www Resource Links
... notification of the change of status to the local IRB and a copy of any responses from the IRB/IEC. If a clinical protocol has been reviewed by the an Institution Biosafety Committee (IBC) or the Recombinant DNA Advisory Committee (RAC), the awardee must provide information about the initial and ong ...
... notification of the change of status to the local IRB and a copy of any responses from the IRB/IEC. If a clinical protocol has been reviewed by the an Institution Biosafety Committee (IBC) or the Recombinant DNA Advisory Committee (RAC), the awardee must provide information about the initial and ong ...
UCSF Cancer Center Phase I Protocol Template
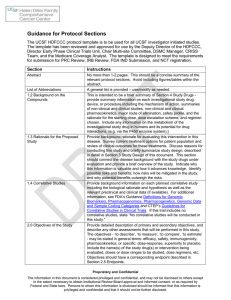
... including the biological rationale and hypothesis as well as the relevant preclinical and clinical data (if available). For additional information, see FDA’s Guidance Definitions for Genomic Biomarkers, Pharmacogenomics, Pharmacogenetics, Genomic Data and Sample Coding Categories and CTEP’s Guidelin ...
... including the biological rationale and hypothesis as well as the relevant preclinical and clinical data (if available). For additional information, see FDA’s Guidance Definitions for Genomic Biomarkers, Pharmacogenomics, Pharmacogenetics, Genomic Data and Sample Coding Categories and CTEP’s Guidelin ...
No. 57 23 July 2015 Dear Healthcare Professional RESTRICTIONS
... efficacy for approved indications of metoclopramide, while the risks of neurological and cardiovascular adverse events are known.1 The review, completed in December 2013, confirmed the relationship between the use of high doses or long-term use of metoclopramide and the increased risks of neurologic ...
... efficacy for approved indications of metoclopramide, while the risks of neurological and cardiovascular adverse events are known.1 The review, completed in December 2013, confirmed the relationship between the use of high doses or long-term use of metoclopramide and the increased risks of neurologic ...
CDASH Serious Adverse Event Supplement Version 1
... the criteria of an SAE. Of those SAEs received by the sponsor, a still smaller subset will meet the regulatory requirements for expedited reporting to the regulatory authorities. These usually are the SAEs that are both unexpected and suspected, also known as Serious Unexpected Suspected Adverse Rea ...
... the criteria of an SAE. Of those SAEs received by the sponsor, a still smaller subset will meet the regulatory requirements for expedited reporting to the regulatory authorities. These usually are the SAEs that are both unexpected and suspected, also known as Serious Unexpected Suspected Adverse Rea ...
Unanticipated Problems, Adverse Events, and Protocol Deviations
... following outcomes: death; a life threatening experience; inpatient hospitalization or prolongation of existing hospitalization; a persistent or significant disability/incapacity; or a congenital anomaly/birth defect. In addition, events that may not result in death, be life-threatening, or require ...
... following outcomes: death; a life threatening experience; inpatient hospitalization or prolongation of existing hospitalization; a persistent or significant disability/incapacity; or a congenital anomaly/birth defect. In addition, events that may not result in death, be life-threatening, or require ...
Adverse events associated with dietary supplements - Direct-MS
... form, to promote uniform records that might not have been otherwise obtained. In particular, the study form assessed causality by standard methods.6 It included questions about confounding factors (such as medical ...
... form, to promote uniform records that might not have been otherwise obtained. In particular, the study form assessed causality by standard methods.6 It included questions about confounding factors (such as medical ...
Dictionary of Pharmaceutical Medicine
... cil for international organisation of medical science) reports always refer to a suspect reaction (in contrast to event or experience), which implies that a physician or other professional health care worker has judged it a reasonable possibility that an observed clinical occurrence has been caused ...
... cil for international organisation of medical science) reports always refer to a suspect reaction (in contrast to event or experience), which implies that a physician or other professional health care worker has judged it a reasonable possibility that an observed clinical occurrence has been caused ...
pharmaceuticals: restrictions in use and availability
... from governments; products which clearly do not meet the criteria have been omitted after consultation with governments. Information received from non-governmental organizations has, in each case, been verified with governments. The information provided also includes references to relevant legal or ...
