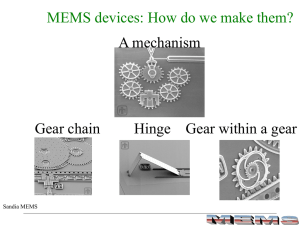
PDF only - at www.arxiv.org.
... Suppose the energy of the photon is E and the height of the barrier is V0 . By analogy with the above example, the photon must borrow at least the energy ΔE = V0 − E to traverse the barrier. Then another question arises: how far can the photon tunnel through the barrier each time? The tunneling dist ...
... Suppose the energy of the photon is E and the height of the barrier is V0 . By analogy with the above example, the photon must borrow at least the energy ΔE = V0 − E to traverse the barrier. Then another question arises: how far can the photon tunnel through the barrier each time? The tunneling dist ...
Hands-On Chemistry Unit
... Explain why arguments are invalid if based on very small samples of data, biased samples, or samples for which there was no control sample. Standard 3 - The Physical Setting Students collect and organize data to identify relationships between physical objects, events, and processes. They use logical ...
... Explain why arguments are invalid if based on very small samples of data, biased samples, or samples for which there was no control sample. Standard 3 - The Physical Setting Students collect and organize data to identify relationships between physical objects, events, and processes. They use logical ...
Atomic Masses: Counting Atoms by Weighing
... To determine the number of oxygen molecules required, we must know how many carbon atoms are present in the pile of carbon. But individual atoms are far too small to see. We must learn to count atoms by weighing samples containing large numbers of them. In the last section we saw that we can easily ...
... To determine the number of oxygen molecules required, we must know how many carbon atoms are present in the pile of carbon. But individual atoms are far too small to see. We must learn to count atoms by weighing samples containing large numbers of them. In the last section we saw that we can easily ...
High-speed spectral-domain optical coherence tomography at 1.3
... (TD-OCT) [1] have indicated that detection sensitivity of greater than 105 dB may be required to provide sufficient penetration depth for accurate diagnosis and quantitative evaluation of tissue properties [2]. Since clinically viable broadband sources are limited in power, high speed operation of T ...
... (TD-OCT) [1] have indicated that detection sensitivity of greater than 105 dB may be required to provide sufficient penetration depth for accurate diagnosis and quantitative evaluation of tissue properties [2]. Since clinically viable broadband sources are limited in power, high speed operation of T ...
CHEM 515 Spectroscopy Vibrational Spectroscopy I
... H35Cl and H37Cl molecules due to the slight difference in their reduced masses. ...
... H35Cl and H37Cl molecules due to the slight difference in their reduced masses. ...
8. Molecular Geometry
... Bonds are polar when one atom is positive and the other negative. Molecules with many atoms have polarity, with one end positive, the other negatively charged. You can predict the polarity of the molecule by looking at the ends of the molecule to see if it has a positive end and a negative end. Lone ...
... Bonds are polar when one atom is positive and the other negative. Molecules with many atoms have polarity, with one end positive, the other negatively charged. You can predict the polarity of the molecule by looking at the ends of the molecule to see if it has a positive end and a negative end. Lone ...
Name: Northwest Vista College Chem 1311
... A) transition metals B) halogens C) alkali metals D) alkaline earth metals E) noble gases 34. Which one of the following elements is a transition element? A) antimony ...
... A) transition metals B) halogens C) alkali metals D) alkaline earth metals E) noble gases 34. Which one of the following elements is a transition element? A) antimony ...
The Advanced Placement Examination in Chemistry Part I – Multiple
... (a) Explain why the student can correctly conclude that the hydrate was heated a sufficient number of times in the experiment. (b) Use the data above to (i) calculate the total number of moles of water lost when the sample was heated, and (ii) determine the formula of the hydrated compound. (c) A di ...
... (a) Explain why the student can correctly conclude that the hydrate was heated a sufficient number of times in the experiment. (b) Use the data above to (i) calculate the total number of moles of water lost when the sample was heated, and (ii) determine the formula of the hydrated compound. (c) A di ...
1 The modern model of the atom is based on the work of (1) one
... What information is necessary to determine the atomic mass of the element chlorine? (1) the atomic mass of each artificially produced isotope of chlorine, only (2) the relative abundance of each naturally occurring isotope of chlorine, only (3) the atomic mass and the relative abundance of each natu ...
... What information is necessary to determine the atomic mass of the element chlorine? (1) the atomic mass of each artificially produced isotope of chlorine, only (2) the relative abundance of each naturally occurring isotope of chlorine, only (3) the atomic mass and the relative abundance of each natu ...
... e0 is the permittivity of free space. At line centre, the refractive index is unity, and the second term in the denominator of equation (1) dominates the ®rst. An important characteristic of the refractive index pro®le is that on resonance the dispersion of the group velocity is zero (see ref. 16), ...
