Shiny, Happy Pretest - Alex LeMay – Science
... 12. Put together the Atomic Theory, established the Law of Multiple Proportions, and explained the difference between a mixture and a compound. ______________________ 13. Worked in Rutherford’s lab on the gold foil experiment, an undergraduate student who worked with Geiger._________________________ ...
... 12. Put together the Atomic Theory, established the Law of Multiple Proportions, and explained the difference between a mixture and a compound. ______________________ 13. Worked in Rutherford’s lab on the gold foil experiment, an undergraduate student who worked with Geiger._________________________ ...
4.1 The Development of a New Atomic Model • Properties of Light o
... o He was able to predict the wavelength of a given particle with m = mass and v = velocity. o Scientists were able to show how an electron stream acted in the same way as a ray of light. o One cannot observe both the particle and wave properties of an electron in the same experiment. • The Heisenber ...
... o He was able to predict the wavelength of a given particle with m = mass and v = velocity. o Scientists were able to show how an electron stream acted in the same way as a ray of light. o One cannot observe both the particle and wave properties of an electron in the same experiment. • The Heisenber ...
6. Quantum Mechanics II
... In any measurement of the observable associated with an operator A, ˆ the only values that can ever be observed are the eigenvalues. Eigenvalues are the possible values of a in the Eigenvalue Equation: ...
... In any measurement of the observable associated with an operator A, ˆ the only values that can ever be observed are the eigenvalues. Eigenvalues are the possible values of a in the Eigenvalue Equation: ...
Lecture 16 - Eunil Won
... In 1905, Einstein proposed: electromagnetic radiation is quantized and exists in elementary amounts (quanta) called photons The quantum of a light wave of frequency f has energy: E = hf (energy of single photon) ...
... In 1905, Einstein proposed: electromagnetic radiation is quantized and exists in elementary amounts (quanta) called photons The quantum of a light wave of frequency f has energy: E = hf (energy of single photon) ...
Lec-23_Strachan
... As a general rule, the order that electrons fill an atom’s subshell is: Once one subshell is filled, the next electron goes into the vacant subshell that is lowest in energy Otherwise, the electron would radiate energy until it reached the subshell with the lowest energy A subshell is filled when it ...
... As a general rule, the order that electrons fill an atom’s subshell is: Once one subshell is filled, the next electron goes into the vacant subshell that is lowest in energy Otherwise, the electron would radiate energy until it reached the subshell with the lowest energy A subshell is filled when it ...
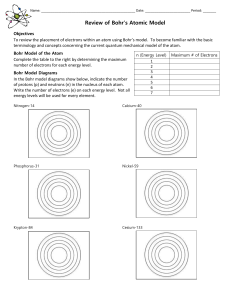
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... Review of Bohr’s Atomic Model Objectives ...
... Review of Bohr’s Atomic Model Objectives ...
Cosmic Medium and Leo Sapogin`s Unitary Quantum Theory
... figurativeness and common sense excluded from physics by the Bohr’s antiquated complementarity principle. Significant advances in quantum mechanics (especially in stationary conditions) started from a prime relation between De Broglie wavelength and geometric properties of potentials. The particle w ...
... figurativeness and common sense excluded from physics by the Bohr’s antiquated complementarity principle. Significant advances in quantum mechanics (especially in stationary conditions) started from a prime relation between De Broglie wavelength and geometric properties of potentials. The particle w ...
EMR and the Bohr Model of the Atom
... • stated that EMR could be viewed as a stream of particles “photons” • photon- a quantum of light • energy of these photons could be calculated by Planck’s equation • stated that the photons strike the electrons therefore ejecting them from the metal ...
... • stated that EMR could be viewed as a stream of particles “photons” • photon- a quantum of light • energy of these photons could be calculated by Planck’s equation • stated that the photons strike the electrons therefore ejecting them from the metal ...
Chapter 5 Homework
... (a) The atoms of very few elements contain neutrons in their nuclei. (b) Its existence was not suspected until Rutherford's gold foil experiment. (c) It was difficult to detect because it has no charge. (d) Because its mass is similar to a proton's mass, both are affected similarly by an electric fi ...
... (a) The atoms of very few elements contain neutrons in their nuclei. (b) Its existence was not suspected until Rutherford's gold foil experiment. (c) It was difficult to detect because it has no charge. (d) Because its mass is similar to a proton's mass, both are affected similarly by an electric fi ...