... from governments; products which clearly do not meet the criteria have been omitted after consultation with governments. Information received from non-governmental organizations has, in each case, been verified with governments. The information provided also includes references to relevant legal or ...
Methods for assessing the preventability of Linköping University Post Print
... Preventable ADEs occur in both outpatient and inpatient settings (4-7). The estimated frequencies of preventable ADEs and the supposed preventability rates of ADEs vary considerably in different studies. A systematic review of preventable ADEs in ambulatory care found that per 1000 patient-months th ...
... Preventable ADEs occur in both outpatient and inpatient settings (4-7). The estimated frequencies of preventable ADEs and the supposed preventability rates of ADEs vary considerably in different studies. A systematic review of preventable ADEs in ambulatory care found that per 1000 patient-months th ...
1.1. An overview of reports on agomelatine Introduction
... yet to be determined [2]. Since Valdoxan has obtained a market authorization from EMA, there has been some discussion regarding the risk-benefit balance of this drug. According to a recently published article, several international experts are not convinced agomelatine has additional value in the tr ...
... yet to be determined [2]. Since Valdoxan has obtained a market authorization from EMA, there has been some discussion regarding the risk-benefit balance of this drug. According to a recently published article, several international experts are not convinced agomelatine has additional value in the tr ...
FINDING POTENTIALLY UNSAFE NUTRITIONAL SUPPLEMENTS
... the ADR dictionary could only have been chosen from a marked subset of topics. Though we do not label topics, we guarantee that tokens that match the tokens in the ADR dictionary are restricted to those subsets. The intent of this restriction is that the subset of topics containing ADR tokens will a ...
... the ADR dictionary could only have been chosen from a marked subset of topics. Though we do not label topics, we guarantee that tokens that match the tokens in the ADR dictionary are restricted to those subsets. The intent of this restriction is that the subset of topics containing ADR tokens will a ...
Adverse Reactions to Radiopharmaceuticals
... Adverse Reaction/ Adverse Drug Reaction (ADR) means a harmful and unintended response to a human medicine, occurring at doses normally used for the diagnosis or treatment of a disease, or for the restoration, correction or modification of a physiological function. In this context, an adverse reactio ...
... Adverse Reaction/ Adverse Drug Reaction (ADR) means a harmful and unintended response to a human medicine, occurring at doses normally used for the diagnosis or treatment of a disease, or for the restoration, correction or modification of a physiological function. In this context, an adverse reactio ...
Final Program - International Society for Pharmacoepidemiology
... and Women’s Hospital & Harvard Medical School u Division of Pharmacy Practice & Administrative Sciences, College of Pharmacy, University of Cincinnati u Drug Safety Research Unit, Associated Department, School of Pharmacy, Portsmouth University u Harvard School of Public Health u London School of Hy ...
... and Women’s Hospital & Harvard Medical School u Division of Pharmacy Practice & Administrative Sciences, College of Pharmacy, University of Cincinnati u Drug Safety Research Unit, Associated Department, School of Pharmacy, Portsmouth University u Harvard School of Public Health u London School of Hy ...
Division of Clinical Pharmacology Department of Medicine and Health Sciences Linköping University
... method to facilitate the detection of adverse drug interaction signals in the WHO Global ICSR Database, VigiBase. Methods: All six studies included in this thesis are based on ICSRs available in VigiBase. Two studies aimed to characterise drug interactions reported in VigiBase. In the first study we ...
... method to facilitate the detection of adverse drug interaction signals in the WHO Global ICSR Database, VigiBase. Methods: All six studies included in this thesis are based on ICSRs available in VigiBase. Two studies aimed to characterise drug interactions reported in VigiBase. In the first study we ...
Electronic Yellow Card Reporting Integration into GP Systems
... Agency”), Member States and interested parties to draw up guidance on the collection, verification and presentation of adverse reaction reports in order to facilitate the exchange of information about human pharmacovigilance within the Community. Similarly, Article 26 of Regulation (EC) No 726/2004 ...
... Agency”), Member States and interested parties to draw up guidance on the collection, verification and presentation of adverse reaction reports in order to facilitate the exchange of information about human pharmacovigilance within the Community. Similarly, Article 26 of Regulation (EC) No 726/2004 ...
