Clustering and unsupervised learning
... find an assignment of each object to one of the clusters that minimizes “within cluster” point scatter cost function The number of possible assignments scales exponentially (again ) and in fact clustering problem is another instance of global optimization problem K-means is an iterative greedy desce ...
... find an assignment of each object to one of the clusters that minimizes “within cluster” point scatter cost function The number of possible assignments scales exponentially (again ) and in fact clustering problem is another instance of global optimization problem K-means is an iterative greedy desce ...
Effects of Macromolecular Crowding on Protein Folding
... kDa). A theoretical crowding model was used to investigate the origins of this stabilization. In this model, Dextran and Ficoll were modeled as elongated rods and the protein was represented as a sphere, with the folded sphere representation being smaller than the unfolded sphere representation. Not ...
... kDa). A theoretical crowding model was used to investigate the origins of this stabilization. In this model, Dextran and Ficoll were modeled as elongated rods and the protein was represented as a sphere, with the folded sphere representation being smaller than the unfolded sphere representation. Not ...
Youngs, Noah: Progress in the Side-Chain Prediction Problem
... clear. Drug-designers would be able create specific structures and target specific protein interactions on an as-yet unrealized scale. In addition, several currently incurable diseases are believed to be caused by protein misfolding, including cystic fibrosis, Alzheimer’s, and Parkinson’s [Cohen and ...
... clear. Drug-designers would be able create specific structures and target specific protein interactions on an as-yet unrealized scale. In addition, several currently incurable diseases are believed to be caused by protein misfolding, including cystic fibrosis, Alzheimer’s, and Parkinson’s [Cohen and ...
Tubulin folding is altered by mutations in a putative GTP binding motif
... stacked rings of 8 non-identical subunits (Lewis et al., 1992; Kubota et al., 1994, 1995). Recently, TCP1 particles have been implicated in actin and tubulin folding (Frydman et al., 1992; Gao et al., 1992; Melki et al., 1993; Rommelaere et al., 1993; Sternlicht et al., 1993; Yaffe et al., 1992). Ad ...
... stacked rings of 8 non-identical subunits (Lewis et al., 1992; Kubota et al., 1994, 1995). Recently, TCP1 particles have been implicated in actin and tubulin folding (Frydman et al., 1992; Gao et al., 1992; Melki et al., 1993; Rommelaere et al., 1993; Sternlicht et al., 1993; Yaffe et al., 1992). Ad ...
An Improved Ant Colony Optimisation Algorithm for the 2D HP
... predict protein structure from sequence information would greatly simplify the tasks of interpreting the sequence data collected, of designing drugs with specific therapeutic properties, and of developing biological polymers with specific material properties. Currently, protein structures are primar ...
... predict protein structure from sequence information would greatly simplify the tasks of interpreting the sequence data collected, of designing drugs with specific therapeutic properties, and of developing biological polymers with specific material properties. Currently, protein structures are primar ...
Early events in protein folding
... polypeptide chain searches out its final native conformation from an inconceivably large number of available conformations. A polypeptide chain of 101 amino acid residues would have to sample 3100 = 5 × 1047 conformations, if each bond connecting two consecutive residues has only three possible conf ...
... polypeptide chain searches out its final native conformation from an inconceivably large number of available conformations. A polypeptide chain of 101 amino acid residues would have to sample 3100 = 5 × 1047 conformations, if each bond connecting two consecutive residues has only three possible conf ...
The Generic Nature of Protein Folding and Misfolding
... (Dobson and Karplus, 1999; Fersht and Daggett, 2002). The ultimate objective is to describe complete energy landscapes for folding reactions, and to understand exactly how these are defined by the sequences involved. Recently, experimental data have been incorporated directly in computer simulations ...
... (Dobson and Karplus, 1999; Fersht and Daggett, 2002). The ultimate objective is to describe complete energy landscapes for folding reactions, and to understand exactly how these are defined by the sequences involved. Recently, experimental data have been incorporated directly in computer simulations ...
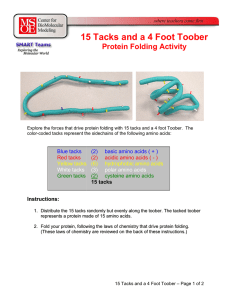
15 Tacks and a 4 Foot Toober
... After everyone has folded their toober as best they can, the teacher can point out: • Every toober had a different random sequence of tacks (amino acids) and therefore each toober (protein) folded into a different structure. • Some sequences of tacks were more easily folded into a reasonable structu ...
... After everyone has folded their toober as best they can, the teacher can point out: • Every toober had a different random sequence of tacks (amino acids) and therefore each toober (protein) folded into a different structure. • Some sequences of tacks were more easily folded into a reasonable structu ...
