Electron configuration of atoms
... This meant that it was now possible to identify the chemical composition of distant objects like the sun and other stars. They concluded that the Fraunhofer lines in the solar spectrum were due to the absorption of light by the atoms of various elements in the sun's ...
... This meant that it was now possible to identify the chemical composition of distant objects like the sun and other stars. They concluded that the Fraunhofer lines in the solar spectrum were due to the absorption of light by the atoms of various elements in the sun's ...
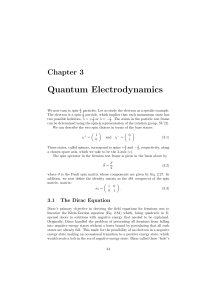
Quantum Electrodynamics
... These states, called spinors, correspond to spins + 21 and − 21 , respectively, along a chosen space axis, which we take to be the 3-axis (z). The spin operator in the fermion rest frame is given in the basis above by ...
... These states, called spinors, correspond to spins + 21 and − 21 , respectively, along a chosen space axis, which we take to be the 3-axis (z). The spin operator in the fermion rest frame is given in the basis above by ...
The Inverse Square Law The Inverse Square Law
... same area diminishes inversely with the square of the radius. This result is true not only for electromagnetic waves, but for any mechanical wave produced by a point source. It is the reason why you cannot read a book by starlight or hear what the players on the field are saying from up in the stand ...
... same area diminishes inversely with the square of the radius. This result is true not only for electromagnetic waves, but for any mechanical wave produced by a point source. It is the reason why you cannot read a book by starlight or hear what the players on the field are saying from up in the stand ...
Electrons in Atoms
... seperated into subshells each of which is represented with angular momentum quantum number “l” .This determines the geometrical shape of the electron probability distribution. The number “l” can have all values ranging from 0, 1, 2 to n-1. For n=1 the maximum and unique value of “l” is 0 which means ...
... seperated into subshells each of which is represented with angular momentum quantum number “l” .This determines the geometrical shape of the electron probability distribution. The number “l” can have all values ranging from 0, 1, 2 to n-1. For n=1 the maximum and unique value of “l” is 0 which means ...
12.3 Assembly of distinguishable Particles
... • The most “disordered” macrostate is the state with the highest probability. • The macrostate with the highest thermodynamic probability will be the observed equilibrium state of the system. • The statistical model suggests that systems tend to change spontaneously from states with low thermodynam ...
... • The most “disordered” macrostate is the state with the highest probability. • The macrostate with the highest thermodynamic probability will be the observed equilibrium state of the system. • The statistical model suggests that systems tend to change spontaneously from states with low thermodynam ...
Posttest for Uncertainty Principle Part 1
... 3. Suppose at time t=0, the position space wavefunction for a particle is not given explicitly but its momentum space wavefunction is given. Is it possible to determine the uncertainty in the position of the particle at time t>0 without knowing the Hamiltonian of the system? Explain. ...
... 3. Suppose at time t=0, the position space wavefunction for a particle is not given explicitly but its momentum space wavefunction is given. Is it possible to determine the uncertainty in the position of the particle at time t>0 without knowing the Hamiltonian of the system? Explain. ...
PHY4605–Introduction to Quantum Mechanics II Spring 1997 Problem Set 4 Jan. 31, 2005
... point-proton model to be δV (r) = V1 (r) − V0 (r). Use 1st-order perturbation theory to find the correction δE0 to the ground state energy due to the finite size of the proton. Obtain a numerical value for the fractional correction δE0 /E0 . (Hint: you can simplify the calculation by noting that r0 ...
... point-proton model to be δV (r) = V1 (r) − V0 (r). Use 1st-order perturbation theory to find the correction δE0 to the ground state energy due to the finite size of the proton. Obtain a numerical value for the fractional correction δE0 /E0 . (Hint: you can simplify the calculation by noting that r0 ...
File
... (13) Define wavelength and frequency and state the units used to measure each quantity. (14) Perform calculations involving wavelength, frequency, and energy, giving answers with the appropriate units and significant figures. (15) Describe the experiment used to show the photoelectric effect and the ...
... (13) Define wavelength and frequency and state the units used to measure each quantity. (14) Perform calculations involving wavelength, frequency, and energy, giving answers with the appropriate units and significant figures. (15) Describe the experiment used to show the photoelectric effect and the ...