Signal-to-Signal-to-Noise-Ratio of Full-Field Fourier
... (DFT). This concept implementation has been recently demonstrated as spectral-domain OCT (SD-OCT) [4,5] and swept-source OCT (SS-OCT) [6]. It has been also demonstrated that the signal-to-noise ratio (SNR) of this new parallel data acquisition approach is improved as compared with that of TD-OCT, wh ...
... (DFT). This concept implementation has been recently demonstrated as spectral-domain OCT (SD-OCT) [4,5] and swept-source OCT (SS-OCT) [6]. It has been also demonstrated that the signal-to-noise ratio (SNR) of this new parallel data acquisition approach is improved as compared with that of TD-OCT, wh ...
Review Unit 5
... CHEMICALLY STABLE: Elements that are nonreactive because their last electron shell is completely filled with 8 electrons. (e.g. Neon, Argon, Krypton.) ISOTOPE: ...
... CHEMICALLY STABLE: Elements that are nonreactive because their last electron shell is completely filled with 8 electrons. (e.g. Neon, Argon, Krypton.) ISOTOPE: ...
Hybridization of atomic orbitals In general VSEPR predicts the
... Hybridization of atomic orbitals In general VSEPR predicts the shape of molecules and ions accurately CH4 : tetrahedral Four equal bonds with equal HCH angles A covalent bond is formed by sharing two electrons by two atoms Imagine an orbital (containing 1 electron) from one atom overlaps with an orb ...
... Hybridization of atomic orbitals In general VSEPR predicts the shape of molecules and ions accurately CH4 : tetrahedral Four equal bonds with equal HCH angles A covalent bond is formed by sharing two electrons by two atoms Imagine an orbital (containing 1 electron) from one atom overlaps with an orb ...
Hybridization of atomic orbitals In general VSEPR predicts the
... Hybridization of atomic orbitals In general VSEPR predicts the shape of molecules and ions accurately CH4 : tetrahedral Four equal bonds with equal HCH angles A covalent bond is formed by sharing two electrons by two atoms Imagine an orbital (containing 1 electron) from one atom overlaps with an orb ...
... Hybridization of atomic orbitals In general VSEPR predicts the shape of molecules and ions accurately CH4 : tetrahedral Four equal bonds with equal HCH angles A covalent bond is formed by sharing two electrons by two atoms Imagine an orbital (containing 1 electron) from one atom overlaps with an orb ...
Deep subwavelength nanolithography using localized surface plasmon modes on
... of the illumination light. According to Abbe’s theory, the resolution can be improved by using either shorter wavelengths or higher numerical apertures. Although the semiconductor industry has made significant progress in increasing the lithography resolution in the past decades, further improvement ...
... of the illumination light. According to Abbe’s theory, the resolution can be improved by using either shorter wavelengths or higher numerical apertures. Although the semiconductor industry has made significant progress in increasing the lithography resolution in the past decades, further improvement ...
Gaussian_calculations
... out of plane hydrogens in the plane of the molecule (select HCCC dihedral angle). This should look more or less right. Then go under edit and select point group. If you use the loose or very loose criterion, you will see the molecule is recognized as having D3h symmetry. Click the symmetrize button ...
... out of plane hydrogens in the plane of the molecule (select HCCC dihedral angle). This should look more or less right. Then go under edit and select point group. If you use the loose or very loose criterion, you will see the molecule is recognized as having D3h symmetry. Click the symmetrize button ...
Covalent Chemical Modification of Self
... has proven to be of significant interest. The fundamental types of radiation mentioned above produce modifications in a number of ways. Bonds at the surface may be broken such that molecular fragments are lost, unsaturated groups may be formed or destroyed, and cross-linking may occur as a result of ...
... has proven to be of significant interest. The fundamental types of radiation mentioned above produce modifications in a number of ways. Bonds at the surface may be broken such that molecular fragments are lost, unsaturated groups may be formed or destroyed, and cross-linking may occur as a result of ...
Chiral specific electron vortex beam spectroscopy
... We can now address the physical meaning of the double summation over p and p0 in Eq. (13) and the implications of this for the chiral-specific spectroscopy of atoms located away from the vortex beam axis. The off-axis case is illustrated in Fig. (1)(b). It can be seen that the features uncovered abo ...
... We can now address the physical meaning of the double summation over p and p0 in Eq. (13) and the implications of this for the chiral-specific spectroscopy of atoms located away from the vortex beam axis. The off-axis case is illustrated in Fig. (1)(b). It can be seen that the features uncovered abo ...
Plasma Orbital Expansion of the Electrons in Water
... or conduction band, has some spots with no electrons in it, otherwise known as unoccupied. The HOMO is full of electrons; it cannot push them along because it is full. Therefore, in order for the material to conduct, the material needs to excite electrons from the HOMO to the LUMO so they can move t ...
... or conduction band, has some spots with no electrons in it, otherwise known as unoccupied. The HOMO is full of electrons; it cannot push them along because it is full. Therefore, in order for the material to conduct, the material needs to excite electrons from the HOMO to the LUMO so they can move t ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.