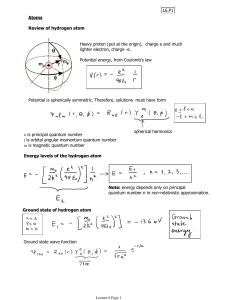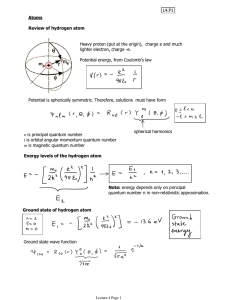
ATOMIC PHYSICS
... 7. Explain the relation between the energy of a quantum of electromagnetic radiation absorbed/released and the energy levels, relate it to the hydrogen model ...
... 7. Explain the relation between the energy of a quantum of electromagnetic radiation absorbed/released and the energy levels, relate it to the hydrogen model ...
CH160: Professor Peter Sadler Introduction to inorganic chemistry
... 1927 Heisenberg's uncertainty principle : product of uncertainty in position and uncertainty in momentum (mass x velocity) of a particle can be no smaller than Planck's constant divided by 4 (x)((p) > h/4 The more precisely you know the position, the less precisely you know the momentum Electron ...
... 1927 Heisenberg's uncertainty principle : product of uncertainty in position and uncertainty in momentum (mass x velocity) of a particle can be no smaller than Planck's constant divided by 4 (x)((p) > h/4 The more precisely you know the position, the less precisely you know the momentum Electron ...
VIII. Other Types of Notations or Configurations
... • Predicted how the spectrum of light changes with temp • Energy absorbed or emitted is quantized in nature (See IV. A) • E = h (h = Planck’s Constant = 6.626*10-34 J*s) ...
... • Predicted how the spectrum of light changes with temp • Energy absorbed or emitted is quantized in nature (See IV. A) • E = h (h = Planck’s Constant = 6.626*10-34 J*s) ...
Advanced Quantum Physics - Theory of Condensed Matter
... gases; degeneracy pressure in neutron stars; Bose-Einstein condensation in ultracold atomic gases. ...
... gases; degeneracy pressure in neutron stars; Bose-Einstein condensation in ultracold atomic gases. ...
Generalized Momentum Operators
... my own attention while writing the final pages of “E. T. Whittaker’s quantum formalism” (). I make reference there to (among other papers) Peter D. Robinson & Joseph O. Hirschfelder, “Generalized momentum operators in quantum mechanics,” J. Math. Phys. 4, 338 (1963) and Peter D. Robinson, “Integ ...
... my own attention while writing the final pages of “E. T. Whittaker’s quantum formalism” (). I make reference there to (among other papers) Peter D. Robinson & Joseph O. Hirschfelder, “Generalized momentum operators in quantum mechanics,” J. Math. Phys. 4, 338 (1963) and Peter D. Robinson, “Integ ...
Chemistry 102 Summary June 25th - Bohr model only works for one
... Chemistry 102 Summary June 25th ...
... Chemistry 102 Summary June 25th ...
Laboratory Exercise: The Electronic Structure of the Hydrogen Atom
... where h represents Planck’s constant; h = 6.62608 x 10-34 Jsec. Photons can cover a wide range of energies, from very low energy Radio Waves to high energy Gamma Radiation. This Electromagnetic Spectrum of photon wavelengths is represented below: ...
... where h represents Planck’s constant; h = 6.62608 x 10-34 Jsec. Photons can cover a wide range of energies, from very low energy Radio Waves to high energy Gamma Radiation. This Electromagnetic Spectrum of photon wavelengths is represented below: ...
Quantum Tunneling - Santa Rosa Junior College
... Particles that we had assumed to be definite in their properties, (position, energy, time, momentum…) can only be described as distributions of probability. These distributions have another limitation. Due to our methods of detection, we are restricted to never knowing two properties of a particle s ...
... Particles that we had assumed to be definite in their properties, (position, energy, time, momentum…) can only be described as distributions of probability. These distributions have another limitation. Due to our methods of detection, we are restricted to never knowing two properties of a particle s ...
6. Quantum Mechanics II
... In any measurement of the observable associated with an operator A, ˆ the only values that can ever be observed are the eigenvalues. Eigenvalues are the possible values of a in the Eigenvalue Equation: ...
... In any measurement of the observable associated with an operator A, ˆ the only values that can ever be observed are the eigenvalues. Eigenvalues are the possible values of a in the Eigenvalue Equation: ...
Multi-Electron Atoms Helium Schrödinger Equation
... More general principle: Particles fall into two kinds that exclusively described by either antisymmetric (Fermions) or symmetric (Bosons) total eigenfunctions. Empirical Finding: ...
... More general principle: Particles fall into two kinds that exclusively described by either antisymmetric (Fermions) or symmetric (Bosons) total eigenfunctions. Empirical Finding: ...
Document
... electric and magnetic fields. In general, the energy per unit volume in an electric field is given by: O In a magnetic field, the energy per unit volume is: O An electromagnetic wave has both electric and magnetic ...
... electric and magnetic fields. In general, the energy per unit volume in an electric field is given by: O In a magnetic field, the energy per unit volume is: O An electromagnetic wave has both electric and magnetic ...
A Fresh View for Maxwell`s Equations and Electromagnetic Wave
... for diffraction phenomenon has been suggested where the separation between two wave fronts is not infinitesimally small but it is found to be λ/2 where the density of photons or particles is maximum. This has explained the observed diffraction pattern successfully for circular aperture without ad ho ...
... for diffraction phenomenon has been suggested where the separation between two wave fronts is not infinitesimally small but it is found to be λ/2 where the density of photons or particles is maximum. This has explained the observed diffraction pattern successfully for circular aperture without ad ho ...
By: 3rd Period Chemistry Actinide Ionization Energy Probability
... of four quantum numbers Random set of numbers ...
... of four quantum numbers Random set of numbers ...
Fall 2011 CHEM 760: Introductory Quantum Chemistry Homework 9
... this hypothetical universe. The spin portion of your wave function should be in terms of the spin functions (ms = 1), (ms = 1), and (ms = -1). b) For each of the trial functions you wrote down above, what is the value of the projection of the total spin angular momentum vector onto the z-axis, ...
... this hypothetical universe. The spin portion of your wave function should be in terms of the spin functions (ms = 1), (ms = 1), and (ms = -1). b) For each of the trial functions you wrote down above, what is the value of the projection of the total spin angular momentum vector onto the z-axis, ...
CHAPTER 7 LEARNING OBJECTIVES - crypt
... Annotate the graph with the answers to the following questions. Q1. Add a line to the graph for a metal with a lower work function than the one depicted. Q2. Show how you would determine the work function for a metal from the graph. Q3. Highlight the point on the graph corresponding to irradiation o ...
... Annotate the graph with the answers to the following questions. Q1. Add a line to the graph for a metal with a lower work function than the one depicted. Q2. Show how you would determine the work function for a metal from the graph. Q3. Highlight the point on the graph corresponding to irradiation o ...
Lecture 6 - physics.udel.edu
... L6.P6 The excited state can be both triplet or singlet state since the electrons are in different states. We can constrict both symmetric and antisymmetric spatial wave functions. Symmetric spatial wave function will go with singlet spin state (parahelium) and antisymmetric one will be triplet (ort ...
... L6.P6 The excited state can be both triplet or singlet state since the electrons are in different states. We can constrict both symmetric and antisymmetric spatial wave functions. Symmetric spatial wave function will go with singlet spin state (parahelium) and antisymmetric one will be triplet (ort ...