Chapter 2
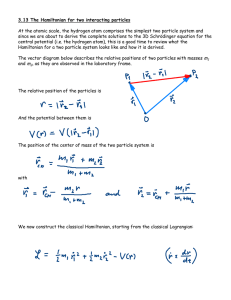
... Plugging in the values of the momentum and the positions gives the energy of the system. The Hamiltonian is the energy written in terms of p and q. The potential Energy (V) is only a function of the position. Again, apply to the H.O. case and determine the energy,(E). The Hamilton form for the energ ...
... Plugging in the values of the momentum and the positions gives the energy of the system. The Hamiltonian is the energy written in terms of p and q. The potential Energy (V) is only a function of the position. Again, apply to the H.O. case and determine the energy,(E). The Hamilton form for the energ ...
Document
... If the lowest energy level is zero, this violates the uncertainty principle. The wave function solutions are where Hn(x) are Hermite polynomials of order n. ...
... If the lowest energy level is zero, this violates the uncertainty principle. The wave function solutions are where Hn(x) are Hermite polynomials of order n. ...
Solutions for class #5 from Yosumism website Problem 1: Problem 27: YOUR NOTES:
... QM in verse... Two operators, both alike in state functions, In fair bases, where we lay our scene, From ancient grudge break new mutiny... Two operators unlike in eigenvalues Yet star-crossed lovers commute. So anyway, the problem gives eigenstate . ...
... QM in verse... Two operators, both alike in state functions, In fair bases, where we lay our scene, From ancient grudge break new mutiny... Two operators unlike in eigenvalues Yet star-crossed lovers commute. So anyway, the problem gives eigenstate . ...
Note-4
... For 1 , they are called px and py, respectively. In this case, Y10 is proportional to pz. The parity of Ym is (1) . For small m for a given , the function peaks more along the z-direction (quantization axis). For the maximum m, it peaks perpendicular to it. Terminology in spectroscopy: ...
... For 1 , they are called px and py, respectively. In this case, Y10 is proportional to pz. The parity of Ym is (1) . For small m for a given , the function peaks more along the z-direction (quantization axis). For the maximum m, it peaks perpendicular to it. Terminology in spectroscopy: ...
File
... c. atomic emission spectra d. the deflection of cathode rays by an electric field e. absorption of beta particles 3. Rutherford's gold foil experiment showed that the atom is mostly empty space because a. some alpha particles were reflected right back b. some alpha particles were deflected c. most a ...
... c. atomic emission spectra d. the deflection of cathode rays by an electric field e. absorption of beta particles 3. Rutherford's gold foil experiment showed that the atom is mostly empty space because a. some alpha particles were reflected right back b. some alpha particles were deflected c. most a ...
Particle in the box
... The wave functions represent stationary states of the particle in a one-dimensional box. The wave functions for the five lowest energies En are presented in Fig. Notice that the number of nodes of the wave functions increase by one in going from one state to the state with the next higher energy En. ...
... The wave functions represent stationary states of the particle in a one-dimensional box. The wave functions for the five lowest energies En are presented in Fig. Notice that the number of nodes of the wave functions increase by one in going from one state to the state with the next higher energy En. ...
Application of Quantum Theory 1- Particle in 1
... d. ψ2 < ψ when ψ have small values while ψ2 = ψ when ψ has the maximum value. (why) e. Node = the point where wave function passes through zero, or the position where probability of finding particle = 0 (No. of nodes = n-1) f. The probability of finding the particle between two points x 1 and x2 are ...
... d. ψ2 < ψ when ψ have small values while ψ2 = ψ when ψ has the maximum value. (why) e. Node = the point where wave function passes through zero, or the position where probability of finding particle = 0 (No. of nodes = n-1) f. The probability of finding the particle between two points x 1 and x2 are ...
Midterm Solution
... 1b. Does the improbability she/he mentions mean that there is still a finite probability that a quantum mechanical object could be in a place where its total energy is less than its potential energy? Yes P in principle No (no is acceptable if well argued due to the measurement problem, it’s no in pr ...
... 1b. Does the improbability she/he mentions mean that there is still a finite probability that a quantum mechanical object could be in a place where its total energy is less than its potential energy? Yes P in principle No (no is acceptable if well argued due to the measurement problem, it’s no in pr ...
Physics 451 - BYU Physics and Astronomy
... I have noticed in recent homeworks that more students quit to do entire problem(s). They are either short in time or overwhelmed by the length of the problems. It is understandable that this is an intense course, and the homework is time consuming. And as it is approaching the middle of the semester ...
... I have noticed in recent homeworks that more students quit to do entire problem(s). They are either short in time or overwhelmed by the length of the problems. It is understandable that this is an intense course, and the homework is time consuming. And as it is approaching the middle of the semester ...
