PPT format
... r = a0(n2/Z) so that for the same value of n r a a0(1/Zeff) When electrons are added to the same shell (same value of n) they are about the same distance from the nucleus as the other electrons in the shell. The electrons in a shell with the same n are spread out and do not shield each other from th ...
... r = a0(n2/Z) so that for the same value of n r a a0(1/Zeff) When electrons are added to the same shell (same value of n) they are about the same distance from the nucleus as the other electrons in the shell. The electrons in a shell with the same n are spread out and do not shield each other from th ...
Suppression of optical damage at 532 nm in
... beam propagating through Sample 1 distorts along the Z axis and preferentially smears toward the –Z direction [4]. This type of unidirectional photorefractive distortion is consistent with a diffusion dominant mechanism such as the bulk photovoltaic effect [15]. Interestingly, the smearing of the be ...
... beam propagating through Sample 1 distorts along the Z axis and preferentially smears toward the –Z direction [4]. This type of unidirectional photorefractive distortion is consistent with a diffusion dominant mechanism such as the bulk photovoltaic effect [15]. Interestingly, the smearing of the be ...
Reducing multi-photon rates in pulsed down
... the process is spontaneous, there is a probability of emitting more than a single photon into the same spatio-temporal mode [21]. This effect is intensified when strong pump pulses are used to drive the down-conversion. These multi-photon, or higher-order, emissions have detrimental effects in appli ...
... the process is spontaneous, there is a probability of emitting more than a single photon into the same spatio-temporal mode [21]. This effect is intensified when strong pump pulses are used to drive the down-conversion. These multi-photon, or higher-order, emissions have detrimental effects in appli ...
Chapter 2 cont’
... do not turn into other elements ◦ Dalton’s Atomic Theory since the number of protons determines the kind of element, the number of protons in the atom does not change in a chemical reaction however, many reactions involve transferring electrons from one atom to another ...
... do not turn into other elements ◦ Dalton’s Atomic Theory since the number of protons determines the kind of element, the number of protons in the atom does not change in a chemical reaction however, many reactions involve transferring electrons from one atom to another ...
LEWIS DOT STRUCTURES , MOLECULAR SHAPES, AND
... How to Construct Lewis Dot Structures for Molecules: 1. Determine the type and number of atoms present in the molecule 2. Determine the number of valence electrons each atom will supply (you may wish to draw the electron dot diagram for that atom) If you are asked to do the structure of a polyatomic ...
... How to Construct Lewis Dot Structures for Molecules: 1. Determine the type and number of atoms present in the molecule 2. Determine the number of valence electrons each atom will supply (you may wish to draw the electron dot diagram for that atom) If you are asked to do the structure of a polyatomic ...
Document
... A) frequency x-rays; atomic weight B) frequency x-rays; atomic number C) frequency of -rays; atomic weight D) frequency of -rays; atomic number ...
... A) frequency x-rays; atomic weight B) frequency x-rays; atomic number C) frequency of -rays; atomic weight D) frequency of -rays; atomic number ...
Worksheet 4 - Periodic Trends A number of physical and chemical
... These properties all involve the outer shell (valence) electrons as well as the inner shell (shielding) electrons. Electrons are held in the atom by their electrostatic attraction to the positively charged protons, the nuclear charge, Z. However, not all electrons in an atom experience the same nucl ...
... These properties all involve the outer shell (valence) electrons as well as the inner shell (shielding) electrons. Electrons are held in the atom by their electrostatic attraction to the positively charged protons, the nuclear charge, Z. However, not all electrons in an atom experience the same nucl ...
Definitions - Loreto Science
... • that when building up the electronic configuration of an atom in its ground state, the electrons occupy the lowest available energy level. ...
... • that when building up the electronic configuration of an atom in its ground state, the electrons occupy the lowest available energy level. ...
AVERAGE ATOMIC MASS LAB
... “One unique property of Beanium should make these experiments particularly easy—unlike normal atoms, Beanium atoms are very large.” says Mr. Smithers. “They can be easily seen, and different isotopes can be sorted by hand.” Scientists are expecting a complete, comprehensive summary of this new eleme ...
... “One unique property of Beanium should make these experiments particularly easy—unlike normal atoms, Beanium atoms are very large.” says Mr. Smithers. “They can be easily seen, and different isotopes can be sorted by hand.” Scientists are expecting a complete, comprehensive summary of this new eleme ...
NLS Theory-Gariaev - NLS BIORRESONANCIA
... has resulted in their significant ageing, imposing an additional damage factor. Nevertheless, a "revitalization" effect is observed, and it demonstrates that DNA- radiowave radiation can carry in itself reparative genetic (metabolic) information that confirms our early work on wave biosign reparativ ...
... has resulted in their significant ageing, imposing an additional damage factor. Nevertheless, a "revitalization" effect is observed, and it demonstrates that DNA- radiowave radiation can carry in itself reparative genetic (metabolic) information that confirms our early work on wave biosign reparativ ...
Question 2
... 2. A mixture of H2(g), O2(g), and 2 milliliters of H2O(l) is present in 0.500-liter rigid container at 25 °C. The number of moles of H2 and the number of moles of O2 are equal. The total pressure is 1,146 millimeters of mercury. (The equilibrium vapor pressure of pure water is 24 millimeters mercury ...
... 2. A mixture of H2(g), O2(g), and 2 milliliters of H2O(l) is present in 0.500-liter rigid container at 25 °C. The number of moles of H2 and the number of moles of O2 are equal. The total pressure is 1,146 millimeters of mercury. (The equilibrium vapor pressure of pure water is 24 millimeters mercury ...
