Matter and Energy Identify a chemical physical change Identify a
... Energy, frequency Orbital Bohr model Paramagnetic vs. diamagnetic Isoelectronic Aufbau Principle Heisenberg Uncertainty Principle Hund’s rule Pauli exclusion Principle Ground and excited state Sublevels s p d f ...
... Energy, frequency Orbital Bohr model Paramagnetic vs. diamagnetic Isoelectronic Aufbau Principle Heisenberg Uncertainty Principle Hund’s rule Pauli exclusion Principle Ground and excited state Sublevels s p d f ...
Lighting the Way
... • Infrared lamps produce heat for personal and industrial use. Now that feels better than saving money! ...
... • Infrared lamps produce heat for personal and industrial use. Now that feels better than saving money! ...
41 Chapter 4 Atomic Structure 4.1 The Nuclear Atom J. J. Thomson
... Geiger and Marsden (1911) shot alpha particles at thin (much less than the thickness of a human hair) gold foils. Alpha particles are helium atoms minus their electrons, so they have a charge of +2e. In the Thomson model, the electric charge is smeared out over the atomic volume, and no (or very wea ...
... Geiger and Marsden (1911) shot alpha particles at thin (much less than the thickness of a human hair) gold foils. Alpha particles are helium atoms minus their electrons, so they have a charge of +2e. In the Thomson model, the electric charge is smeared out over the atomic volume, and no (or very wea ...
Quantum Theory of the Atom
... A. The Quantum Mechanical Model assigns quantum numbers to indicate the relative sizes and energies of atomic orbitals. B. There are three things for every electron 1. Principal energy level (principal quantum number, ...
... A. The Quantum Mechanical Model assigns quantum numbers to indicate the relative sizes and energies of atomic orbitals. B. There are three things for every electron 1. Principal energy level (principal quantum number, ...
Chapter 30 Quantum Physics
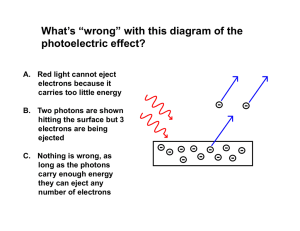
... 1. Any beam of light of any color can eject electrons if it is intense enough. 2. The maximum kinetic energy of an ejected electron should increase as the intensity increases. Observations: 1. Light must have a certain minimum frequency in order to eject electrons. 2. More intensity results in more ...
... 1. Any beam of light of any color can eject electrons if it is intense enough. 2. The maximum kinetic energy of an ejected electron should increase as the intensity increases. Observations: 1. Light must have a certain minimum frequency in order to eject electrons. 2. More intensity results in more ...
rev8thgrade - PAMS
... A chemical equation represents the change that takes place in a chemical reaction • In a chemical equation, the chemical formulas of the reactants are written on the left; an arrow indicates a change to a new substance; and the chemical formulas of the products are written on the right ...
... A chemical equation represents the change that takes place in a chemical reaction • In a chemical equation, the chemical formulas of the reactants are written on the left; an arrow indicates a change to a new substance; and the chemical formulas of the products are written on the right ...
Quantum Theory
... Quantum Theory, in physics, description of the particles that make up matter and how they interact with each other and with energy. Quantum theory explains in principle how to calculate what will happen in any experiment involving physical or biological systems, and how to understand how our world w ...
... Quantum Theory, in physics, description of the particles that make up matter and how they interact with each other and with energy. Quantum theory explains in principle how to calculate what will happen in any experiment involving physical or biological systems, and how to understand how our world w ...
The Bohr model for the electrons
... the atom lay in accepting the wave properties of electrons De Broglie wave-particle duality All particles have a wavelength – wavelike nature. – Significant only for very small particles – like electrons or photons – As mass increases, wavelength decreases ...
... the atom lay in accepting the wave properties of electrons De Broglie wave-particle duality All particles have a wavelength – wavelike nature. – Significant only for very small particles – like electrons or photons – As mass increases, wavelength decreases ...
Atomic Structure and the Periodic Table Atomic Structure and the
... properties of the elements are a periodic function of their atomic number. Periodic Table modern relates chemical and physical properties of Groups of elements ...
... properties of the elements are a periodic function of their atomic number. Periodic Table modern relates chemical and physical properties of Groups of elements ...
with x
... A cat is placed in a closed box. Inside the box a radioactive source is placed in which on average once per hour a radioactive decay takes place. If the decay takes place, a bottle of poison breaks, killing the cat. In quantum-physical sense, the cat is 50% dead and 50 alive after half an hour. Sinc ...
... A cat is placed in a closed box. Inside the box a radioactive source is placed in which on average once per hour a radioactive decay takes place. If the decay takes place, a bottle of poison breaks, killing the cat. In quantum-physical sense, the cat is 50% dead and 50 alive after half an hour. Sinc ...
General Scattering and Resonance – Getting Started
... Each boundary has two conditions: the wave function must be continuous across the boundary and the first derivative (with respect to x) must be continuous across the boundary. Exercise 2: Write down the four boundary conditions for the situation shown in figure 2, using the wave functions of exerci ...