Do your homework on a separate piece of paper, or
... 20. What is meant by the term “wave-particle duality” and to what is it applied? Matter and energy can both act like waves and act like particles. 21. What is a matter wave? It is the wave part of the duality manifest in matter. = h/p. 22. State the de Broglie hypothesis, and then write its mathem ...
... 20. What is meant by the term “wave-particle duality” and to what is it applied? Matter and energy can both act like waves and act like particles. 21. What is a matter wave? It is the wave part of the duality manifest in matter. = h/p. 22. State the de Broglie hypothesis, and then write its mathem ...
Light PPT - Paso Robles High School
... In 1900 Max Planck came up with a revolutionary way to resolve the problem by assuming that energy came in discrete amounts (quanta). This was the beginning of quantum mechanics. Each quantum of light is called a photon, and its energy is given by E = h f, where f is the frequency of the radiation a ...
... In 1900 Max Planck came up with a revolutionary way to resolve the problem by assuming that energy came in discrete amounts (quanta). This was the beginning of quantum mechanics. Each quantum of light is called a photon, and its energy is given by E = h f, where f is the frequency of the radiation a ...
Light and Photons - Continuum Center
... Evan Harris Walker (the Physics of Consciousness): “When we carry out a complete measurement loop the math will force the whole thing to have nice, steady real solutions only if one of the states happens and all the other states vanish. When the loop closes state vector collapse is forced to happen. ...
... Evan Harris Walker (the Physics of Consciousness): “When we carry out a complete measurement loop the math will force the whole thing to have nice, steady real solutions only if one of the states happens and all the other states vanish. When the loop closes state vector collapse is forced to happen. ...
atomsagain
... Many-electron atoms •The nuclear charge is large •Electron charges are relatively small •Maybe treat each electron as orbiting in a Hydrogen-like atom •Goal: Figure out which electrons will be there, list which states they are in ...
... Many-electron atoms •The nuclear charge is large •Electron charges are relatively small •Maybe treat each electron as orbiting in a Hydrogen-like atom •Goal: Figure out which electrons will be there, list which states they are in ...
Chapter 5
... His son, George Thomson won the Nobel prize for describing the wave-like nature of the electron. The electron is a particle! ...
... His son, George Thomson won the Nobel prize for describing the wave-like nature of the electron. The electron is a particle! ...
tutorial 12 - UBC Physics
... equation". lt determines the specific shape that the wavefunction must have at t=0 so that it will just oscillate with frequency E/h as a function of time (and therefore have a definite energy E). tt is simpler than the full Schrodinger equation since it only depends on space and not time. Linear eq ...
... equation". lt determines the specific shape that the wavefunction must have at t=0 so that it will just oscillate with frequency E/h as a function of time (and therefore have a definite energy E). tt is simpler than the full Schrodinger equation since it only depends on space and not time. Linear eq ...
... 11) Two loud speakers are working in phase, producing waves with a wavelength of 1.44m. A listener is standing half way between the two speakers. In order to get to a destructive interference point, he needs to move toward one of the speakers by a distance of 0.18m 0.36m 0.72m 1.44m 2.88m 12) In ord ...
... 11) Two loud speakers are working in phase, producing waves with a wavelength of 1.44m. A listener is standing half way between the two speakers. In order to get to a destructive interference point, he needs to move toward one of the speakers by a distance of 0.18m 0.36m 0.72m 1.44m 2.88m 12) In ord ...
1 What Is the Measurement Problem Anyway?
... • Wave–Particle Duality. Subject any particle to an experiment set to measure waves and it will manifest unmistakably undulatory properties. Perform on it an experiment designed to measure corpuscular properties and you will end up with a particle. Both results are equivocal – and mutually exclusive ...
... • Wave–Particle Duality. Subject any particle to an experiment set to measure waves and it will manifest unmistakably undulatory properties. Perform on it an experiment designed to measure corpuscular properties and you will end up with a particle. Both results are equivocal – and mutually exclusive ...
Chapter 3 Electromagnetic Theory, Photons, and Light
... Maxwell in ~1865 found that EM wave must move at speed v ...
... Maxwell in ~1865 found that EM wave must move at speed v ...