Ionic Bonding
... electronic structures of noble gases like neon or argon which have eight electrons in their outer energy levels (or two in the case of helium). These noble gas structures are thought of as being in some way a "desirable" thing for an atom to have. You may well have been left with the strong impressi ...
... electronic structures of noble gases like neon or argon which have eight electrons in their outer energy levels (or two in the case of helium). These noble gas structures are thought of as being in some way a "desirable" thing for an atom to have. You may well have been left with the strong impressi ...
14-3 Temperature
... function of temperature for a constantbecause the atoms or molecules have no kinetic energy. This volume situation. Extrapolating the graph to is not quite true, although applying ideas of quantum zero pressure shows that absolute zero mechanics is necessary to understand why not. If the atoms corre ...
... function of temperature for a constantbecause the atoms or molecules have no kinetic energy. This volume situation. Extrapolating the graph to is not quite true, although applying ideas of quantum zero pressure shows that absolute zero mechanics is necessary to understand why not. If the atoms corre ...
Quantum fluctuations and the Casimir effect
... keep the ratio between old shot-noise and the amplified signal constant, and not much smaller than unity. In this way the new shot-noise, the one that appears due to the coupling with the signal, will be of the same order of the old shot-noise and the amplified signal and not much larger. ...
... keep the ratio between old shot-noise and the amplified signal constant, and not much smaller than unity. In this way the new shot-noise, the one that appears due to the coupling with the signal, will be of the same order of the old shot-noise and the amplified signal and not much larger. ...
topic 03 outline YT 2010 test
... 1. Explaining the existence of line spectra The Bohr model was based on a simple postulate, Bohr applied to the hydrogen atom the concept that the electron can exist only in certain energy levels without an energy change but that, when the electron changes its state, it must absorb or emit the exa ...
... 1. Explaining the existence of line spectra The Bohr model was based on a simple postulate, Bohr applied to the hydrogen atom the concept that the electron can exist only in certain energy levels without an energy change but that, when the electron changes its state, it must absorb or emit the exa ...
CHE 106 Chapter 6
... With a principal quantum number, 3s electrons will experience less screening/shielding than the 3d electrons. So the 3d electrons have less Zeff. In a many electron atom, for a given ‘n’.. Zeff ...
... With a principal quantum number, 3s electrons will experience less screening/shielding than the 3d electrons. So the 3d electrons have less Zeff. In a many electron atom, for a given ‘n’.. Zeff ...
Extreme Sensitivity in Photoacoustics by Using Optical Cantilever
... Following measurements were used to test the accuracy of the derived theoretical freguency responce of the cantilever, given by equation (6). The chambers were filled with gas (N2 , CO2 , CH4 or air) to several different pressures. The signal was produced either by changing the ...
... Following measurements were used to test the accuracy of the derived theoretical freguency responce of the cantilever, given by equation (6). The chambers were filled with gas (N2 , CO2 , CH4 or air) to several different pressures. The signal was produced either by changing the ...
Bourdel-3 (doc, 273 KiB)
... observation time is demanded in order to obtain the best accuracy. This time is limited not only by the expansion speed of the sample (i.e. its temperature, 100 nK corresponds to expansion speed of ~3 m/s for Rubidium 87), but also by the size of the vacuum chamber in which the measurement takes pla ...
... observation time is demanded in order to obtain the best accuracy. This time is limited not only by the expansion speed of the sample (i.e. its temperature, 100 nK corresponds to expansion speed of ~3 m/s for Rubidium 87), but also by the size of the vacuum chamber in which the measurement takes pla ...
document
... The cluster of atoms can be seen as a bright ball of light due to the reemission of photons. ...
... The cluster of atoms can be seen as a bright ball of light due to the reemission of photons. ...
Nature template - PC Word 97
... observation time is demanded in order to obtain the best accuracy. This time is limited not only by the expansion speed of the sample (i.e. its temperature, 100 nK corresponds to expansion speed of ~3 m/s for Rubidium 87), but also by the size of the vacuum chamber in which the measurement takes pla ...
... observation time is demanded in order to obtain the best accuracy. This time is limited not only by the expansion speed of the sample (i.e. its temperature, 100 nK corresponds to expansion speed of ~3 m/s for Rubidium 87), but also by the size of the vacuum chamber in which the measurement takes pla ...
P. LeClair
... work like this, there is no resolution! Why not protons, though, since they can be accelerated by potentials? Electrons, we found, are bound to their atomic nuclei with energies on the order of a few or a dozen electron volts - they are easy enough to remove from atoms for acceleration and focusing. ...
... work like this, there is no resolution! Why not protons, though, since they can be accelerated by potentials? Electrons, we found, are bound to their atomic nuclei with energies on the order of a few or a dozen electron volts - they are easy enough to remove from atoms for acceleration and focusing. ...
File
... 65. The volume of 400 mL of chlorine gas at 400mmHg is decreased to 200mL at constant temperature. What is the new gas pressure? A. 400 mmHg B. 300 mmHg C. 800 mmHg D. 650 mmHg 66. If a sealed bag of chips is left in a hot car, what happens to the volume of bag? A. volume increases B. volume decreas ...
