavailability or bioequivalence study in humans using a drug product that contains an already approved, non-new chemical entity and the study will involve a multiple-dose study on an extended release product on which no single-dose study has been completed. Yes No For tests in vitro or in laboratory research animals, the following items must be found to be “Yes” for an IND to not be required. N/A Is the drug/biologic is intended solely for tests in vitro or in laboratory research animals? Yes No Will the drug/biologic be shipped in accordance with FDA Regulations for Drugs for investigational use in laboratory research animals or in vitro tests (21 CFR 312.160) Yes No FINAL IRB DETRMINATION An IND is required for this investigation. Proper documentation has been received. An IND is not required for this investigation (an FDA exemption letter has been received). The IRB feels an IND is not required for this investigation; however, the investigator should formally request an exemption determination from the FDA. Page 3 of 3 (rev. 7/4/11)