08_lecture_ppt - Chemistry at Winthrop University
... • Bohr theory only modeled the line spectrum of H • Further experiments established waveparticle duality of light and matter – Young’s two slit experiment produced interference patterns for both photons and electrons. ...
... • Bohr theory only modeled the line spectrum of H • Further experiments established waveparticle duality of light and matter – Young’s two slit experiment produced interference patterns for both photons and electrons. ...
x - Purdue Physics
... A careful analysis of the process of observation in atomic physics has shown that the subatomic particles have no meaning as isolated entities, but can only be understood as interconnections between the preparation of an experiment and the subsequent measurement. - Erwin Schrödinger ...
... A careful analysis of the process of observation in atomic physics has shown that the subatomic particles have no meaning as isolated entities, but can only be understood as interconnections between the preparation of an experiment and the subsequent measurement. - Erwin Schrödinger ...
Physics 200 Class #1 Outline
... Problem: The peak of the blackbody curve is measured to be at 1000 nm for a temperature of 2900 K. Find the temperature of the surface of the sun if the peak of the solar spectrum is at 500 nm. And now, the beginning of quantum mechanics: All attempts to predict the blackbody curve using classical p ...
... Problem: The peak of the blackbody curve is measured to be at 1000 nm for a temperature of 2900 K. Find the temperature of the surface of the sun if the peak of the solar spectrum is at 500 nm. And now, the beginning of quantum mechanics: All attempts to predict the blackbody curve using classical p ...
Introduction to PHY 855 “Introduction to field theory as it
... “Theory of relativistic quantum fields and renormalization with emphasis on applications for particle physics. Offered second half of semester.” ...
... “Theory of relativistic quantum fields and renormalization with emphasis on applications for particle physics. Offered second half of semester.” ...
6 I – Rocket Science
... But it wasn’t until the end of the 19th century, when English scientist James Clerk Maxwell found out that the two belong together. They are just two different phenomena of the same physical interaction, which we nowadays call “the electromagnetic field”. The electric and magnetic force belong toget ...
... But it wasn’t until the end of the 19th century, when English scientist James Clerk Maxwell found out that the two belong together. They are just two different phenomena of the same physical interaction, which we nowadays call “the electromagnetic field”. The electric and magnetic force belong toget ...
R - University of St Andrews
... Thus, energy levels turn out to be dependent on two quantum numbers, but only when one takes relativistic considerations into account. Without relativity, we get the same formula for E as before. Relativistic correction: electrons in very eccentric orbits have large velocities when they are near the ...
... Thus, energy levels turn out to be dependent on two quantum numbers, but only when one takes relativistic considerations into account. Without relativity, we get the same formula for E as before. Relativistic correction: electrons in very eccentric orbits have large velocities when they are near the ...
photoelectric effect
... When metal surfaces are exposed to electromagnetic radiation with sufficient energy they absorb the photons of energy and emit electrons. This process is called the photoelectric effect. ...
... When metal surfaces are exposed to electromagnetic radiation with sufficient energy they absorb the photons of energy and emit electrons. This process is called the photoelectric effect. ...
Discussion Question 13B
... (d) Write down an expression for the magnetic field B(x,y,z,t). Express your answer algebraically (i.e. no numbers) in terms of the symbols k, ω, and B0 (the latter being the magnetic field amplitude). Be sure to indicate the direction of the magnetic field. The E and B fields of an electromagnetic ...
... (d) Write down an expression for the magnetic field B(x,y,z,t). Express your answer algebraically (i.e. no numbers) in terms of the symbols k, ω, and B0 (the latter being the magnetic field amplitude). Be sure to indicate the direction of the magnetic field. The E and B fields of an electromagnetic ...
Broglie and Schrodinger Atomic Model
... Educational, Scientific and Cultural Organization). To help understand Broglie’s theories and ideas better this is Broglie’s theory of quantum physics. “Thus I arrived at the following general idea which has guided my researches: for matter, just as much as for radiation, in particular light, we mus ...
... Educational, Scientific and Cultural Organization). To help understand Broglie’s theories and ideas better this is Broglie’s theory of quantum physics. “Thus I arrived at the following general idea which has guided my researches: for matter, just as much as for radiation, in particular light, we mus ...