Protein Folding at the Exit Tunnel
... options are not entirely clear yet, and represent an outstanding challenge in in vitro protein folding. On the other hand, there are two apparent emerging trends. (1) Collapse is slower when it occurs concomitantly with secondary structure formation, pointing to the kinetic difficulties in assemblin ...
... options are not entirely clear yet, and represent an outstanding challenge in in vitro protein folding. On the other hand, there are two apparent emerging trends. (1) Collapse is slower when it occurs concomitantly with secondary structure formation, pointing to the kinetic difficulties in assemblin ...
Contribution of molecular chaperones to protein folding in the
... usually not native, proteins not only protects unfolded protein conformers from aggregation, but most importantly, through cycles of timed release and rebinding, also allows productive interactions to occur which are required for correct folding. In the event that such productive interactions do not ...
... usually not native, proteins not only protects unfolded protein conformers from aggregation, but most importantly, through cycles of timed release and rebinding, also allows productive interactions to occur which are required for correct folding. In the event that such productive interactions do not ...
ET4718 - Computer Programming 7
... Overfitting and overgeneralization in Classification Algorithms have resulted in classification and prediction systems that are highly accurate or they are not so accurate for no apparent reason. A growing belief is that the root to that problem is the overfitting and overgeneralization behavior of ...
... Overfitting and overgeneralization in Classification Algorithms have resulted in classification and prediction systems that are highly accurate or they are not so accurate for no apparent reason. A growing belief is that the root to that problem is the overfitting and overgeneralization behavior of ...
Amino Acids
... e.g. alcohol groups of Ser and Thr, can form H-bonds with electron rich atoms e.g., oxygen of a carboxyl group or carbonyl group of a peptide bond – Formation of H-bonds b/w polar groups on protein surface and aqueous solvent enhances solubility of protein ...
... e.g. alcohol groups of Ser and Thr, can form H-bonds with electron rich atoms e.g., oxygen of a carboxyl group or carbonyl group of a peptide bond – Formation of H-bonds b/w polar groups on protein surface and aqueous solvent enhances solubility of protein ...
NMR spectroscopy brings invisible protein states into
... rotations about unhindered dihedral angles16, to substantial chain displacements17. In some favorable cases such picosecond to nanosecond dynamics can be inferred from temperature factors derived from crystal structures18 or often more rigorously from NMR spin relaxation properties19–22. In addition ...
... rotations about unhindered dihedral angles16, to substantial chain displacements17. In some favorable cases such picosecond to nanosecond dynamics can be inferred from temperature factors derived from crystal structures18 or often more rigorously from NMR spin relaxation properties19–22. In addition ...
Cortegra Purchases New Vijuk Large Format Folder for
... A subsidiary of Menasha Corporation, Cortegra, headquartered in Fairfield, New Jersey, brings single-source excellence to healthcare packaging materials. With more than six decades of pharmaceutical printing experience, the company has become one of the recognized leaders in providing packaging and ...
... A subsidiary of Menasha Corporation, Cortegra, headquartered in Fairfield, New Jersey, brings single-source excellence to healthcare packaging materials. With more than six decades of pharmaceutical printing experience, the company has become one of the recognized leaders in providing packaging and ...
Repeat proteins challenge the concept of structural domains
... defining structural domains [1]. These domains typically correlate with biological activities and many modern proteins can be described as composed by novel ‘domain arrangements’ [2]. For globular proteins, this fact facilitates the description, evolution and construction of single amino acid chains ...
... defining structural domains [1]. These domains typically correlate with biological activities and many modern proteins can be described as composed by novel ‘domain arrangements’ [2]. For globular proteins, this fact facilitates the description, evolution and construction of single amino acid chains ...
Mechanisms of Translocation of Legionella pneumophila Effectors
... IcmS for translocation within the effectors studied and have some evidence that this phenotype is unrelated to direct binding of IcmS to the effector protein. Of particular interest, it was found that the translocation defect in a DHFR-effector fusion is exacerbated in an icmS deletion mutant. These ...
... IcmS for translocation within the effectors studied and have some evidence that this phenotype is unrelated to direct binding of IcmS to the effector protein. Of particular interest, it was found that the translocation defect in a DHFR-effector fusion is exacerbated in an icmS deletion mutant. These ...
Protein Folding
... like hemoglobin subunit you have to break some bonds with the environment. i. Bonds must be broken from polypeptide chain and all bonds with water must be broken. ii. New bonds must form as protein folds in on itself. c) When a protein folds on itself (globular protein) there isn’t a lot of space, t ...
... like hemoglobin subunit you have to break some bonds with the environment. i. Bonds must be broken from polypeptide chain and all bonds with water must be broken. ii. New bonds must form as protein folds in on itself. c) When a protein folds on itself (globular protein) there isn’t a lot of space, t ...