... 65. The volume of 400 mL of chlorine gas at 400mmHg is decreased to 200mL at constant temperature. What is the new gas pressure? A. 400 mmHg B. 300 mmHg C. 800 mmHg D. 650 mmHg 66. If a sealed bag of chips is left in a hot car, what happens to the volume of bag? A. volume increases B. volume decreas ...
Interference with correlated photons: Five quantum mechanics
... which are based on the fact that E⫽ប ⫽បkc⫽hc/ for a photon, where is the angular frequency of the light and k is the magnitude of its wave number in vacuum. The wave number k is related to the wavelength in vacuum by k ⫽2 /⫽ /c, where c is the speed of light. High detection efficiency of ...
... which are based on the fact that E⫽ប ⫽បkc⫽hc/ for a photon, where is the angular frequency of the light and k is the magnitude of its wave number in vacuum. The wave number k is related to the wavelength in vacuum by k ⫽2 /⫽ /c, where c is the speed of light. High detection efficiency of ...
optical transitions and excitonic coupling in a covalently linked
... We present a quantum chemical study of the electronic excitation spectrum of a novel luminescent bichromophoric dye [1] which is made up of a diphenylbutadiene and an indolone unit which is carrying a dansyl moiety. Experimentally, a broad fluorescence band with a maximum at 511 nm has been observed ...
... We present a quantum chemical study of the electronic excitation spectrum of a novel luminescent bichromophoric dye [1] which is made up of a diphenylbutadiene and an indolone unit which is carrying a dansyl moiety. Experimentally, a broad fluorescence band with a maximum at 511 nm has been observed ...
Section 7: Free electron model
... The question that caused the greatest difficulty in the early development of the electron theory of metals concerns the heat capacity of the conduction electrons. Classical statistical mechanics predicts that a free particle should have a heat capacity of 3/2kB, where kB is the Boltzmann constant. I ...
... The question that caused the greatest difficulty in the early development of the electron theory of metals concerns the heat capacity of the conduction electrons. Classical statistical mechanics predicts that a free particle should have a heat capacity of 3/2kB, where kB is the Boltzmann constant. I ...
Charge Transfer in Collisions of Ions with atoms and - Indico
... required to treat the dynamics of electron capture processes in a collision. When the centre of mass (CM) energy is of the order of a few hundred eV/amu or greater, the de Broglie wave-length of the nuclear motion is much less than the Bohr radius . So while, a quantum description of the internal el ...
... required to treat the dynamics of electron capture processes in a collision. When the centre of mass (CM) energy is of the order of a few hundred eV/amu or greater, the de Broglie wave-length of the nuclear motion is much less than the Bohr radius . So while, a quantum description of the internal el ...
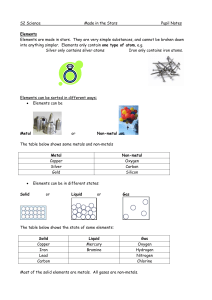
Made in the Stars Notes
... at room temperature except for mercury, which is a liquid. Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graph ...
... at room temperature except for mercury, which is a liquid. Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graph ...
ONE HUNDRED YEARS OF LIGHT QUANTA
... tion we have ever developed. That overwhelming and continuing success may eventually have led to a certain complacency. It seemed to imply that the field of optics, by the middle of the 20th century, scarcely needed to take any notice of the granular nature of light. Studying the behavior of light ...
... tion we have ever developed. That overwhelming and continuing success may eventually have led to a certain complacency. It seemed to imply that the field of optics, by the middle of the 20th century, scarcely needed to take any notice of the granular nature of light. Studying the behavior of light ...
Interaction of Photons with Matter - Faculty
... ii) The lowest energy state of neutral sodium, Na I, has an e− configuration of 1s2 2s2 2p6 3s. (NOTE: the exponents indicate the number of e−s in that subshell, no number ≡ 1.) Here, the K- and Lshells are completely filled — the 3s e− is called a valence e−. E. Photon-Matter Interactions. 1. We ha ...
... ii) The lowest energy state of neutral sodium, Na I, has an e− configuration of 1s2 2s2 2p6 3s. (NOTE: the exponents indicate the number of e−s in that subshell, no number ≡ 1.) Here, the K- and Lshells are completely filled — the 3s e− is called a valence e−. E. Photon-Matter Interactions. 1. We ha ...
Atomic Structure Practice Test
... (Note: h = 6.626 × 10-34 J ∙ s, e = 1.602 × 10-19 C, and m = 9.110 × 10-31 kg.) 30) An atom with atomic number 9 is in its ground state. How many electrons are in its outermost shell? ...
... (Note: h = 6.626 × 10-34 J ∙ s, e = 1.602 × 10-19 C, and m = 9.110 × 10-31 kg.) 30) An atom with atomic number 9 is in its ground state. How many electrons are in its outermost shell? ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.