Operators and meaning of wave function
... In this paper we focus our attention on different interpretations in quantum theory mainly how is the meaning of a wave function. Here are not substantially new results, we want to show that the most best acceptable interpretation of the wave function to take it as an operator. We have shown also a ...
... In this paper we focus our attention on different interpretations in quantum theory mainly how is the meaning of a wave function. Here are not substantially new results, we want to show that the most best acceptable interpretation of the wave function to take it as an operator. We have shown also a ...
Wave function

A wave function in quantum mechanics describes the quantum state of an isolated system of one or more particles. There is one wave function containing all the information about the entire system, not a separate wave function for each particle in the system. Its interpretation is that of a probability amplitude. Quantities associated with measurements, such as the average momentum of a particle, can be derived from the wave function. It is a central entity in quantum mechanics and is important in all modern theories, like quantum field theory incorporating quantum mechanics, while its interpretation may differ. The most common symbols for a wave function are the Greek letters ψ or Ψ (lower-case and capital psi).For a given system, once a representation corresponding to a maximal set of commuting observables and a suitable coordinate system is chosen, the wave function is a complex-valued function of the system's degrees of freedom corresponding to the chosen representation and coordinate system, continuous as well as discrete. Such a set of observables, by a postulate of quantum mechanics, are Hermitian linear operators on the space of states representing a set of physical observables, like position, momentum and spin that can, in principle, be simultaneously measured with arbitrary precision. Wave functions can be added together and multiplied by complex numbers to form new wave functions, and hence are elements of a vector space. This is the superposition principle of quantum mechanics. This vector space is endowed with an inner product such that it is a complete metric topological space with respect to the metric induced by the inner product. In this way the set of wave functions for a system form a function space that is a Hilbert space. The inner product is a measure of the overlap between physical states and is used in the foundational probabilistic interpretation of quantum mechanics, the Born rule, relating transition probabilities to inner products. The actual space depends on the system's degrees of freedom (hence on the chosen representation and coordinate system) and the exact form of the Hamiltonian entering the equation governing the dynamical behavior. In the non-relativistic case, disregarding spin, this is the Schrödinger equation.The Schrödinger equation determines the allowed wave functions for the system and how they evolve over time. A wave function behaves qualitatively like other waves, such as water waves or waves on a string, because the Schrödinger equation is mathematically a type of wave equation. This explains the name ""wave function"", and gives rise to wave–particle duality. The wave of the wave function, however, is not a wave in physical space; it is a wave in an abstract mathematical ""space"", and in this respect it differs fundamentally from water waves or waves on a string.For a given system, the choice of which relevant degrees of freedom to use are not unique, and correspondingly the domain of the wave function is not unique. It may be taken to be a function of all the position coordinates of the particles over position space, or the momenta of all the particles over momentum space, the two are related by a Fourier transform. These descriptions are the most important, but they are not the only possibilities. Just like in classical mechanics, canonical transformations may be used in the description of a quantum system. Some particles, like electrons and photons, have nonzero spin, and the wave function must include this fundamental property as an intrinsic discrete degree of freedom. In general, for a particle with half-integer spin the wave function is a spinor, for a particle with integer spin the wave function is a tensor. Particles with spin zero are called scalar particles, those with spin 1 vector particles, and more generally for higher integer spin, tensor particles. The terminology derives from how the wave functions transform under a rotation of the coordinate system. No elementary particle with spin 3⁄2 or higher is known, except for the hypothesized spin 2 graviton. Other discrete variables can be included, such as isospin. When a system has internal degrees of freedom, the wave function at each point in the continuous degrees of freedom (e.g. a point in space) assigns a complex number for each possible value of the discrete degrees of freedom (e.g. z-component of spin). These values are often displayed in a column matrix (e.g. a 2 × 1 column vector for a non-relativistic electron with spin 1⁄2).In the Copenhagen interpretation, an interpretation of quantum mechanics, the squared modulus of the wave function, |ψ|2, is a real number interpreted as the probability density of measuring a particle as being at a given place at a given time or having a definite momentum, and possibly having definite values for discrete degrees of freedom. The integral of this quantity, over all the system's degrees of freedom, must be 1 in accordance with the probability interpretation, this general requirement a wave function must satisfy is called the normalization condition. Since the wave function is complex valued, only its relative phase and relative magnitude can be measured. Its value does not in isolation tell anything about the magnitudes or directions of measurable observables; one has to apply quantum operators, whose eigenvalues correspond to sets of possible results of measurements, to the wave function ψ and calculate the statistical distributions for measurable quantities.The unit of measurement for ψ depends on the system, and can be found by dimensional analysis of the normalization condition for the system. For one particle in three dimensions, its units are [length]−3/2, because an integral of |ψ|2 over a region of three-dimensional space is a dimensionless probability.