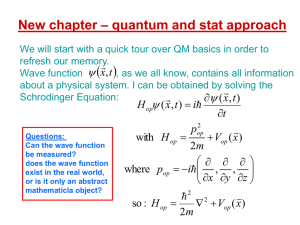
Read Notes #1 - Faculty Website Listing
... uncertainty in energy is related to the time required for the transition (t). An emission with zero uncertainty in energy would require a transition time (t) of infinity (i.e. it would never happen). Thus Quantum Physics is Not Deterministic. It is Probabilistic. We have verified the Heisenberg r ...
... uncertainty in energy is related to the time required for the transition (t). An emission with zero uncertainty in energy would require a transition time (t) of infinity (i.e. it would never happen). Thus Quantum Physics is Not Deterministic. It is Probabilistic. We have verified the Heisenberg r ...
14 - University of Utah Physics
... One of the strange features of quantum mechanics is that the behavior that something exhibits can depend on what we try to find out about it. Thus, an electron can behave like a particle or like a wave, depending on which experimental setup we subject it to. For example, in some situations particlel ...
... One of the strange features of quantum mechanics is that the behavior that something exhibits can depend on what we try to find out about it. Thus, an electron can behave like a particle or like a wave, depending on which experimental setup we subject it to. For example, in some situations particlel ...
Chapter 1. Fundamental Theory
... Postulate III describes the basic principle of quantum measurement, which is the foundation of quantum interpretation. While the mathematical structure of quantum mechanics is extremely successful, its interpretation remains controversial. In this class we adopt the standard Copenhagen interpretatio ...
... Postulate III describes the basic principle of quantum measurement, which is the foundation of quantum interpretation. While the mathematical structure of quantum mechanics is extremely successful, its interpretation remains controversial. In this class we adopt the standard Copenhagen interpretatio ...
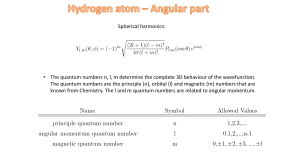
Spherical harmonics: • The quantum numbers n, l, m determine the
... The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum. ...
... The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 11. a) If a particle’s kinetic energy is equal to its rest mass energy, what is its speed? b) Obtain the relation between the relativistic energy and momentum. (3 ½ +4) 12. Explain how the components of electric field transform as viewed from another inertial frame. 13. Outline the wave mechanical p ...
... 11. a) If a particle’s kinetic energy is equal to its rest mass energy, what is its speed? b) Obtain the relation between the relativistic energy and momentum. (3 ½ +4) 12. Explain how the components of electric field transform as viewed from another inertial frame. 13. Outline the wave mechanical p ...
Syllabus PHYS 441
... A survey of concepts in particle and nuclear physics. We will learn about particles and forces that make up this universe, modern theories about these forces, culminating into an "almost theory of everything" known as the standard model of particle physics. We will learn about the Higgs boson and, t ...
... A survey of concepts in particle and nuclear physics. We will learn about particles and forces that make up this universe, modern theories about these forces, culminating into an "almost theory of everything" known as the standard model of particle physics. We will learn about the Higgs boson and, t ...
Presentation
... There exists an unitary operator U (not unique), acting on some larger space formed by system and environment, corresponding to every quantum operation. ...
... There exists an unitary operator U (not unique), acting on some larger space formed by system and environment, corresponding to every quantum operation. ...
The Learnability of Quantum States
... Consider the class of point functions: fs(x)=1 if x=s, fs(x)=0 otherwiseThis scheme is provably secure Theorem: ...
... Consider the class of point functions: fs(x)=1 if x=s, fs(x)=0 otherwiseThis scheme is provably secure Theorem: ...
Materiality: Is It Real?
... our material reality is the result of man’s practical nature. Physics is concerned with physical phenomena for which it is necessary to have practical explanations. Every physical substance has mass, or its essential existence . Its mass is what is pushed, pulled, spun, or just allowed to lie there, ...