Rare events - The Conference Exchange
... of an adverse event, for the eventual cure might require ongoing therapy that must cease if an adverse event occurs. In this sense, the adverse event might represent informative censoring of any patient followed for a cure. (Wu 1988) Second, the investigator cannot focus on one outcome alone in any ...
... of an adverse event, for the eventual cure might require ongoing therapy that must cease if an adverse event occurs. In this sense, the adverse event might represent informative censoring of any patient followed for a cure. (Wu 1988) Second, the investigator cannot focus on one outcome alone in any ...
Protocol Template Guidelines
... withdrawal could be related to the safety profile of the study drug. If a subject withdraws consent to participate in the study, attempts should be made to obtain permission to record at least survival data up to the protocol-described end of subject follow-up period. IT MUST BE A HIGH PRIORITY TO T ...
... withdrawal could be related to the safety profile of the study drug. If a subject withdraws consent to participate in the study, attempts should be made to obtain permission to record at least survival data up to the protocol-described end of subject follow-up period. IT MUST BE A HIGH PRIORITY TO T ...
Ophthalmic adverse drug reactions to systemic drugs
... We performed risk of bias assessment for each included study and recorded it in a standardized form created to assess ADR studies (in a previous work10) and adapted to Ophthalmology after a pilot study. We did not use scales (discouraged by the Cochrane approach20) but criteria from Cochrane, STROBE ...
... We performed risk of bias assessment for each included study and recorded it in a standardized form created to assess ADR studies (in a previous work10) and adapted to Ophthalmology after a pilot study. We did not use scales (discouraged by the Cochrane approach20) but criteria from Cochrane, STROBE ...
Guideline on Risk Management Systems for Medicinal Products for
... relatively limited. This is due to many factors including the small numbers of subjects in clinical trials, restricted population in terms of age, gender and ethnicity, restricted co-morbidity, restricted comedication, restricted conditions of use, relatively short duration of exposure and follow up ...
... relatively limited. This is due to many factors including the small numbers of subjects in clinical trials, restricted population in terms of age, gender and ethnicity, restricted co-morbidity, restricted comedication, restricted conditions of use, relatively short duration of exposure and follow up ...
Strengths and weaknesses of available methods for assessing the
... Harm is usually measured in terms of the occurrence of adverse events. Adverse events have been defined as injuries related to medical management (in contrast to complications of disease) (4). According to the Institute of Medicine, a preventable adverse event is defined as one attributable to error ...
... Harm is usually measured in terms of the occurrence of adverse events. Adverse events have been defined as injuries related to medical management (in contrast to complications of disease) (4). According to the Institute of Medicine, a preventable adverse event is defined as one attributable to error ...
Clinical Research Protocol
... withdrawal could be related to the safety profile of the study drug. If a subject withdraws consent to participate in the study, attempts should be made to obtain permission to record at least survival data up to the protocol-described end of subject follow-up period. IT MUST BE A HIGH PRIORITY TO T ...
... withdrawal could be related to the safety profile of the study drug. If a subject withdraws consent to participate in the study, attempts should be made to obtain permission to record at least survival data up to the protocol-described end of subject follow-up period. IT MUST BE A HIGH PRIORITY TO T ...
Adverse Drug Reactions - CUA Law Scholarship Repository
... of adequate post-approval surveillance. Each year, the FDA receives approximately 230,000 reports of possible adverse drug reactions, and approximately ten percent of these reports raise concerns about serious reactions that pre-approval clinical trials failed to detect. 13 Yet the FDA only devotes ...
... of adequate post-approval surveillance. Each year, the FDA receives approximately 230,000 reports of possible adverse drug reactions, and approximately ten percent of these reports raise concerns about serious reactions that pre-approval clinical trials failed to detect. 13 Yet the FDA only devotes ...
Safety of allopurinol compared with other urate
... In the electronic search, a total of 617 citations were retrieved: 396 from MEDLINE, 148 from EMBASE, and 73 from the Cochrane Library. A flowchart summarizing the search results is presented in Fig. 1. In a first review based on abstracts and titles, we excluded 539 citations. Of the 40 full texts ...
... In the electronic search, a total of 617 citations were retrieved: 396 from MEDLINE, 148 from EMBASE, and 73 from the Cochrane Library. A flowchart summarizing the search results is presented in Fig. 1. In a first review based on abstracts and titles, we excluded 539 citations. Of the 40 full texts ...