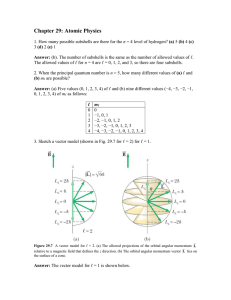
Chapter 29: Atomic Physics
... change and (ii), the minimum wavelength of the bremsstrahlung (a) increases, (b) decreases, or (c) does not change. Answer: (i), (c). The wavelengths of the characteristic x-rays are determined by the separation between energy levels in the atoms of the target, which is unrelated to the energy with ...
... change and (ii), the minimum wavelength of the bremsstrahlung (a) increases, (b) decreases, or (c) does not change. Answer: (i), (c). The wavelengths of the characteristic x-rays are determined by the separation between energy levels in the atoms of the target, which is unrelated to the energy with ...
Course Poster
... Instructor: Prof. Dio Margetis ([email protected], x 5-5455) FOCUS: Mathematical concepts and analytical tools used in classical mechanics as well as quantum mechanics and quantum field theories. Applications from: fluid mechanics, elasticity, electromagnetism, atomic and particle physics. ...
... Instructor: Prof. Dio Margetis ([email protected], x 5-5455) FOCUS: Mathematical concepts and analytical tools used in classical mechanics as well as quantum mechanics and quantum field theories. Applications from: fluid mechanics, elasticity, electromagnetism, atomic and particle physics. ...
Simple Harmonic Oscillator
... The planetary atom will emit light of a particular frequency. An electron in an orbit will emit a light wave at the orbital frequency. ...
... The planetary atom will emit light of a particular frequency. An electron in an orbit will emit a light wave at the orbital frequency. ...
Electron Configuration Class Notes
... 1. Main, or Principal, quantum number (n) values from 1 ------> infinity describes the average distance of the electron from the nucleus 2. angular momentum, orbital, or azimuthal, quantum number (l) values from 0 to n - 1 if n = 3, then l can equal 0, 1, or 2 describes the actual electron path (sha ...
... 1. Main, or Principal, quantum number (n) values from 1 ------> infinity describes the average distance of the electron from the nucleus 2. angular momentum, orbital, or azimuthal, quantum number (l) values from 0 to n - 1 if n = 3, then l can equal 0, 1, or 2 describes the actual electron path (sha ...
Example solution to the exercise 1
... Hint: The acceleration is caused by the electrostatic interaction between the electron and the nucleus. Write the total energy as the sum of kinetic and potential energy: ...
... Hint: The acceleration is caused by the electrostatic interaction between the electron and the nucleus. Write the total energy as the sum of kinetic and potential energy: ...
Handout - UNT Chemistry
... perfectly for H (as well as He+, Li2+, etc.). And it’s so much EASIER than the Schrödinger Equation. The only problem with the Bohr Theory is that it fails as soon as you try to use it on an atom as “complex” as helium. ...
... perfectly for H (as well as He+, Li2+, etc.). And it’s so much EASIER than the Schrödinger Equation. The only problem with the Bohr Theory is that it fails as soon as you try to use it on an atom as “complex” as helium. ...
Many Worlds Theory/ `Relative State` formation of Quantum Mechanics
... How can he determine that? • The original idea is that small particles travel as probability waves ~ a particle gives up its position when it has momentum, we can figure out its position when it has zero momentum. - position and momentum cannot be known at the same time • Then comes along Hugh Evere ...
... How can he determine that? • The original idea is that small particles travel as probability waves ~ a particle gives up its position when it has momentum, we can figure out its position when it has zero momentum. - position and momentum cannot be known at the same time • Then comes along Hugh Evere ...
Wave packets Uncertainty - cranson
... the Uncertainty Principle. The interaction time is known to a high degree of precision. The same variation is vital in the field of QUANTUM ELECTRODYNAMICS, where an apparent violation of energy conservation can be rationalized by an interaction time within the uncertainty limits imposed. This leads ...
... the Uncertainty Principle. The interaction time is known to a high degree of precision. The same variation is vital in the field of QUANTUM ELECTRODYNAMICS, where an apparent violation of energy conservation can be rationalized by an interaction time within the uncertainty limits imposed. This leads ...
Periodic boundary physics etc
... September 2009 by K.A. Olive (University of Minnesota) and J.A. Peacock (University of Edinburgh). ...
... September 2009 by K.A. Olive (University of Minnesota) and J.A. Peacock (University of Edinburgh). ...