3 section 4.2
... metal- the light must be powerful enough. Electrons in the metal absorb the energy. The electrons become excited, and they jump out of the metal. ...
... metal- the light must be powerful enough. Electrons in the metal absorb the energy. The electrons become excited, and they jump out of the metal. ...
Document
... All matter is made of atoms. Atoms are the smallest form of elements. About 100 elements • Hydrogen is an element that accounts for about 90% of total mass of the universe. • Hydrogen makes up about 1% of Earth’s crust and most of that is in water. ...
... All matter is made of atoms. Atoms are the smallest form of elements. About 100 elements • Hydrogen is an element that accounts for about 90% of total mass of the universe. • Hydrogen makes up about 1% of Earth’s crust and most of that is in water. ...
Zero-Point Energy and Interstellar Travel
... some pretty high frequencies with this portion of the electromagnetic spectrum, weÕre talking about some really high energy intensities. In fact, Nobel Laureate Richard Feynman and one of EinsteinÕs protgs, John Wheeler, calculated that there is more than enough energy in a cup of coffee to evapor ...
... some pretty high frequencies with this portion of the electromagnetic spectrum, weÕre talking about some really high energy intensities. In fact, Nobel Laureate Richard Feynman and one of EinsteinÕs protgs, John Wheeler, calculated that there is more than enough energy in a cup of coffee to evapor ...
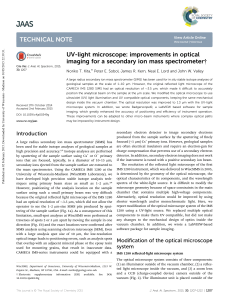
UV-light microscope: improvements in optical imaging for a
... same UV-light microscope system (Fig. S2, ESI-2†). Blue-LED illumination on the new UV-light microscope system resolved 2 mm lines, but not 1.5 mm lines (Fig. S2, ESI-2†), which indicates that the optical resolution is between 1.5 mm and 2 mm. These data indicate that the optical resolution is impro ...
... same UV-light microscope system (Fig. S2, ESI-2†). Blue-LED illumination on the new UV-light microscope system resolved 2 mm lines, but not 1.5 mm lines (Fig. S2, ESI-2†), which indicates that the optical resolution is between 1.5 mm and 2 mm. These data indicate that the optical resolution is impro ...
Document
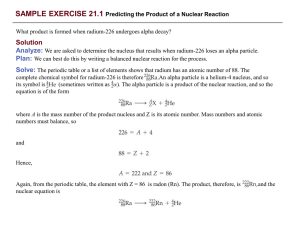
... masses of the nuclei in the reaction. We calculate these by taking account of the masses of the electrons that contribute to the atomic masses. atom has 27 electrons. The mass of an electron is 5.4858 10–4 amu. (See the list of fundamental constants in the back inside cover). We subtract the mass ...
... masses of the nuclei in the reaction. We calculate these by taking account of the masses of the electrons that contribute to the atomic masses. atom has 27 electrons. The mass of an electron is 5.4858 10–4 amu. (See the list of fundamental constants in the back inside cover). We subtract the mass ...
Luminescence model with quantum impact parameter for low energy ions H.S. Cruz-Galindo
... energy deposition profile qðrÞ, the M–M model considers two basic assumptions; first, that the regional density of electron–hole pairs (e–h) or excited molecular structures (defined in the M–M theory as energy carriers) created in the scintillator material is, in the absence of quenching effects, propor ...
... energy deposition profile qðrÞ, the M–M model considers two basic assumptions; first, that the regional density of electron–hole pairs (e–h) or excited molecular structures (defined in the M–M theory as energy carriers) created in the scintillator material is, in the absence of quenching effects, propor ...
Chapter 8 Notes - Bonding: General Concepts 8.1 Types of
... a. Attractive forces (proton - electron) b. Repulsive forces (electron - electron, proton - proton) 3. Energy is given off (bond energy) when two atoms achieve greater stability together than apart D. Covalent Bonds 1. Electrons are shared by nuclei 2. Pure covalent (non-polar covalent) a. Electrons ...
... a. Attractive forces (proton - electron) b. Repulsive forces (electron - electron, proton - proton) 3. Energy is given off (bond energy) when two atoms achieve greater stability together than apart D. Covalent Bonds 1. Electrons are shared by nuclei 2. Pure covalent (non-polar covalent) a. Electrons ...
Atomic Theory
... For d and f electron the shielding from underlying groups is 1.00 for each electron in the underlying group. For s and p electrons the shielding from the immediately underlying shell (n - 1) is 0.85 for each electron, and that from groups further in is 1.00 for each electron. ...
... For d and f electron the shielding from underlying groups is 1.00 for each electron in the underlying group. For s and p electrons the shielding from the immediately underlying shell (n - 1) is 0.85 for each electron, and that from groups further in is 1.00 for each electron. ...
Atomic Spectra - Rutgers Physics
... different n values, approximating the difference between initial and final state relativistic shifts by that for the final state (smaller n) only gives a result approximately in the range of fit error for the experimental vacuum Rydberg constant, the theoretical shift being usually toward shorter wa ...
... different n values, approximating the difference between initial and final state relativistic shifts by that for the final state (smaller n) only gives a result approximately in the range of fit error for the experimental vacuum Rydberg constant, the theoretical shift being usually toward shorter wa ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.