7.12. PROTEIN FOLDING AND MISFOLDING43
... folding indicative of the absence of any significant energy barriers on the path between unfolded and native states. At suboptimal temperatures or in structurally more complex proteins, the “correct” nucleation may present an energy barrier due to the presence of multiple similar energy states displ ...
... folding indicative of the absence of any significant energy barriers on the path between unfolded and native states. At suboptimal temperatures or in structurally more complex proteins, the “correct” nucleation may present an energy barrier due to the presence of multiple similar energy states displ ...
Molecular Chaperones in the Cytosol: from Nascent Chain to Folded
... phobic forces and interchain hydrogen bonding (1, 6). This aggregation process irreversibly removes proteins from their productive folding pathways, and must be prevented in vivo by molecular chaperones. A certain level of protein aggregation does occur in cells despite the presence of an exclusive ...
... phobic forces and interchain hydrogen bonding (1, 6). This aggregation process irreversibly removes proteins from their productive folding pathways, and must be prevented in vivo by molecular chaperones. A certain level of protein aggregation does occur in cells despite the presence of an exclusive ...
Protein folding
... aggregation, and early degradation and thus to a loss of functional PAH proteins • BH4 – molecular chaperone – the restoration of enzyme function which might be transmitted by correction of protein misfolding. • About 75% of PAH mutations, characterized by high residual activity, have been found to ...
... aggregation, and early degradation and thus to a loss of functional PAH proteins • BH4 – molecular chaperone – the restoration of enzyme function which might be transmitted by correction of protein misfolding. • About 75% of PAH mutations, characterized by high residual activity, have been found to ...
Indian Journal of Chemistry
... control. However, we show that a large ribozyme can fold all the way to the biological active state in 0.1 second (orders of magnitude faster than previously observed) without falling into kinetic traps. We ...
... control. However, we show that a large ribozyme can fold all the way to the biological active state in 0.1 second (orders of magnitude faster than previously observed) without falling into kinetic traps. We ...
Chaperone-assisted protein folding: the path to discovery from a
... major conformational changes in the interacting GroEL subunits22 (Fig. 2e). The idea that the folding reaction might take place in the central cavity was soon reinforced. In 1993, we showed that the GroEL-GroES complex is asymmetrical and highly dynamic, with GroES binding and unbinding in a mechani ...
... major conformational changes in the interacting GroEL subunits22 (Fig. 2e). The idea that the folding reaction might take place in the central cavity was soon reinforced. In 1993, we showed that the GroEL-GroES complex is asymmetrical and highly dynamic, with GroES binding and unbinding in a mechani ...
Part 1
... Entropy helps in predicting the spontaneity of any process. An unfolded polypeptide chain has high entropy which goes on decreasing as the protein folds into its native state. 2. Free energy: The free energy, also known as Gibbs free energy, is the maximum amount of mechanical work that can be done ...
... Entropy helps in predicting the spontaneity of any process. An unfolded polypeptide chain has high entropy which goes on decreasing as the protein folds into its native state. 2. Free energy: The free energy, also known as Gibbs free energy, is the maximum amount of mechanical work that can be done ...
Folding@home

Folding@home (FAH or F@h) is a distributed computing project for disease research that simulates protein folding, computational drug design, and other types of molecular dynamics. The project uses the idle processing resources of thousands of personal computers owned by volunteers who have installed the software on their systems. Its primary purpose is to determine the mechanisms of protein folding, which is the process by which proteins reach their final three-dimensional structure, and to examine the causes of protein misfolding. This is of significant academic interest with major implications for medical research into Alzheimer's disease, Huntington's disease, and many forms of cancer, among other diseases. To a lesser extent, Folding@home also tries to predict a protein's final structure and determine how other molecules may interact with it, which has applications in drug design. Folding@home is developed and operated by the Pande Laboratory at Stanford University, under the direction of Prof. Vijay Pande, and is shared by various scientific institutions and research laboratories across the world.The project has pioneered the use of GPUs, PlayStation 3s, Message Passing Interface (used for computing on multi-core processors), as well as some Sony Xperia smartphones for distributed computing and scientific research. The project uses statistical simulation methodology that is a paradigm shift from traditional computational approaches. As part of the client-server network architecture, the volunteered machines each receive pieces of a simulation (work units), complete them, and return them to the project's database servers where the units are compiled into an overall simulation. Volunteers can track their contributions on the Folding@home website, which makes volunteers' participation competitive and encourages long-term involvement.Folding@home is one of the world's fastest computing systems, with a speed of approximately 40 petaFLOPS: greater than all projects running on the BOINC distributed computing platform combined. This performance from its large-scale computing network has allowed researchers to run computationally expensive atomic-level simulations of protein folding thousands of times longer than previously achieved. Since its launch on October 1, 2000, the Pande Lab has produced 118 scientific research papers as a direct result of Folding@home. Results from the project's simulations agree favorably with experiments.