... Each boundary has two conditions: the wave function must be continuous across the boundary and the first derivative (with respect to x) must be continuous across the boundary. Exercise 2: Write down the four boundary conditions for the situation shown in figure 2, using the wave functions of exerci ...
Higher Level Multi A 1. Natalie measures the mass and speed of a
... A resistor is connected in series with an alternating current supply of negligible internal resistance. The peak value of the supply voltage is Vo and the peak value of the current in the resistor is I0. The average power dissipation in the resistor is A. ...
... A resistor is connected in series with an alternating current supply of negligible internal resistance. The peak value of the supply voltage is Vo and the peak value of the current in the resistor is I0. The average power dissipation in the resistor is A. ...
electron
... A portion of the complete spectrum of hydrogen is shown here. The lines cannot be explained by the Rutherford theory. ...
... A portion of the complete spectrum of hydrogen is shown here. The lines cannot be explained by the Rutherford theory. ...
Quiz 9
... The atomic number, Z; i.e. the number of protons in the nucleus of the atom determines the ground state energy of an atom. The reason is that the potential experienced by an electron depends on the total charge, hence Z, of the nucleus. 2. In your own words, what are degenerate states. ...
... The atomic number, Z; i.e. the number of protons in the nucleus of the atom determines the ground state energy of an atom. The reason is that the potential experienced by an electron depends on the total charge, hence Z, of the nucleus. 2. In your own words, what are degenerate states. ...
K - Christian J. Bordé
... by direct comparison with Z0 (Lampard experiment), but also determine KJ thanks to the watt balance used with Z0 instead of RK, without any hypothesis on the formulas which connect these constants to e, h and ...
... by direct comparison with Z0 (Lampard experiment), but also determine KJ thanks to the watt balance used with Z0 instead of RK, without any hypothesis on the formulas which connect these constants to e, h and ...
The hydrogen line spectrum explained as Raman shift
... visible region and the alleged corresponding quantum jumps of the electron between quantazized energy levels according to Bohr’s atomic model. [hy] Does the hydrogen spectrum indicate some features of the atomic structure? Indeed, physicists observed patterns or series of spectral lines of hydrogen. ...
... visible region and the alleged corresponding quantum jumps of the electron between quantazized energy levels according to Bohr’s atomic model. [hy] Does the hydrogen spectrum indicate some features of the atomic structure? Indeed, physicists observed patterns or series of spectral lines of hydrogen. ...
The Photoelectric Effect: Determination of Planck`s Constant
... would require a delay from when the light first hit the surface to when the first electron was ejected. But, experimentally it is seen that the emission of electrons occurs very shortly after the arrival of the radiation. Motivated by these, Einstein produced one of his seminal papers in 1905, “A he ...
... would require a delay from when the light first hit the surface to when the first electron was ejected. But, experimentally it is seen that the emission of electrons occurs very shortly after the arrival of the radiation. Motivated by these, Einstein produced one of his seminal papers in 1905, “A he ...
semester ii
... –expression for heat and work – rotational energy levels for diatomic molecules – vibrational energy levels for diatomic molecules – factorizing the partition function – equipartition theorem – minimizing the free energy. Statistics of Identical Particles (4 Hrs) Identical particles – symmetric and ...
... –expression for heat and work – rotational energy levels for diatomic molecules – vibrational energy levels for diatomic molecules – factorizing the partition function – equipartition theorem – minimizing the free energy. Statistics of Identical Particles (4 Hrs) Identical particles – symmetric and ...
Chemistry: Matter and Change
... Matter can gain or lose energy only in small, specific amounts called quanta. ...
... Matter can gain or lose energy only in small, specific amounts called quanta. ...
DeBroglie Hypothesis
... Heisenberg Uncertainty Principle b) A particle does have a definite location at a specific time, but it does not have a frequency or wavelength. c) Inbetween case: a group of sine waves can add together (via Fourier analysis) to give a semi-definite location: a result of Fourier analysis is this: t ...
... Heisenberg Uncertainty Principle b) A particle does have a definite location at a specific time, but it does not have a frequency or wavelength. c) Inbetween case: a group of sine waves can add together (via Fourier analysis) to give a semi-definite location: a result of Fourier analysis is this: t ...
Section1 Final Key
... 5. (10 pts) Postulates and Principles: True/False. T / F : According to the variational principle, the quantum mechanical energy is always lower than the classical energy. T / F: A spherical harmonic function Ylm (θ, φ) is an eigenfunction of the L̂2 operator with eigenvalue h̄2 l(l + 1). T / F : A ...
... 5. (10 pts) Postulates and Principles: True/False. T / F : According to the variational principle, the quantum mechanical energy is always lower than the classical energy. T / F: A spherical harmonic function Ylm (θ, φ) is an eigenfunction of the L̂2 operator with eigenvalue h̄2 l(l + 1). T / F : A ...