... our material reality is the result of man’s practical nature. Physics is concerned with physical phenomena for which it is necessary to have practical explanations. Every physical substance has mass, or its essential existence . Its mass is what is pushed, pulled, spun, or just allowed to lie there, ...
Quantum Reality
... only refuse to share a room but also insist on rooms as far as possible from each other. On the other hand, boson siblings prefer to share the same room. (Since fermions rent more rooms than bosons, motel owners prefer doing business with fermions. Some motels even refuse to rent rooms to bosons!) ...
... only refuse to share a room but also insist on rooms as far as possible from each other. On the other hand, boson siblings prefer to share the same room. (Since fermions rent more rooms than bosons, motel owners prefer doing business with fermions. Some motels even refuse to rent rooms to bosons!) ...
Part I - TTU Physics
... • For a system with f degrees of freedom, the many particle wavefunction is formally: ...
... • For a system with f degrees of freedom, the many particle wavefunction is formally: ...
Copenhagen Interpretation (of quantum physics)
... the package of ideas that became the Copenhagen Interpretation, But [Neils] Bohr was always its most evangelical proponent. The package was essentially complete by 1930…But it rests on some quite bizarre concepts. The key concept is the so-called ‘collapse of the wave function’, In seeking to explai ...
... the package of ideas that became the Copenhagen Interpretation, But [Neils] Bohr was always its most evangelical proponent. The package was essentially complete by 1930…But it rests on some quite bizarre concepts. The key concept is the so-called ‘collapse of the wave function’, In seeking to explai ...
COMPLEXITY OF QUANTUM FIELD THEORIES 1. Introduction
... operator whose eigenstates are the states with definite values of energy. The energy of an eigenstate is its eigenvalue). We require locality of our field theory, so the Hamiltonian of our system is of the form Z H = d3 H(x), where H is called the “Hamiltonian density” of our system[3]. 2Technically ...
... operator whose eigenstates are the states with definite values of energy. The energy of an eigenstate is its eigenvalue). We require locality of our field theory, so the Hamiltonian of our system is of the form Z H = d3 H(x), where H is called the “Hamiltonian density” of our system[3]. 2Technically ...
Modern Physics
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
Modern Physics
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
... We cannot specify the precise location of the particle in space and time We deal with averages of physical properties Particles passing through a slit will form a diffraction pattern Any given particle can fall at any point on the receiving screen It is only by building up a picture based on many ob ...
p = mv
... Eventually these light quanta became known as photons. In 1924 Louis de Broglie proposed that if light (a classical wave) can display particle-like behavior, then matter (classical particles) should likewise display wave-like behavior under particular experimental conditions. ...
... Eventually these light quanta became known as photons. In 1924 Louis de Broglie proposed that if light (a classical wave) can display particle-like behavior, then matter (classical particles) should likewise display wave-like behavior under particular experimental conditions. ...
Chapter 11 Quantum statistics
... manifests itself in the statistical interpretation of the wave function and in the uncertainty relation between qi and pi , which cannot be anymore sharply measured at the same time. The concepts of phase space and phase trajectory have no meaning anymore. ...
... manifests itself in the statistical interpretation of the wave function and in the uncertainty relation between qi and pi , which cannot be anymore sharply measured at the same time. The concepts of phase space and phase trajectory have no meaning anymore. ...
De Broglie Waves.
... Bohr's success in describing the line spectrum of hydrogen atom when he assumed that the electron moves in orbit (circular shape) around stationary nucleus by angular momentum (quantized values) ...
... Bohr's success in describing the line spectrum of hydrogen atom when he assumed that the electron moves in orbit (circular shape) around stationary nucleus by angular momentum (quantized values) ...
slides
... Let us denote by the Hilbert space of cylindrical functions defined on a graph which contains a number of loops such the one introduced by the action of the Euclidean part of .Which means that each loop is associated to a pair of links originating from the same node. ...
... Let us denote by the Hilbert space of cylindrical functions defined on a graph which contains a number of loops such the one introduced by the action of the Euclidean part of .Which means that each loop is associated to a pair of links originating from the same node. ...