Quanta and Waves - Calderglen High School
... As the temperature increases, the amount of emitted radiation increases and the peaks of the wavelength distributions shift towards higher frequencies (shorter wavelengths). The graph of specific intensity against wavelength is similar to the frequency graph but the shape is reversed. For the wavel ...
... As the temperature increases, the amount of emitted radiation increases and the peaks of the wavelength distributions shift towards higher frequencies (shorter wavelengths). The graph of specific intensity against wavelength is similar to the frequency graph but the shape is reversed. For the wavel ...
Corso di Fisica Moderna
... It is now apparent why Rydberg atoms have such peculiar properAes: the radius of the orbit scales as n2 (the n = 137 state of hydrogen has an atomic radius ~1 µm) and the geometric cross-‐secA ...
... It is now apparent why Rydberg atoms have such peculiar properAes: the radius of the orbit scales as n2 (the n = 137 state of hydrogen has an atomic radius ~1 µm) and the geometric cross-‐secA ...
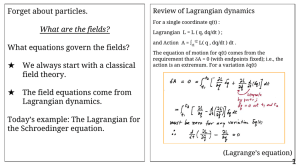
Forget about particles. What equations govern the fields? What are the fields?
... For many degrees of freedom… q(t) ⟶ Q(t) ≡ { qi(t) ; i = 1 2 3 … i … N } ...
... For many degrees of freedom… q(t) ⟶ Q(t) ≡ { qi(t) ; i = 1 2 3 … i … N } ...
L 32 Light and Optics-4 Light “rays” travel in straight lines Wave or
... • Thus far we have been dealing only with geometrical optics • In geometrical optics we deal only with the behavior of light rays it either travels in a straight line or is reflected by a mirror, or bent (refracted) when it travels from one medium into another. • However, light is a WAVE, and ther ...
... • Thus far we have been dealing only with geometrical optics • In geometrical optics we deal only with the behavior of light rays it either travels in a straight line or is reflected by a mirror, or bent (refracted) when it travels from one medium into another. • However, light is a WAVE, and ther ...
L32.ppt
... • Today we will look at effects related to the wave nature of light – physical optics – polarization – interference • thin film interference • diffraction • resolving close objects ...
... • Today we will look at effects related to the wave nature of light – physical optics – polarization – interference • thin film interference • diffraction • resolving close objects ...
L 32.ppt
... • Today we will look at effects related to the wave nature of light – physical optics – polarization – interference • thin film interference • diffraction • resolving close objects ...
... • Today we will look at effects related to the wave nature of light – physical optics – polarization – interference • thin film interference • diffraction • resolving close objects ...
final exam review pdf
... b. charging by polarization d. neutralization. 8) A difference between insulators and conductors is that in conductors a. Electrons can be removed from atoms easily. b. Electrons are free to move around. c. Electrons carry electric charge d. All of the above 9) When I throw an apple up into the air. ...
... b. charging by polarization d. neutralization. 8) A difference between insulators and conductors is that in conductors a. Electrons can be removed from atoms easily. b. Electrons are free to move around. c. Electrons carry electric charge d. All of the above 9) When I throw an apple up into the air. ...
4.quantumorbitals
... Quantum Theory The electron is like a cloud of negative energy or a wave. Orbitals are areas in 3D space where the electrons most probably are. The energy of the electron is in its vibrational modes- like notes on a guitar string. Photons are produced when high energy modes change to lower energy mo ...
... Quantum Theory The electron is like a cloud of negative energy or a wave. Orbitals are areas in 3D space where the electrons most probably are. The energy of the electron is in its vibrational modes- like notes on a guitar string. Photons are produced when high energy modes change to lower energy mo ...
PPT
... Here's the key point: if you have just one particle, going through two slits, the two paths show interference only if they get to the same place. The (x,y,z,t) coordinates must all be the same. The wave function representing MANY particles is a function of ALL their coordinates, so if there are two ...
... Here's the key point: if you have just one particle, going through two slits, the two paths show interference only if they get to the same place. The (x,y,z,t) coordinates must all be the same. The wave function representing MANY particles is a function of ALL their coordinates, so if there are two ...