* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download WS for 10/14 Sun (dot structures, geometry)
Survey
Document related concepts
Transcript
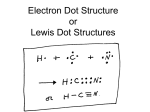
Lewis Dot Structures, Molecular Geometries Supplemental Instruction Iowa State University Leader: Course: Instructor: Date: Danielle Chem 167 Schewe-Miller 10/14/12 1. Indicate how many valence electrons are in the following compounds/elements. C ___ electrons F ___ electrons SO2 ___ electrons CH3 ___ electrons 3 PO4 ___ electrons NH4+ ___ electrons 2. Draw Lewis structures for the following compounds. CS2 SCl2 SeI4 XeF2 NO2- 1060 Hixson-Lied Student Success Center 515-294-6624 [email protected] http://www.si.iastate.edu 3. Draw the Lewis dot structure for SF4. How many lone pairs are there? How many total bonding sites are there? 4. What is the molecular geometry of SF4? What would it be if there was one more lone pair? 5. In each group of three bonds, circle the one which is most likely to be polar. C–H O–H S –H C – Cl Cl – Cl H – Cl F–F O–F C–F N–H N–O N – Cl 6. Fill in the blanks or circle the correct term. Bonds are likely to be ionic if there is a great/small difference in ________________ between the atoms. When a structure can be represented in multiple ways, we say that it has a certain number of ______________ structures. The concept of an atom trying to have ____ valence electrons is called the _______________ rule. The attraction of an atom for the shared electrons in a covalent bond is called ___________________. 7. Some exceptions to the octet rule: Hydrogen and Helium are stable with only ____ electrons. Beryllium (Be) is stable when surrounded by ____ electrons. Boron (B) is stable when surrounded by ____ electrons.